Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of Fruit Quality
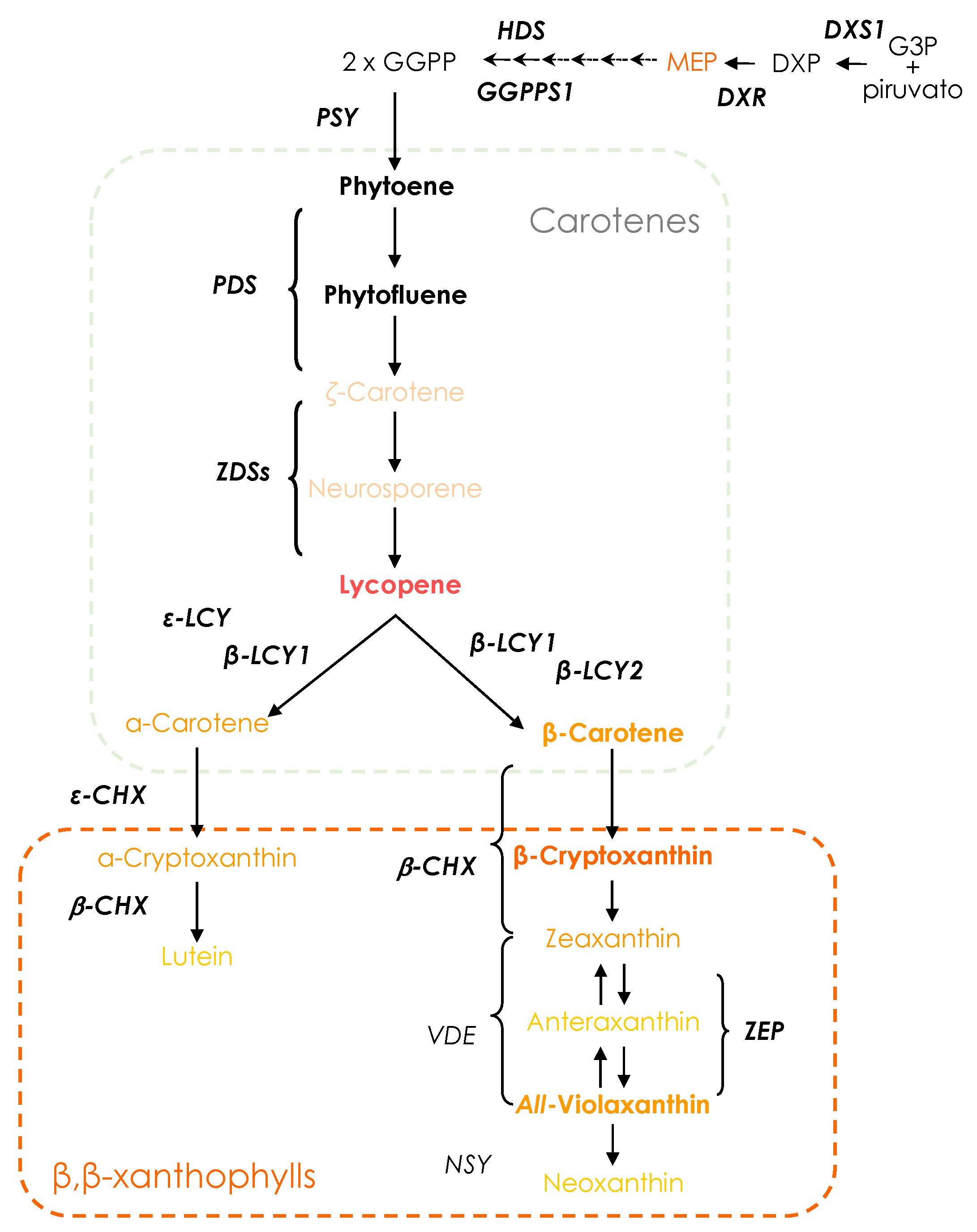
2.3. Carotenoid Extraction and UPLC Analysis of Individual Carotenoids
2.4. Total RNA Isolation and Quantitative RT-PCR Analysis
3. Results
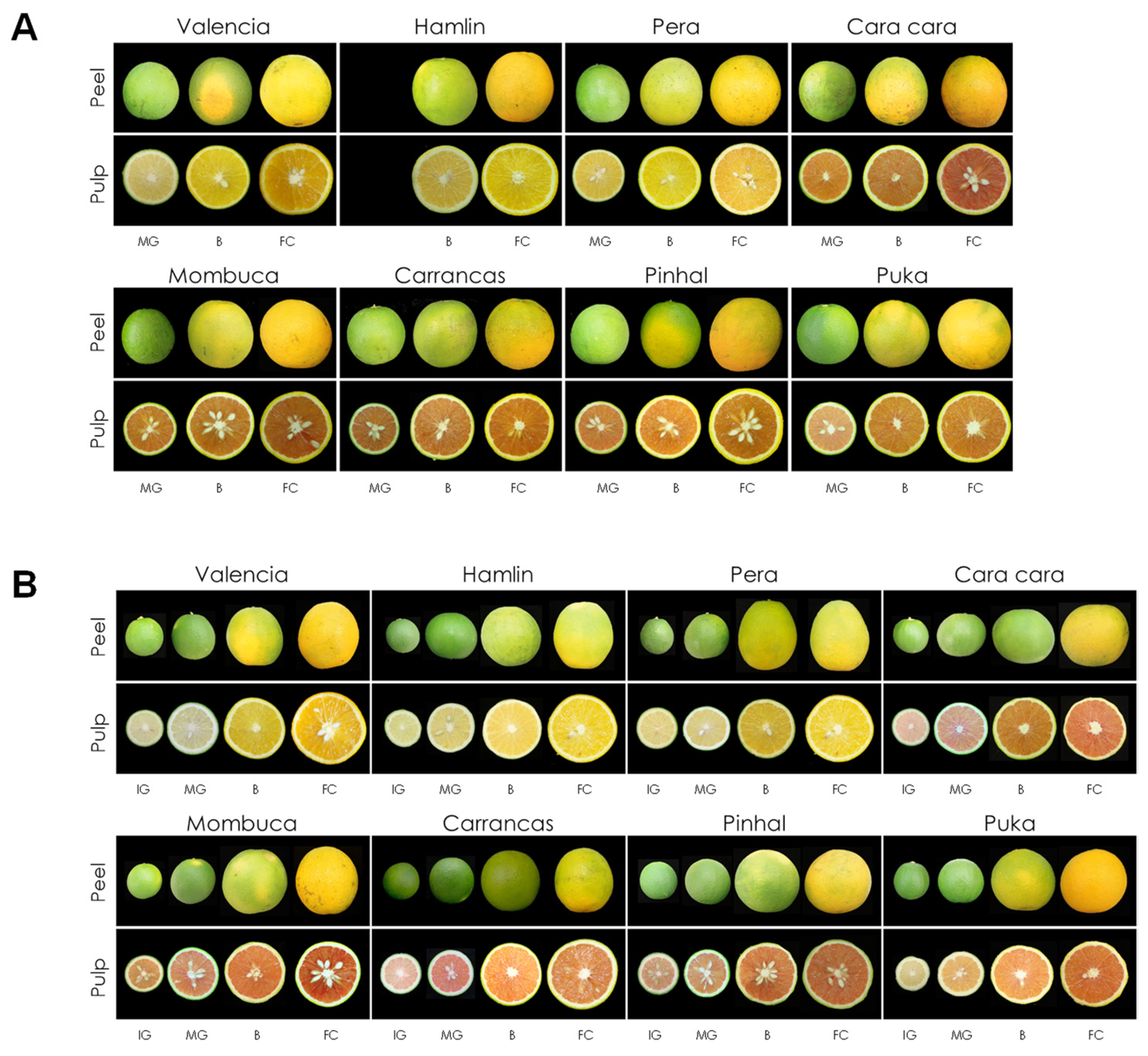
3.1. Fruit Appearance at Different Developmental Stages and Fruit Quality Parameters of New Red-Fleshed Sweet Orange Mutants
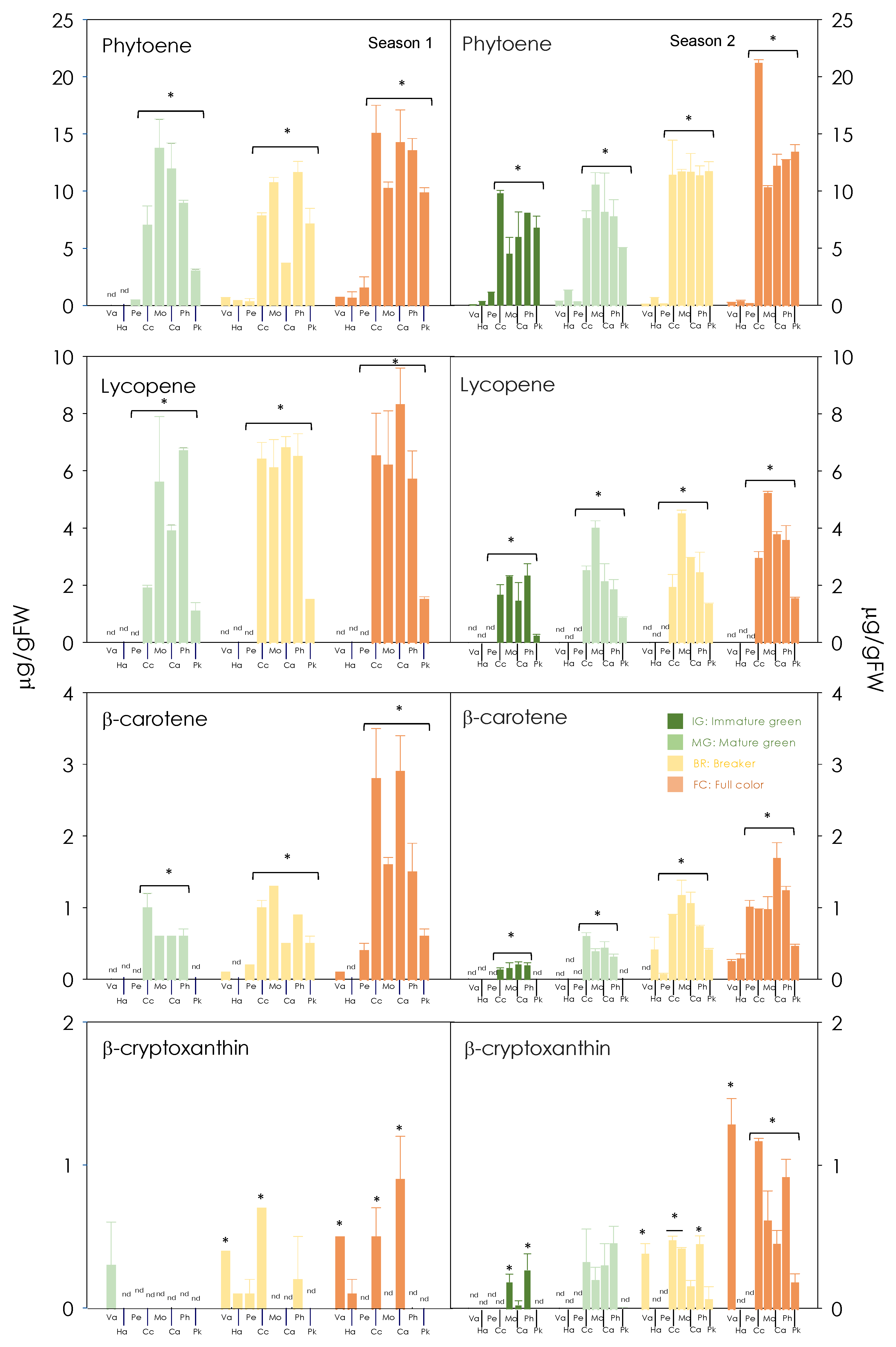
3.2. Carotenoid Composition of Red-Fleshed Sweet Orange Pulp during Maturation
