A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases
Abstract
:1. Introduction
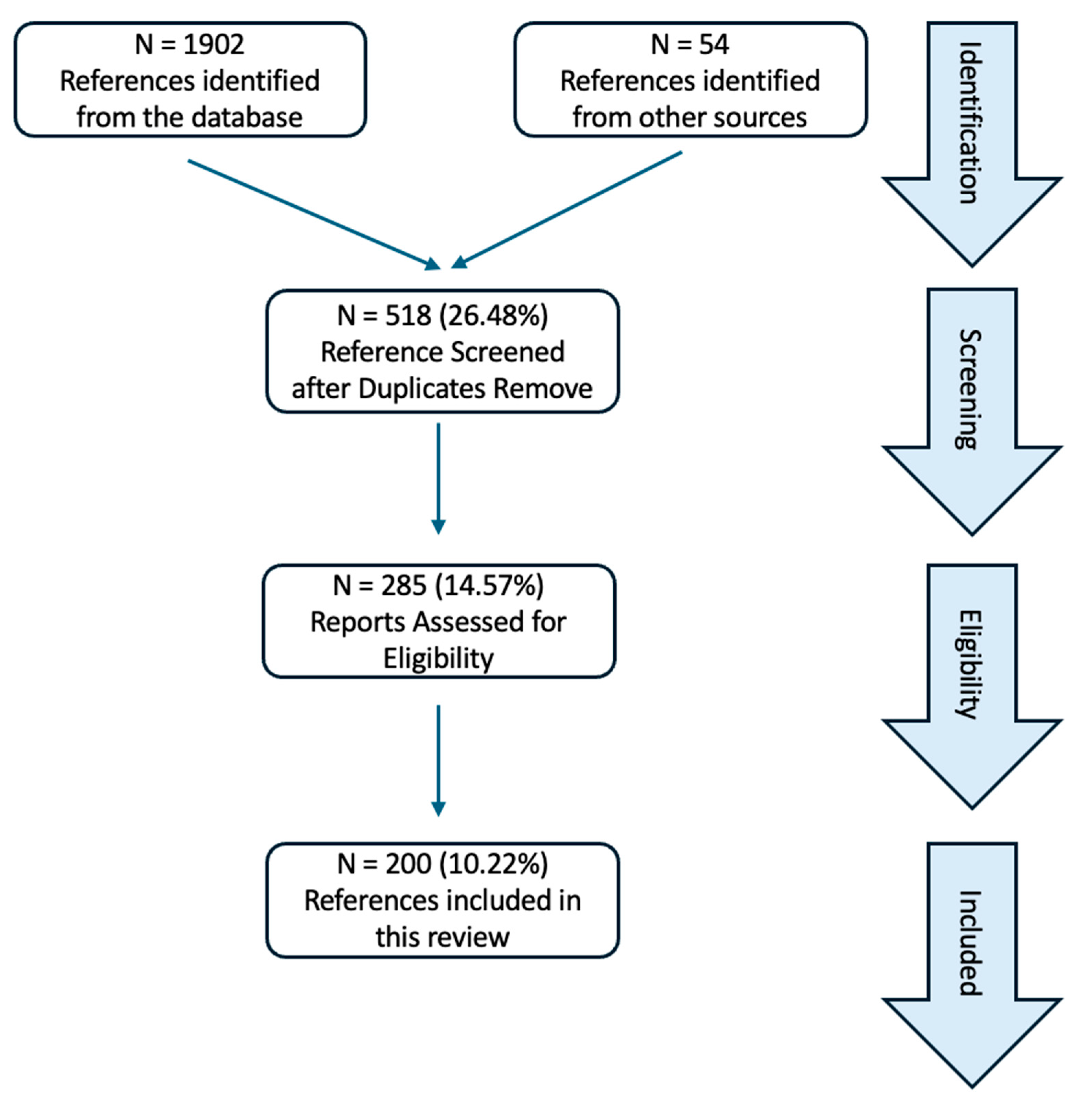
2. Method
3. Oxidative Stress in Cardiovascular Diseases (Figure 2)
3.1. Oxidative Stress
3.2. Sources of Oxidative Stress in the Cardiovascular System (Table 1)
| Source | Role in ROS Production | Model Examined |
|---|---|---|
| NADPH Oxidase (NOX) | NOX generates superoxide radicals by transferring electrons from NADPH to oxygen, where dysregulation can contribute to oxidative stress and cardiovascular pathologies [20]. NOX1 in vascular smooth muscle cells and NOX2 in neutrophils and cardiovascular cells are generally harmful, while NOX4, broadly expressed in cardiovascular cells, might be protective, with NOX5 less implicated in pathology. | Human endothelial cells Human vascular smooth muscle cells |
| Xanthine Oxidase (XO) | XO catalyzes the conversion of hypoxanthine to xanthine and oxidation of xanthaine to uric acid, producing superoxide and hydrogen peroxide as byproducts, especially in ischemic injury and inflammatory responses in CVD [21]. | Human endothelial cells Human cardiomyocytes |
| Nitric Oxide (NO) | NO causes peroxynitrite formation and highly reactive molecules, which contributes to endothelial dysfunction and cardiovascular disease under oxidative stress conditions [22]. | Human endothelial cells |
| Lipoxygenases | Lipoxygenases catalyze oxidation of polyunsaturated fatty acids, producing lipid hydroperoxides, which contributes to inflammation and oxidative damage [23]. Increased lipoxygenase activity is linked to the progression of atherosclerosis and inflammation in human atherosclerotic lesions | Human aortic endothelial cells Mouse ApoE−/− and Ldlr−/− mouse models |
| Myeloperoxidase | Myeloperoxidase is released by neutrophils and generates hypochlorous acid, leading to oxidative stress and vascular function impairment [24]. | Human plasma levels |
3.3. Role of Oxidative Stress in the Pathogenesis of CVDs (Figure 3)
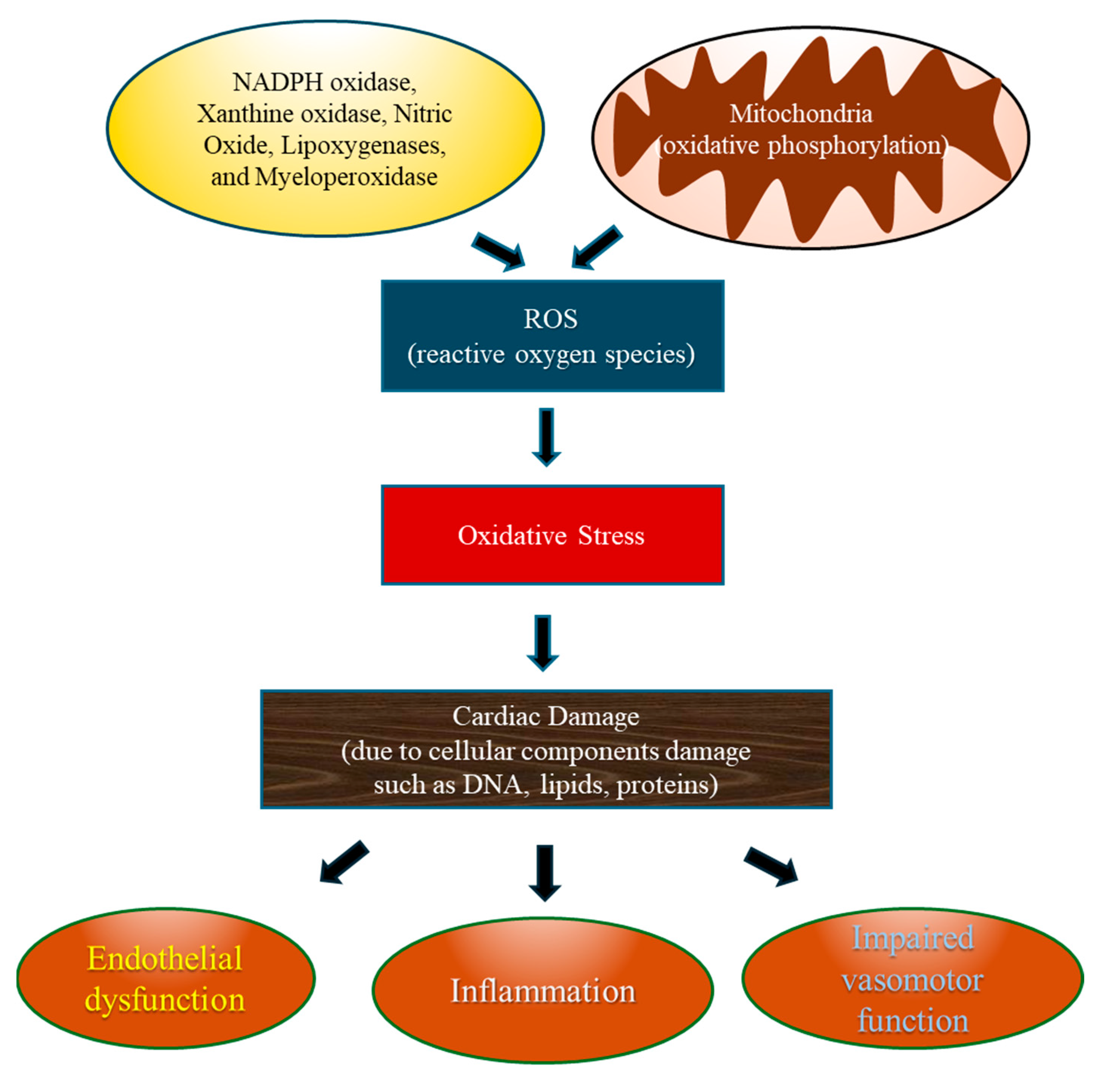
4. Cardiovascular Diseases Associated with Oxidative Stress
4.1. Myocardial Ischemia/Reperfusion Injury (MI/RI) and Oxidative Stress
4.2. Atherosclerosis and Oxidative Stress (Figure 4)
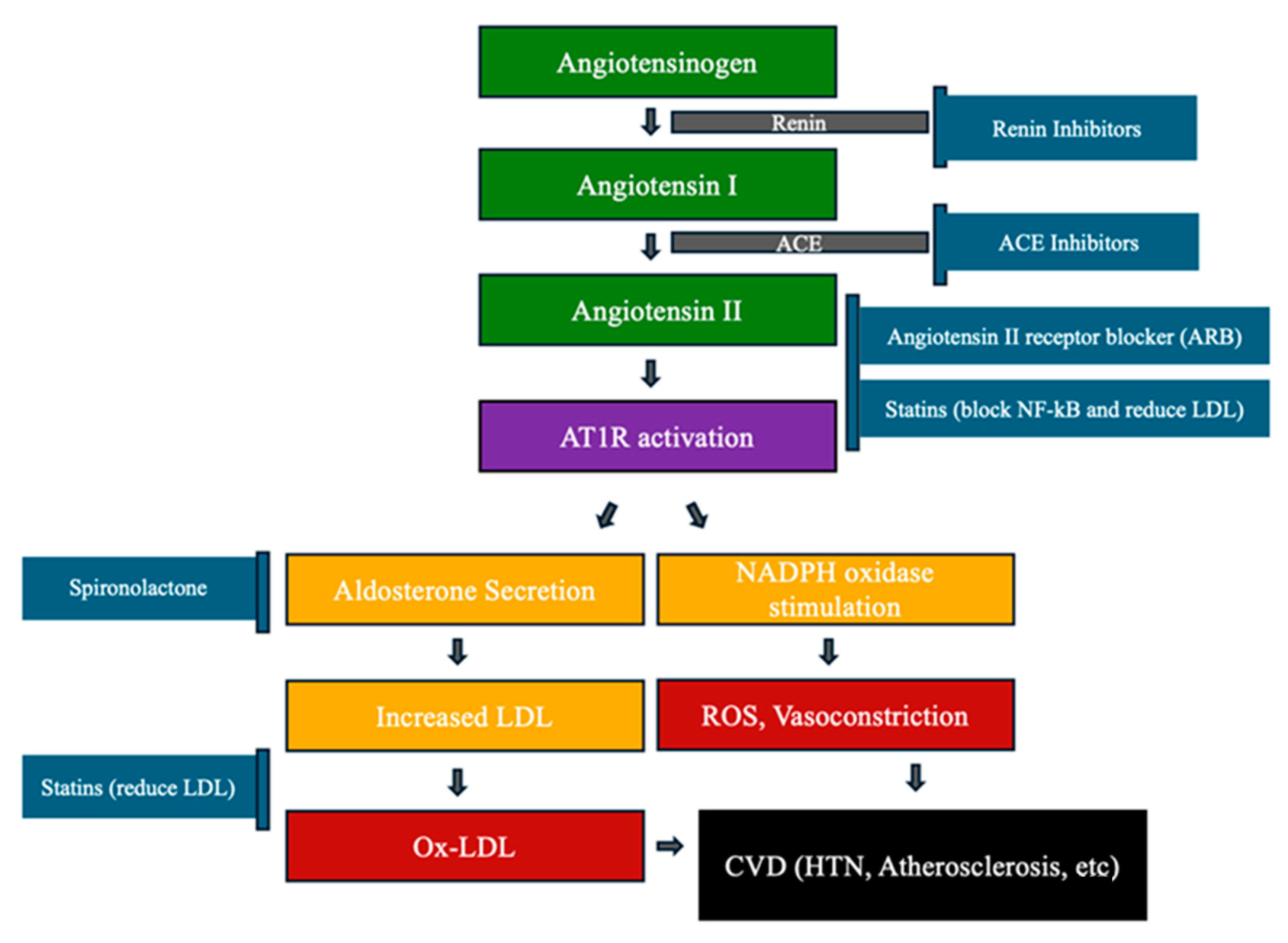
4.3. Heart Failure (HF) and Oxidative Stress
4.4. Hypertension (HTN) and Oxidative Stress
5. Antioxidant Supplements in CVD (Table 2)
| Antioxidant Supplement | Description |
|---|---|
| Resveratrol | - A natural polyphenolic compound - ↓ ferroptosis and the USP19/Beclin1-mediated autophagy pathway - ↑ the Nrf2 pathway |
| Vitamins C, D, and E | - Scavenge free radicals, ↓ oxidative damage, regulate RAAS activity - ↓ parathyroid hormone levels. |
| Omega-3 (EPA and DHA) | - ↑ antioxidant molecule via upregulation (↑) of NRF2 pathway - ↑ glutathione peroxidase (GPx) and superoxide dismutase (SOD) - ↓ levels of malondialdehyde (MDA) |
| Flavanoids | - ↓ xanthine oxidase activity - ↓ platelet adhesion, ↑ endothelial function by vasodilation |
| Coenzyme Q-10 | - ↑ antioxidant production, ↓ lipid peroxidation - Protect blood vessels by preserving NO |
| Curcumin | - ↓ COX-2, LOX, NF-kB, and iNOS - ↓ inflammatory markers like CRP, TNF-α, and IL-6 |
5.1. Resveratrol
5.2. Vitamins
5.3. Omega-3
5.4. Flavanoids
5.5. CoEnzyme Q-10 (CoQ10)
5.6. Curcumin
6. Pharmacological Management of Oxidative Stress-Medicated Cardiovascular Diseases (Table 3)
6.1. Antioxidants
| 1. Pharmacological Antioxidants | |
| N-acetyl-cysteine (NAC) | - A synthetic derivative of L-cysteine - Mitigates cardio-renal syndrome type 3 |
| Puerarin | - Target AMPK-mediated ferroptosis signaling, |
| Melatonin | - Reduce oxidative stress and improving vascular function. |
| Irisin | - Reducing oxidative stress and apoptosis |
| Cannabinoids | - Interact with the endocannabinoid system |
| 2. Nanoparticles | |