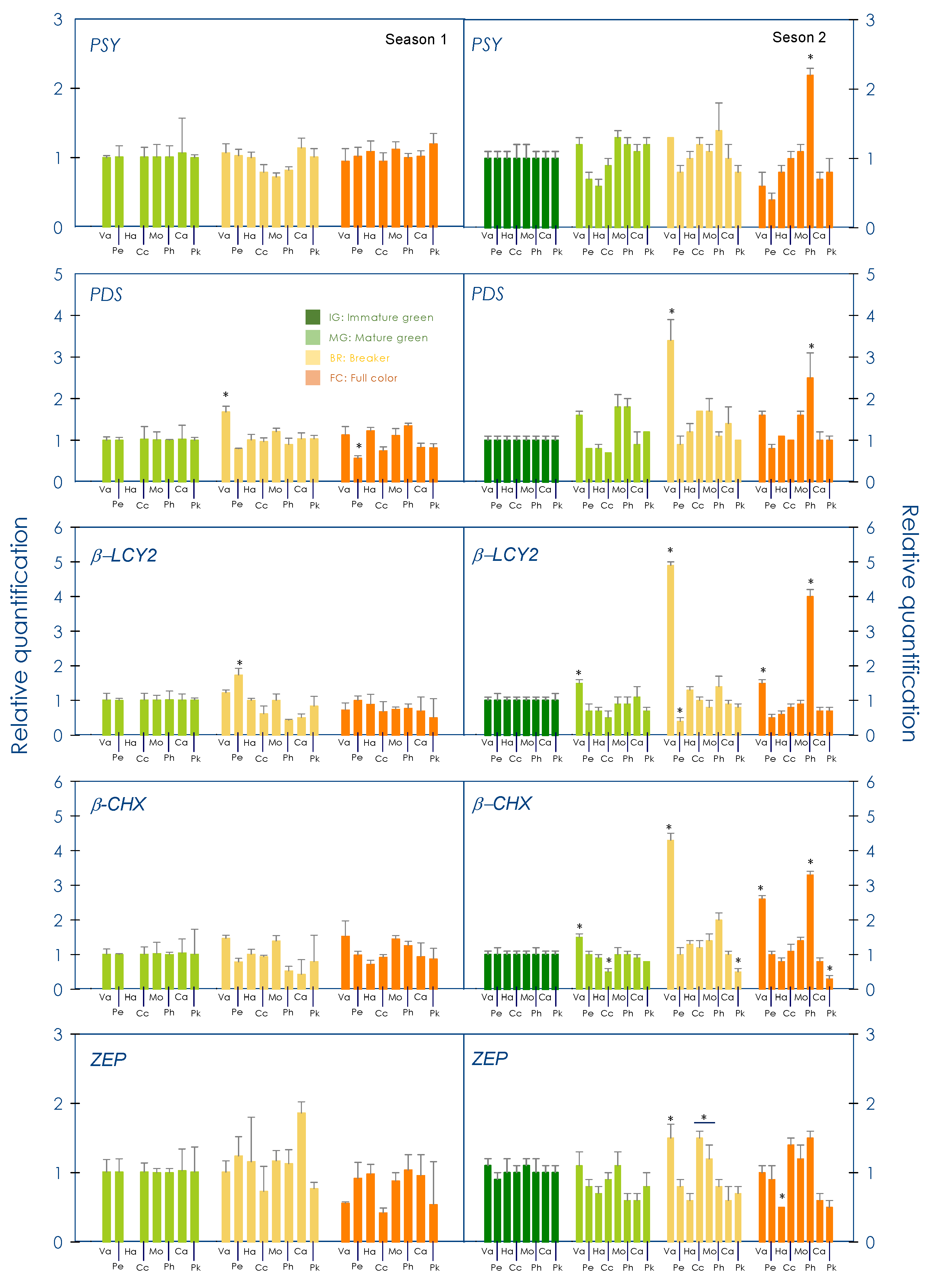
3.3. Gene Expression Profile of Carotenogenic Genes during Development and Maturation in the Pulp of Red-Fleshed Sweet Oranges
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demmig-Adams, B.; Gilmore, A.M.; Iii, W.W.A. Carotenoids 3: In Vivo Function of Carotenoids in Higher Plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The Biosynthesis and Nutritional Uses of Carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer Prevention by Carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Uchiyama, S. Osteoporosis Prevention by β-Cryptoxanthin. ACS Symp. Ser. 2008, 993, 408–418. [Google Scholar]
- Bohn, T.; Balbuena, E.; Ulus, H.; Iddir, M.; Wang, G.; Crook, N.; Eroglu, A. Carotenoids in Health as Studied by Omics-Related Endpoints. Adv. Nutr. 2023, 14, 1538–1578. [Google Scholar] [CrossRef]
- Terao, J. Revisiting Carotenoids as Dietary Antioxidants for Human Health and Disease Prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Edem, D.O. Vitamin A: A Review. Asian J. Clin. Nutr. 2009, 1, 65–82. [Google Scholar] [CrossRef]
- Burri, B.J. Beta-Cryptoxanthin as a Source of Vitamin A. J. Sci. Food Agric. 2015, 95, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Color and Carotenoid Profile of Spanish Valencia Late Ultrafrozen Orange Juices. Food Res. Int. 2005, 38, 931–936. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid Biosynthesis in Flowering Plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Dellapenna, D.; Pogson, B.J. Vitamin Synthesis in Plants: Tocopherols and Carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/ (accessed on 1 June 2023).
- Meléndez-Martínez, A.; Vicario, I.M.; Heredia, F.J. Carotenoid Pigments: Structural and Physicochemical Considerations. Arch. Latinoam. Nutr. 2007, 57, 109–117. [Google Scholar] [PubMed]
- Stewart, I. Provitamin A and Carotenoid Content of Citrus Juices. J. Am. Chem. Soc. Chromatogr. 1976, 77, 1132–1137. [Google Scholar] [CrossRef]
- Stewart, I.; Wheaton, T.A. Conversion of β-Citraurin to Reticulataxanthin and β-Apo-8′-Carotenal to Citranaxanthin during the Isolation of Carotenoids from Citrus. Phytochemistry 1973, 12, 2947–2951. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Fruits; Academic Press: London, UK, 1987. [Google Scholar]
- Alquézar, B.; Jesús Rodrigo, M.; Zacarías, L. Carotenoid Biosynthesis and Their Regulation in Citrus Fruits. Tree For. Sci. Biotechnol. 2008, 2, 23–35. [Google Scholar]
- Ikoma, Y.; Matsumoto, H.; Kato, M. Diversity in the Carotenoid Profiles and the Expression of Genes Related to Carotenoid Accumulation among Citrus Genotypes. Breed. Sci. 2016, 66, 139–147. [Google Scholar] [CrossRef]
- Römer, S.; Fraser, P.D. Recent Advances in Carotenoid Biosynthesis, Regulation and Manipulation. Planta 2005, 221, 305–308. [Google Scholar] [CrossRef]
- Kato, M.; Ikoma, Y.; Matsumoto, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of Carotenoids and Expression of Carotenoid Biosynthetic Genes during Maturation in Citrus Fruit. Plant Physiol. 2004, 134, 824–837. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Dhuique-Mayer, C.; Luro, F.; Morillon, R.; Ollitrault, P. Carotenoid Biosynthetic Pathway in the Citrus Genus: Number of Copies and Phylogenetic Diversity of Seven Genes. J. Agric. Food Chem. 2007, 55, 7405–7417. [Google Scholar] [CrossRef]
- Mendes, A.F.D.S.; Soares, V.L.F.; Costa, M.G.C. Carotenoid Biosynthesis Genomics. In Pigments in Fruits and Vegetables; Springer: New York, NY, USA, 2015; pp. 9–29. [Google Scholar]
- Rodríguez-Concepció, M.; Boronat, A. Elucidation of the Methylerythritol Phosphate Pathway for Isoprenoid Biosynthesis in Bacteria and Plastids. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M. Early Steps in Isoprenoid Biosynthesis: Multilevel Regulation of the Supply of Common Precursors in Plant Cells. Phytochem. Rev. 2006, 5, 1–15. [Google Scholar] [CrossRef]
- James, S. Citrus Varieties of the World: An Illustrated Guide; Sinclair International Ltd.: Norwich, UK, 2000. [Google Scholar]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective Effects of Lycopene in Cancer, Cardiovascular, and Neurodegenerative Diseases: An Update on Epidemiological and Mechanistic Perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Shafe, M.O.; Gumede, N.M.; Nyakudya, T.T.; Chivandi, E. Lycopene: A Potent Antioxidant with Multiple Health Benefits. J. Nutr. Metab. 2024, 2024, 6252426. [Google Scholar] [CrossRef] [PubMed]
- Monselise, S.P.; Halevy, A.H. Detection of Lycopene in Pink Orange Fruit. Science 1961, 133, 1478. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, J.; Liu, Y.; Zhao, X.; Deng, X.; Guo, L.; Gu, J. A Novel Bud Mutation That Confers Abnormal Patterns of Lycopene Accumulation in Sweet Orange Fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 2007, 58, 4161–4171. [Google Scholar] [CrossRef]