| PVAX | - Nanoparticles containing vanillyl alcohol - Reduce ROS, inflammation, and apoptosis |
| Curcumin Nanomicelle | - ↓ COX-2, LOX, NF-kB, and iNOS - ↓ inflammatory markers like CRP, TNF-α, and IL-6 |
6.1.1. N-Acetyl-Cysteine (NAC)
6.1.2. Puerarin
6.1.3. Melatonin
6.1.4. Irisin
6.1.5. Cannabinoids
6.2. Nanoparticle Therapies
6.2.1. Polyoxalate-Based Targeted Nanoparticles
6.2.2. Nanomicelle Delivery System for Curcumin
7. Biomarkers for Oxidative Stress Assessment
7.1. Soluble Transferrin Receptor
7.2. Transthyretin (TTR)
7.3. Cystatin C
8. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Juge, R.; Breugnot, J.; Da Silva, C.; Bordes, S.; Closs, B.; Aouacheria, A. Quantification and Characterization of UVB-Induced Mitochondrial Fragmentation in Normal Primary Human Keratinocytes. Sci. Rep. 2016, 6, 35065. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Matschke, V.; Theiss, C.; Matschke, J. Oxidative stress: The lowest common denominator of multiple diseases. Neural Regen. Res. 2019, 14, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Preiser, J.C. Oxidative stress. J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp. 2011, 57, e3357. [Google Scholar] [CrossRef]
- Stevens, J.L.; Feelisch, M.; Martin, D.S. Perioperative Oxidative Stress: The Unseen Enemy. Anesth. Analg. 2019, 129, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H. The centennial of the Fenton reaction. Free Radic. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombie, S.; Gibon, Y.; Petriacq, P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.H.; Rathore, M.G.; Allende-Vega, N.; Vo, D.N.; Belkhala, S.; Orecchioni, S.; Talarico, G.; Bertolini, F.; Cartron, G.; Lecellier, C.H.; et al. Human Leukemic Cells performing Oxidative Phosphorylation (OXPHOS) Generate an Antioxidant Response Independently of Reactive Oxygen species (ROS) Production. EBioMedicine 2016, 3, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Loperena, R.; Harrison, D.G. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Martins, D.; Garcia, L.R.; Queiroz, D.A.R.; Lazzarin, T.; Tonon, C.R.; Balin, P.D.S.; Polegato, B.F.; de Paiva, S.A.R.; Azevedo, P.S.; Minicucci, M.F.; et al. Oxidative Stress as a Therapeutic Target of Cardiac Remodeling. Antioxidants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Forte, A.; Finicelli, M.; Grossi, M.; Vicchio, M.; Alessio, N.; Sante, P.; De Feo, M.; Cotrufo, M.; Berrino, L.; Rossi, F.; et al. DNA damage and repair in a model of rat vascular injury. Clin. Sci. 2010, 118, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Group, E.S.C.S.D. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Beamish, R.E.; Dhalla, N.S. Depression of heart sarcolemmal Ca2+-pump activity by oxygen free radicals. Am. J. Physiol. 1989, 256, H368–H374. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Kimchi, A. Autophagy and cell death. Curr. Top. Dev. Biol. 2007, 78, 217–245. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Gillet, G.; Prudent, J.; Popgeorgiev, N. Bcl-2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int. J. Mol. Sci. 2021, 22, 3730. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, C.H. Effect of Reactive Oxygen Species on the Endoplasmic Reticulum and Mitochondria during Intracellular Pathogen Infection of Mammalian Cells. Antioxidants 2021, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid. Med. Cell. Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef]
- Fuentes, E.; Moore-Carrasco, R.; de Andrade Paes, A.M.; Trostchansky, A. Role of Platelet Activation and Oxidative Stress in the Evolution of Myocardial Infarction. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 509–520. [Google Scholar] [CrossRef]