- Lee, H.S. Characterization of Carotenoids in Juice of Red Navel Orange (Cara Cara). J. Agric. Food Chem. 2001, 49, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, P. The Genus Citrus, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Pompeu, J. Porta Enxertos. In Citros; Mattos, D., de Negri, J.D., Pio, R.M., Pompeu, J., Eds.; Fundag Campinas, Instituto Agronomico: Campinas, Brazil, 2005; pp. 61–104. [Google Scholar]
- Pinheiro, T.; Nishimura, D.; de Nadai, F.; Figueira, A.; Latado, R. Selection of Reference Genes for Expression Analyses of Red-Fleshed Sweet Orange (Citrus sinensis). Genet. Mol. Res. 2015, 14, 18440–18451. [Google Scholar] [CrossRef] [PubMed]
- Pons, E.; Peris, J.E.; Peña, L. Field Performance of Transgenic Citrus Trees: Assessment of the Long-Term Expression of UidA and NptII Transgenes and Its Impact on Relevant Agronomic and Phenotypic Characteristics. BMC Biotechnol. 2012, 12, 41. [Google Scholar] [CrossRef]
- Carmona, L.; Zacarías, L.; Rodrigo, M.J. Stimulation of Coloration and Carotenoid Biosynthesis during Postharvest Storage of ‘Navelina’ Orange Fruit at 12 °C. Postharvest Biol. Technol. 2012, 74, 108–117. [Google Scholar] [CrossRef]
- de Oliveira Caland, R.B.; Cadavid, C.O.M.; Carmona, L.; Peña, L.; de Paula Oliveira, R. Pasteurized Orange Juice Rich in Carotenoids Protects Caenorhabditis Elegans against Oxidative Stress and β-Amyloid Toxicity through Direct and Indirect Mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, 5046280. [Google Scholar] [CrossRef]
- Alquezar, B.; Rodrigo, M.J.; Zacarías, L. Regulation of Carotenoid Biosynthesis during Fruit Maturation in the Red-Fleshed Orange Mutant Cara Cara. Phytochemistry 2008, 69, 1997–2007. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Marcos, J.F.; Zacarías, L. Biochemical and Molecular Analysis of Carotenoid Biosynthesis in Flavedo of Orange (Citrus sinensis L.) during Fruit Development and Maturation. J. Agric. Food Chem. 2004, 2, 6724–6731. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Marcos, J.F.; Alférez, F.; Mallent, M.D.; Zacarías, L. Characterization of Pinalate, a Novel Citrus Sinensis Mutant with a Fruit-Specific Alteration That Results in Yellow Pigmentation and Decreased ABA Content. J. Exp. Bot. 2003, 54, 727–738. [Google Scholar] [CrossRef]
- Rouseff, R.; Raley, L.; Hofsommer, H.J. Application of Diode Array Detection with a C-30 Reversed Phase Column for the Separation and Identification of Saponified Orange Juice Carotenoids. J. Agric. Food Chem. 1996, 44, 2176–2181. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Overview of Carotenoid Biosynthesis. In Biosynthesis and Metabolism; Birkhäuser Verlag: Basel, Switzerland, 1998; Volume 3, pp. 13–148. [Google Scholar]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin Biosynthesis and Accumulation in Blood Oranges during Postharvest Storage at Different Low Temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef]
- Alós, E.; Cercós, M.; Rodrigo, M.J.; Zacarías, L.; Talón, M. Regulation of Color Break in Citrus Fruits. Changes in Pigment Profiling and Gene Expression Induced by Gibberellins and Nitrate, Two Ripening Retardants. J. Agric. Food Chem. 2006, 54, 4888–4895. [Google Scholar] [CrossRef]