- Schanze, N.; Hamad, M.A.; Nuhrenberg, T.G.; Bode, C.; Duerschmied, D. Platelets in Myocardial Ischemia/Reperfusion Injury. Hamostaseologie 2023, 43, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, K.; Hu, P. The Role of Autophagy in Acute Myocardial Infarction. Front. Pharmacol. 2019, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. StatPerarls—Atherosclerosis; StatPerals Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Morrison, A.M.; Sullivan, A.E.; Aday, A.W. Atherosclerotic Disease: Pathogenesis and Approaches to Management. Med. Clin. N. Am. 2023, 107, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Saldeen, T.; Romeo, F.; Mehta, J.L. Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: The potential role of transcription factor NF-kappaB. Circulation 2000, 102, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, patterns of recognition, and pathophysiology. Antioxid. Redox Signal 2010, 13, 39–75. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Dzau, V.J. The renin-angiotensin-aldosterone system: A specific target for hypertension management. Am. J. Hypertens. 1999, 12, 205S–213S. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Renin-Angiotensin System in Pathogenesis of Atherosclerosis and Treatment of CVD. Int. J. Mol. Sci. 2021, 22, 6702. [Google Scholar] [CrossRef]
- Silva, G.M.; Franca-Falcao, M.S.; Calzerra, N.T.M.; Luz, M.S.; Gadelha, D.D.A.; Balarini, C.M.; Queiroz, T.M. Role of Renin-Angiotensin System Components in Atherosclerosis: Focus on Ang-II, ACE2, and Ang-1-7. Front. Physiol. 2020, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Faggiotto, A.; Paoletti, R. State-of-the-Art lecture. Statins and blockers of the renin-angiotensin system: Vascular protection beyond their primary mode of action. Hypertension 1999, 34, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Kiaie, N.; Gorabi, A.M.; Reiner, Z.; Jamialahmadi, T.; Ruscica, M.; Sahebkar, A. Effects of Statins on Renin-Angiotensin System. J. Cardiovasc. Dev. Dis. 2021, 8, 80. [Google Scholar] [CrossRef]
- Mansouri, A.; Reiner, Z.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Piemonte, F. Protein glutathionylation in cardiovascular diseases. Int. J. Mol. Sci. 2013, 14, 20845–20876. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Jurgens, G.; Quehenberger, O.; Koller, E. Autoxidation of human low density lipoprotein: Loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J. Lipid Res. 1987, 28, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Mao, J.; Wang, X.; Yang, R.; Wang, C.; Li, C.; Zhou, X. Advances in treatment strategies based on scavenging reactive oxygen species of nanoparticles for atherosclerosis. J. Nanobiotechnol. 2023, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Taye, A.; Morawietz, H. Spironolactone inhibits NADPH oxidase-induced oxidative stress and enhances eNOS in human endothelial cells. Iran. J. Pharm. Res. 2011, 10, 329–337. [Google Scholar]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; Lopez, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- Seres, T. Heart Failure. In Anesthesia Secrets; Duke, J., Ed.; Mosby: Maryland Heights, MO, USA, 2011; pp. 236–243. [Google Scholar]
- Pazos-Lopez, P.; Peteiro-Vazquez, J.; Carcia-Campos, A.; Garcia-Bueno, L.; de Torres, J.P.; Castro-Beiras, A. The causes, consequences, and treatment of left or right heart failure. Vasc. Health Risk Manag. 2011, 7, 237–254. [Google Scholar] [CrossRef]
- Mongirdiene, A.; Skrodenis, L.; Varoneckaite, L.; Mierkyte, G.; Gerulis, J. Reactive Oxygen Species Induced Pathways in Heart Failure Pathogenesis and Potential Therapeutic Strategies. Biomedicines 2022, 10, 602. [Google Scholar] [CrossRef]
- Nakamura, K.; Murakami, M.; Miura, D.; Yunoki, K.; Enko, K.; Tanaka, M.; Saito, Y.; Nishii, N.; Miyoshi, T.; Yoshida, M.; et al. Beta-Blockers and Oxidative Stress in Patients with Heart Failure. Pharmaceuticals 2011, 4, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W.H.; Henderson, L.S.; Lukas, M.A. Controlled-release carvedilol in the management of systemic hypertension and myocardial dysfunction. Vasc. Health Risk Manag. 2008, 4, 1387–1400. [Google Scholar] [CrossRef]
- Ichihara, S.; Yamada, Y.; Ichihara, G.; Kanazawa, H.; Hashimoto, K.; Kato, Y.; Matsushita, A.; Oikawa, S.; Yokota, M.; Iwase, M. Attenuation of oxidative stress and cardiac dysfunction by bisoprolol in an animal model of dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2006, 350, 105–113. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2022, 13, 1098725. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E. Does increased oxidative stress cause hypertension? Diabetes Care 2008, 31 (Suppl. S2), S185–S189. [Google Scholar] [CrossRef]
- Xiao, L.; Harrison, D.G. Inflammation in Hypertension. Can. J. Cardiol. 2020, 36, 635–647. [Google Scholar] [CrossRef]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef] [PubMed]
- Craighead, D.H.; Heinbockel, T.C.; Freeberg, K.A.; Rossman, M.J.; Jackman, R.A.; Jankowski, L.R.; Hamilton, M.N.; Ziemba, B.P.; Reisz, J.A.; D’Alessandro, A.; et al. Time-Efficient Inspiratory Muscle Strength Training Lowers Blood Pressure and Improves Endothelial Function, NO Bioavailability, and Oxidative Stress in Midlife/Older Adults With Above-Normal Blood Pressure. J. Am. Heart Assoc. 2021, 10, e020980. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.B.; Scott, J.D. Phosphorylation, compartmentalization, and cardiac function. IUBMB Life 2023, 75, 353–369. [Google Scholar] [CrossRef]
- Li, T.; Tan, Y.; Ouyang, S.; He, J.; Liu, L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 2022, 808, 145968. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, I.P.; Laszlo, M.R.; Popescu, T.; Toma, V.; Ion, R.M.; Moldovan, R.; Filip, G.A.; Cainap, C.; Clichici, S.; Muresan, A. The comparative effects of Resveratrol and Curcumin in combination with photodynamic therapy. Med. Pharm. Rep. 2022, 95, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, M.; Qin, C.; Wang, Z.; Chen, J.; Wang, R.; Hu, J.; Zou, Q.; Niu, X. Resveratrol Attenuate Myocardial Injury by Inhibiting Ferroptosis Via Inducing KAT5/GPX4 in Myocardial Infarction. Front. Pharmacol. 2022, 13, 906073. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, T.; Lian, J.; Deng, K.; Qu, C.; Li, E.; Li, G.; Ren, Y.; Wang, Z.; Jiang, Z.; et al. Resveratrol reduces ROS-induced ferroptosis by activating SIRT3 and compensating the GSH/GPX4 pathway. Mol. Med. 2023, 29, 137. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Biray Avci, C.; Rahbarghazi, R.; Rezabakhsh, A.; Nourazarian, A.; Nabat, E.; Fathi, F.; Khaksar, M. Resveratrol reduced the detrimental effects of malondialdehyde on human endothelial cells. J. Cardiovasc. Thorac. Res. 2021, 13, 131–140. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Tang, B.; Kang, P.; Zhang, H.; Shi, C. Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J. Cell. Mol. Med. 2023, 27, 3075–3089. [Google Scholar] [CrossRef]
- Li, D.; Song, C.; Zhang, J.; Zhao, X. Resveratrol alleviated 5-FU-induced cardiotoxicity by attenuating GPX4 dependent ferroptosis. J. Nutr. Biochem. 2023, 112, 109241. [Google Scholar] [CrossRef]
- Luoqian, J.; Yang, W.; Ding, X.; Tuo, Q.Z.; Xiang, Z.; Zheng, Z.; Guo, Y.J.; Li, L.; Guan, P.; Ayton, S.; et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell. Mol. Immunol. 2022, 19, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, Y.; Peng, L.; Rong, X.; Liu, Y.; Li, J.; Luo, J. Ferroptosis in cardiovascular diseases: Role and mechanism. Cell Biosci. 2023, 13, 226. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Ramli, I.; Posadino, A.M.; Giordo, R.; Fenu, G.; Fardoun, M.; Iratni, R.; Eid, A.H.; Zayed, H.; Pintus, G. Effect of Resveratrol on Pregnancy, Prenatal Complications and Pregnancy-Associated Structure Alterations. Antioxidants 2023, 12, 341. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Sekizawa, A.; Koide, K.; Hasegawa, J.; Satoh, K.; Arakaki, T.; Takenaka, S.; Matsuoka, R. Vitamin C Induces the Reduction of Oxidative Stress and Paradoxically Stimulates the Apoptotic Gene Expression in Extravillous Trophoblasts Derived From First-Trimester Tissue. Reprod. Sci. 2015, 22, 783–790. [Google Scholar] [CrossRef]