- Lado, J.; Cronje, P.; Alquézar, B.; Page, A.; Manzi, M.; Gómez-Cadenas, A.; Stead, A.D.; Zacarías, L.; Rodrigo, M.J. Fruit Shading Enhances Peel Color, Carotenes Accumulation and Chromoplast Differentiation in Red Grapefruit. Physiol. Plant 2015, 154, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Mafra, V.; Kubo, K.S.; Alves-Ferreira, M.; Ribeiro-Alves, M.; Stuart, R.M.; Boava, L.P.; Rodrigues, C.M.; Machado, M.A. Reference Genes for Accurate Transcript Normalization in Citrus Genotypes under Different Experimental Conditions. PLoS ONE 2012, 7, e31263. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; de Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Kawai, C.; Zhang, Y.; Lukács, G.; Chu, W.; Zheng, C.; Gao, C.; Gozli, D.; Wang, Y.; Ansorge, U. The Good, the Bad, and the Red: Implicit Color-Valence Associations across Cultures. Psychol. Res. 2023, 87, 704–724. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, J.; van Dijk, E.; Li, H.; Schnall, S. The Color Red Is Implicitly Associated with Social Status in the United Kingdom and China. Front. Psychol. 2018, 9, 1902. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A. Provitamin A Function of Carotenoids: The Conversion of Beta-Carotene into Vitamin A. J. Nutr. 1989, 119, 105–108. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Fraser, P.D.; Wang, W.J.; Bramley, P.M. Differences in the Carotenoid Content of Ordinary Citrus and Lycopene-Accumulating Mutants. J. Agric. Food Chem. 2006, 54, 5474–5481. [Google Scholar] [CrossRef] [PubMed]
- Zacarías-García, J.; Cronje, P.J.; Diretto, G.; Zacarías, L.; Rodrigo, M.J. A Comprehensive Analysis of Carotenoids Metabolism in Two Red-Fleshed Mutants of Navel and Valencia Sweet Oranges (Citrus sinensis). Front. Plant Sci. 2022, 13, 1034204. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, K.; Zhu, A.; Ye, J.; Liu, Q.; Zhang, J.; Deng, X. Comparative Transcripts Profiling Reveals New Insight into Molecular Processes Regulating Lycopene Accumulation in a Sweet Orange (Citrus sinensis) Red-Flesh Mutant. BMC Genom. 2009, 10, 540. [Google Scholar] [CrossRef]
- Enfissi, E.M.A.; Nogueira, M.; Bramley, P.M.; Fraser, P.D. The Regulation of Carotenoid Formation in Tomato Fruit. Plant J. 2017, 89, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P.M. Regulation of Carotenoid Formation during Tomato Fruit Ripening and Development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar] [CrossRef]
- Alquézar, B.; Zacarías, L.; Rodrigo, M.J. Molecular and Functional Characterization of a Novel Chromoplast-Specific Lycopene β-Cyclase from Citrus and Its Relation to Lycopene Accumulation. J. Exp. Bot. 2009, 60, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Zheng, X.; Zhu, K.; Xu, Q.; Deng, X. Isolation and Functional Characterization of a Lycopene β-Cyclase Gene Promoter from Citrus. Front. Plant Sci. 2016, 7, 1367. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef] [PubMed]

| Variety | Total Soluble Solids (°Brix) (TSS) | Titratable Acidity (g/L) (TA) | Maturity Index (MI) | Juice Content Per Orange (JC) (mL) | Fruit Weight (W) (g) | Percentage Juice Content/Fruit Weight (%) | Fruit Diameter (mm) | Fruit Height (mm) | Cortex (mm) | Peel (mm) | Number of Segments | Number of Seed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valencia | 11.7 ± 0.0 | 0.7 ± 0.3 | 13.5 ± 0.3 | 85.0 ± 11.3 | 219.3 ± 20.2 | 38.9 ± 4.6 | 73.7 ± 2.0 | 76.5 ± 3.1 | 4.6 ± 0.2 | 1.7 ± 0.1 | 10.9 ± 0.3 | 56.3 ± 7.4 |