- Renke, G.; Starling-Soares, B.; Baesso, T.; Petronio, R.; Aguiar, D.; Paes, R. Effects of Vitamin D on Cardiovascular Risk and Oxidative Stress. Nutrients 2023, 15, 769. [Google Scholar] [CrossRef]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. Biomed. Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef]
- Shite, J.; Qin, F.; Mao, W.; Kawai, H.; Stevens, S.Y.; Liang, C. Antioxidant vitamins attenuate oxidative stress and cardiac dysfunction in tachycardia-induced cardiomyopathy. J. Am. Coll. Cardiol. 2001, 38, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.R.; Anderson, R.A.; Lang, D.; Blackman, D.J.; Morris, R.H.; Morris-Thurgood, J.; McDowell, I.F.; Jackson, S.K.; Lewis, M.J.; Frenneaux, M.P. Neutrophil superoxide anion--generating capacity, endothelial function and oxidative stress in chronic heart failure: Effects of short- and long-term vitamin C therapy. J. Am. Coll. Cardiol. 2000, 36, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Hosseini-Esfahani, F.; Esfandiar, Z.; Hosseinpour-Niazi, S.; Azizi, F. Associations between dietary antioxidant intakes and cardiovascular disease. Sci. Rep. 2022, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Dastan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C.; et al. omega-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Ciumarnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Rachisan, A.L.; Negrean, V.; Perne, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Isa, S.S.P.; Ablat, A.; Mohamad, J. The Antioxidant and Xanthine Oxidase Inhibitory Activity of Plumeria rubra Flowers. Molecules 2018, 23, 400. [Google Scholar] [CrossRef]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Ikemura, M.; Sasaki, Y.; Giddings, J.C.; Yamamoto, J. Preventive effects of hesperidin, glucosyl hesperidin and naringin on hypertension and cerebral thrombosis in stroke-prone spontaneously hypertensive rats. Phytother. Res. 2012, 26, 1272–1277. [Google Scholar] [CrossRef]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Drangova, M.; Huff, M.W. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr(-/-) mice. J. Lipid Res. 2018, 59, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Jokura, H.; Hashizume, K.; Ominami, H.; Shibuya, Y.; Suzuki, A.; Hase, T.; Shimotoyodome, A. Hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3'-O-glucuronide, exerts hypotensive, vasodilatory, and anti-inflammatory activities. Food Funct. 2013, 4, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Sapian, S.; Taib, I.S.; Latip, J.; Katas, H.; Chin, K.Y.; Mohd Nor, N.A.; Jubaidi, F.F.; Budin, S.B. Therapeutic Approach of Flavonoid in Ameliorating Diabetic Cardiomyopathy by Targeting Mitochondrial-Induced Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 11616. [Google Scholar] [CrossRef] [PubMed]
- Sood, B.; Patel, P.; Keenaghan, M. Coenzyme Q10. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Cordero, M.D.; Cano-García, F.J.; Alcocer-Gómez, E.; De Miguel, M.; Sánchez-Alcázar, J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: Coenzyme Q₁₀ effect on clinical improvement. PLoS ONE 2012, 7, e35677. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Ruiz, Y.; Llanos-González, E.; Alcain, F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants 2021, 10, 755. [Google Scholar] [CrossRef]
- Hasanloei, M.A.V.; Zeinaly, A.; Rahimlou, M.; Houshyar, H.; Moonesirad, S.; Hashemi, R. Effect of coenzyme Q10 supplementation on oxidative stress and clinical outcomes in patients with low levels of coenzyme Q10 admitted to the intensive care unit. J. Nutr. Sci. 2021, 10, e48. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Canale, R.E.; McCarthy, C.G.; Farney, T.M. Impact of oral ubiquinol on blood oxidative stress and exercise performance. Oxid. Med. Cell. Longev. 2012, 2012, 465020. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Niaz, M.A.; Kumar, A.; Sindberg, C.D.; Moesgaard, S.; Littarru, G.P. Effect on absorption and oxidative stress of different oral Coenzyme Q10 dosages and intake strategy in healthy men. Biofactors 2005, 25, 219–224. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Gutierrez-Mariscal, F.M.; Arenas-de Larriva, A.P.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q(10) Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2020, 21, 7870. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Hatamipour, M.; Sahebkar, A.; Alavizadeh, S.H.; Dorri, M.; Jaafari, M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran. J. Basic Med. Sci. 2019, 22, 282–289. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Reyes, D.R.A.; Gomes, M.J.; Rosa, C.M.; Pagan, L.U.; Damatto, F.C.; Damatto, R.L.; Depra, I.; Campos, D.H.S.; Fernandez, A.A.H.; Martinez, P.F.; et al. N-Acetylcysteine Influence on Oxidative Stress and Cardiac Remodeling in Rats During Transition from Compensated Left Ventricular Hypertrophy to Heart Failure. Cell. Physiol. Biochem. 2017, 44, 2310–2321. [Google Scholar] [CrossRef]
- Tenório, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early Use of N-acetylcysteine With Nitrate Therapy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction Reduces Myocardial Infarct Size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Tossios, P.; Bloch, W.; Huebner, A.; Raji, M.R.; Dodos, F.; Klass, O.; Suedkamp, M.; Kasper, S.M.; Hellmich, M.; Mehlhorn, U. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: Results of a randomized, double-blind, placebo-controlled clinical trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, J.; Chen, Y.; Liu, Y.; Tang, X.; Xia, P.; Yu, P.; Yu, S. Puerarin protects against sepsis-induced myocardial injury through AMPK-mediated ferroptosis signaling. Aging 2022, 14, 3617–3632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, C. Current progress of ferroptosis in cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1259219. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, H.; Wu, Q.Q.; Li, F.F.; Bian, Z.Y.; Deng, W.; Zhou, M.Q.; Tang, Q.Z. Puerarin attenuates the inflammatory response and apoptosis in LPS-stimulated cardiomyocytes. Exp. Ther. Med. 2016, 11, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Gonzalez, J.L.; Sanchez-Quintero, D.; Proano-Bernal, L.; Santana-Apreza, R.; Jimenez-Chavarria, M.A.; Luna-Alvarez-Amezquita, J.A.; Straface, J.I.; Perez-Partida, A.M.; Berarducci, J.; Armenta-Moreno, J.I.; et al. Role of the Antioxidant Activity of Melatonin in Myocardial Ischemia-Reperfusion Injury. Antioxidants 2022, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Sciatti, E.; Favero, G.; Bonomini, F.; Vizzardi, E.; Rezzani, R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. Int. J. Mol. Sci. 2022, 23, 14489. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- McMullan, C.J.; Rimm, E.B.; Schernhammer, E.S.; Forman, J.P. A nested case-control study of the association between melatonin secretion and incident myocardial infarction. Heart 2017, 103, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 888319. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tian, Z.; Boidin, M.; Buckley, B.J.R.; Thijssen, D.H.J.; Lip, G.Y.H. Irisin is an Effector Molecule in Exercise Rehabilitation Following Myocardial Infarction (Review). Front. Physiol. 2022, 13, 935772. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xu, Z.; Pan, S.; Ma, Y.; Li, H.; Wu, F.; Bo, W.; Cai, M.; Tian, Z. Irisin and ALCAT1 mediated aerobic exercise-alleviated oxidative stress and apoptosis in skeletal muscle of mice with myocardial infarction. Free Radic. Biol. Med. 2022, 193, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Elsen, M.; Raschke, S.; Eckel, J. Browning of white fat: Does irisin play a role in humans? J. Endocrinol. 2014, 222, R25–R38. [Google Scholar] [CrossRef]