| Hamlim | 10.8 ± 0.3 | 0.8 ± 0.0 | 14.1 ± 0.5 | 61.3 ± 5.3 | 168.8 ± 5.1 | 36.3 ± 3.2 | 68.9 ± 0.9 | 69.4 ± 0.6 | 4.4 ± 0.4 | 1.6 ± 0.1 | 10.5 ± 0.2 | 51.5 ± 6.7 |
| Pera | 12.6 ± 0.1 | 0.7 ± 0.2 | 12.8 ± 0.3 | 88.5 ± 1.5 | 207.7 ± 5.8 | 42.7 ± 1.8 | 71.1 ± 0.7 | 76.1 ± 0.7 | 5.1 ± 0.1 | 1.6 ± 0.1 | 10.6 ± 0.2 | 61.5 ± 6.4 |
| Cara Cara | 9.5 ± 0.0 | 0.5 ± 0.2 | 17.9 ± 0.7 | 38.1 ± 4.5 | 277.0 ± 26.8 | 14.4 ± 2.8 | 86.1 ± 3.8 | 88.9 ± 1.6 | 7.5 ± 0.8 | 2.5 ± 0.4 | 10.7 ± 0.3 | 0.3 ± 0.3 |
| Mombuca | 8.3 ± 0.0 | 0.5 ± 0.0 | 18.0 ± 0.6 | 92.0 ± 5.3 | 188.9 ± 8.1 | 48.6 ± 0.9 | 71.0 ± 1.2 | 72.0 ± 1.0 | 4.6 ± 0.2 | 1.7 ± 0.1 | 10.6 ± 0.2 | 144.3 ± 3.7 |
| Carrancas | 7.8 ± 0.0 | 0.4 ± 0.3 | 20.1 ± 0.7 | 55.9 ± 1.6 | 144.6 ± 7.0 | 38.7 ± 1.8 | 64.9 ± 1.3 | 65.2 ± 1.6 | 3.2 ± 0.2 | 1.8 ± 0.1 | 10.2 ± 0.4 | 69.0 ± 5.9 |
| Pinhal | 8.8 ± 0.3 | 0.5 ± 0.2 | 18.9 ± 0.3 | 54.3 ± 3.1 | 157.2 ± 2.0 | 34.5 ± 1.8 | 65.5 ± 1.0 | 68.1 ± 1.6 | 4.9 ± 0.2 | 2.2 ± 0.2 | 10.2 ± 0.2 | 137.5 ± 3.8 |
| Puka | 9.6 ± 0.2 | 0.5 ± 0.1 | 18.6 ± 0.3 | 109.1 ± 4.4 | 209.2 ± 5.4 | 52.2 ± 2.9 | 73.3 ± 0.7 | 72.9 ± 0.9 | 4.7 ± 0.1 | 1.6 ± 0.2 | 10.4 ± 0.3 | 72.5 ± 9.9 |
| Valencia | 11.8 ± 0.3 | 0.7 ± 0.2 | 16.1 ± 0.2 | 92.3 ± 1.9 | 190.6 ± 4.3 | 48.4 ± 1.1 | 70.4 ± 0.8 | 72.5 ± 0.7 | 6.1 ± 1.3 | 1.1 ± 0.1 | 10.8 ± 0.2 | 45.0 ± 5.3 |
| Hamlim | 9.5 ± 0.1 | 0.7 ± 0.2 | 14.5 ± 0.2 | 80.0 ± 0.6 | 152.7 ± 2.2 | 52.4 ± 0.8 | 66.3 ± 0.7 | 69.3 ± 1.1 | 4.4 ± 0.2 | 2.0 ± 0.0 | 10.2 ± 0.2 | 49.3 ± 7.4 |
| Pera | 12.6 ± 0.2 | 0.7 ± 0.3 | 17.9 ± 0.5 | 93.8 ± 2.1 | 181.7 ± 1.2 | 51.6 ± 0.9 | 68.2 ± 0.1 | 73.9 ± 0.7 | 4.7 ± 0.2 | 1.6 ± 0.2 | 9.7 ± 0.2 | 43.5 ± 6.5 |
| Cara Cara | 9.9 ± 0.1 | 0.5 ± 0.1 | 18.6 ± 0.4 | 53.8 ± 3.1 | 225.7 ± 5.6 | 27.1 ± 0.7 | 75.0 ± 0.4 | 74.9 ± 0.8 | 6.2 ± 0.3 | 1.4 ± 0.1 | 10.4 ± 0.2 | 7.0 ± 0.4 |
| Mombuca | 8.5 ± 0.2 | 0.5 ± 0.2 | 17.8 ± 0.4 | 72.3 ± 1.7 | 141.2 ± 1.5 | 51.2 ± 0.8 | 64.8 ± 0.3 | 66.8 ± 0.3 | 5.1 ± 0.1 | 2.2 ± 0.1 | 10.4 ± 0.1 | 147.5 ± 5.5 |
| Carrancas | 8.0 ± 0.4 | 0.4 ± 0.3 | 20.4 ± 0.8 | 74.3 ± 6.7 | 143.9 ± 2.3 | 51.7 ± 5.2 | 64.3 ± 0.7 | 66.0 ± 0.6 | 4.6 ± 0.2 | 1.6 ± 0.1 | 10.4 ± 0.2 | 89.0 ± 11.7 |
| Pinhal | 9.1 ± 0.0 | 0.5 ± 0.0 | 19.7 ± 0.1 | 62.8 ± 2.8 | 131.0 ± 3.0 | 47.9 ± 1.3 | 62.5 ± 0.7 | 64.5 ± 0.6 | 4.8 ± 0.2 | 2.0 ± 0.1 | 10.4 ± 0.2 | 116.8 ± 7.8 |
| Puka | 9.6 ± 0.3 | 0.6 ± 0.2 | 17.1 ± 0.2 | 66.8 ± 5.6 | 160.6 ± 6.6 | 41.9 ± 4.9 | 74.7 ± 0.6 | 65.7 ± 0.8 | 4.4 ± 0.1 | 1.9 ± 0.1 | 10.6 ± 0.1 | 35.5 ± 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, L.; Alquézar, B.; Peña, L. Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants. Antioxidants 2024, 13, 994. https://doi.org/10.3390/antiox13080994
Carmona L, Alquézar B, Peña L. Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants. Antioxidants. 2024; 13(8):994. https://doi.org/10.3390/antiox13080994
Chicago/Turabian StyleCarmona, Lourdes, Berta Alquézar, and Leandro Peña. 2024. "Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants" Antioxidants 13, no. 8: 994. https://doi.org/10.3390/antiox13080994
APA StyleCarmona, L., Alquézar, B., & Peña, L. (2024). Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants. Antioxidants, 13(8), 994. https://doi.org/10.3390/antiox13080994