- Liu, C.; Wei, A.; Wang, T. Irisin, an Effective Treatment for Cardiovascular Diseases? J. Cardiovasc. Dev. Dis. 2022, 9, 305. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.T.; Lee, J.H.; Borazjani, A.; Mangum, L.C.; Hou, X.; Ross, M.K. Oxyradical stress increases the biosynthesis of 2-arachidonoylglycerol: Involvement of NADPH oxidase. Am. J. Physiol.-Cell Physiol. 2016, 311, C960–C974. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Sadoughi, M.M.; Abdulmajid Ayatollahi, S.; Ainy, E.; Kiani, R.; Zali, A.; Miri, M. Therapeutic potentials of cannabidiol: Focus on the Nrf2 signaling pathway. Biomed. Pharmacother. 2023, 168, 115805. [Google Scholar] [CrossRef]
- Li, R.; Rhee, S.J.; Bae, S.; Su, S.; Kang, C.S.; Ke, Q.; Koo, Y.E.; Ryu, C.; Song, C.G.; Lee, D.; et al. H2O2-Responsive Antioxidant Nanoparticle Attenuates Whole Body Ischemia/Reperfusion-Induced Multi-Organ Damages. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 279–288. [Google Scholar] [CrossRef]
- Bae, S.; Park, M.; Kang, C.; Dilmen, S.; Kang, T.H.; Kang, D.G.; Ke, Q.; Lee, S.U.; Lee, D.; Kang, P.M. Hydrogen Peroxide-Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003697. [Google Scholar] [CrossRef]
- Lee, D.; Bae, S.; Hong, D.; Lim, H.; Yoon, J.H.; Hwang, O.; Park, S.; Ke, Q.; Khang, G.; Kang, P.M. H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Sci. Rep. 2013, 3, 2233. [Google Scholar] [CrossRef]
- Park, S.; Yoon, J.; Bae, S.; Park, M.; Kang, C.; Ke, Q.; Lee, D.; Kang, P.M. Therapeutic use of H2O2-responsive anti-oxidant polymer nanoparticles for doxorubicin-induced cardiomyopathy. Biomaterials 2014, 35, 5944–5953. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef]
- Mahmood, E.; Bae, S.; Chaudhary, O.; Feng, R.; Mahmood, F.; Robson, S.; Lee, D.; Kang, P.M.; Matyal, R. Neuropeptide Y(3-36) incorporated into PVAX nanoparticle improves angiogenesis in a murine model of myocardial ischemia. Eur. J. Pharmacol. 2020, 882, 173261. [Google Scholar] [CrossRef] [PubMed]
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: A randomized clinical trial. BMC Complement. Med. Ther. 2021, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh Estakhri, M.; Shokrzadeh, M.; Jaafari, M.R.; Karami, M.; Mohammadi, H. Organ toxicity attenuation by nanomicelles containing curcuminoids: Comparing the protective effects on tissues oxidative damage induced by diazinon. Iran. J. Basic Med. Sci. 2019, 22, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Valokola, M.G.; Karimi, G.; Razavi, B.M.; Kianfar, M.; Jafarian, A.H.; Jaafari, M.R.; Imenshahidi, M. The protective activity of nanomicelle curcumin in bisphenol A-induced cardiotoxicity following subacute exposure in rats. Environ. Toxicol. 2019, 34, 319–329. [Google Scholar] [CrossRef]
- Mogharrabi, M.; Rahimi, H.R.; Hasanzadeh, S.; Dastani, M.; Kazemi-Oskuee, R.; Akhlaghi, S.; Soukhtanloo, M. The effects of nanomicelle of curcumin on the matrix metalloproteinase (MMP-2, 9) activity and expression in patients with coronary artery disease (CAD): A randomized controlled clinical trial. ARYA Atheroscler. 2020, 16, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Helli, B.; Gerami, H.; Kavianpour, M.; Heybar, H.; Hosseini, S.K.; Haghighian, H.K. Curcumin Nanomicelle Improves Lipid Profile, Stress Oxidative Factors and Inflammatory Markers in Patients Undergoing Coronary Elective Angioplasty; A Randomized Clinical Trial. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2090–2098. [Google Scholar] [CrossRef]
- Ratnaningsih, T.; Sukirto, N.W.; Wahyuningsih, A.T. Soluble Transferrin Receptor (sTfR) Identifies Iron Deficiency Anemia (IDA) in Pulmonary Tuberculosis Patients. Acta Med. Indones. 2020, 52, 334–343. [Google Scholar] [PubMed]
- Suarez-Ortegon, M.F.; Arbelaez, A.; Moreno-Navarrete, J.M.; Ortega-Avila, J.G.; Mosquera, M.; Fernandez-Real, J.M. Soluble Transferrin Receptor, Antioxidant Status and Cardiometabolic Risk in Apparently Healthy Individuals. Antioxidants 2022, 12, 19. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, C.; Zhao, C.; Chen, G.; Meng, S.; Hong, M.; Xiang, M.; Xie, Y. Increased Serum Soluble Transferrin Receptor Levels Were Associated With High Prevalence of Cardiovascular Diseases: Insights From the National Health and Nutrition Examination Survey 2017–2018. Front. Cell Dev. Biol. 2022, 10, 874846. [Google Scholar] [CrossRef]
- Jain, A.; Zahra, F. Transthyretin Amyloid Cardiomyopathy (ATTR-CM) In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic amyloidosis from A (AA) to T (ATTR): A review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yokochi, T. Transthyretin cardiac amyloidosis: An update on diagnosis and treatment. ESC Heart Fail. 2019, 6, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Buxbaum, J.N.; Reixach, N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry 2013, 52, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Khan, S.; Rahman, S.; Singh, L.R. The Extracellular Protein, Transthyretin Is an Oxidative Stress Biomarker. Front. Physiol. 2019, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Genereux, J.C.; Suh, E.H.; Vartabedian, V.F.; Rius, B.; Qu, S.; Dendle, M.T.A.; Kelly, J.W.; Wiseman, R.L. Endoplasmic Reticulum Proteostasis Influences the Oligomeric State of an Amyloidogenic Protein Secreted from Mammalian Cells. Cell Chem. Biol. 2016, 23, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Fernandez-Irigoyen, J.; Santamaria, E.; Nieto, M.L.; Bravo-San Pedro, J.M.; Cachofeiro, V. Mitochondrial Oxidative Stress Induces Cardiac Fibrosis in Obese Rats through Modulation of Transthyretin. Int. J. Mol. Sci. 2022, 23, 8080. [Google Scholar] [CrossRef]
- Ortore, G.; Orlandini, E.; Braca, A.; Ciccone, L.; Rossello, A.; Martinelli, A.; Nencetti, S. Targeting Different Transthyretin Binding Sites with Unusual Natural Compounds. ChemMedChem 2016, 11, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Rimbas, R.C.; Balinisteanu, A.; Magda, S.L.; Visoiu, S.I.; Ciobanu, A.O.; Beganu, E.; Nicula, A.I.; Vinereanu, D. New Advanced Imaging Parameters and Biomarkers-A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy. J. Clin. Med. 2022, 11, 2360. [Google Scholar] [CrossRef]
- Bhat, P.; Tang, W.W.H. Biomarkers to Assess and Guide the Management of Heart Failure. In Biomarkers in Cardiovascular Disease; Nambi, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–108. [Google Scholar]
- Ceglarek, U.; Schellong, P.; Rosolowski, M.; Scholz, M.; Willenberg, A.; Kratzsch, J.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Buttner, P.; et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur. Heart J. 2021, 42, 2344–2352. [Google Scholar] [CrossRef]
- Blok, I.M.; van Riel, A.C.; Schuuring, M.J.; de Bruin-Bon, R.H.; van Dijk, A.P.; Hoendermis, E.S.; Zwinderman, A.H.; Mulder, B.J.; Bouma, B.J. The role of cystatin C as a biomarker for prognosis in pulmonary arterial hypertension due to congenital heart disease. Int. J. Cardiol. 2016, 209, 242–247. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Kang, P.M. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants 2024, 13, 923. https://doi.org/10.3390/antiox13080923
Jin S, Kang PM. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants. 2024; 13(8):923. https://doi.org/10.3390/antiox13080923
Chicago/Turabian StyleJin, Soyeon, and Peter M. Kang. 2024. "A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases" Antioxidants 13, no. 8: 923. https://doi.org/10.3390/antiox13080923






