Pattern of Expression of Genes Involved in Systemic Inflammation and Glutathione Metabolism Reveals Exacerbation of COPD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Individuals
2.2. RNA Extraction from PBMCs
2.3. cDNA Synthesis
2.4. Measurement of mRNA Expression
2.5. Statistical Analysis
3. Results
3.1. Association of Enzymes’ Expression with Lung Function Parameters, Exacerbations, and Smoking History
3.2. Differences in Enzyme Expression between GOLD 1–4 Stratification
3.3. Differences in Enzyme Expression between GOLD A–D Stratification
3.4. Grouping Ability of the Enzymes
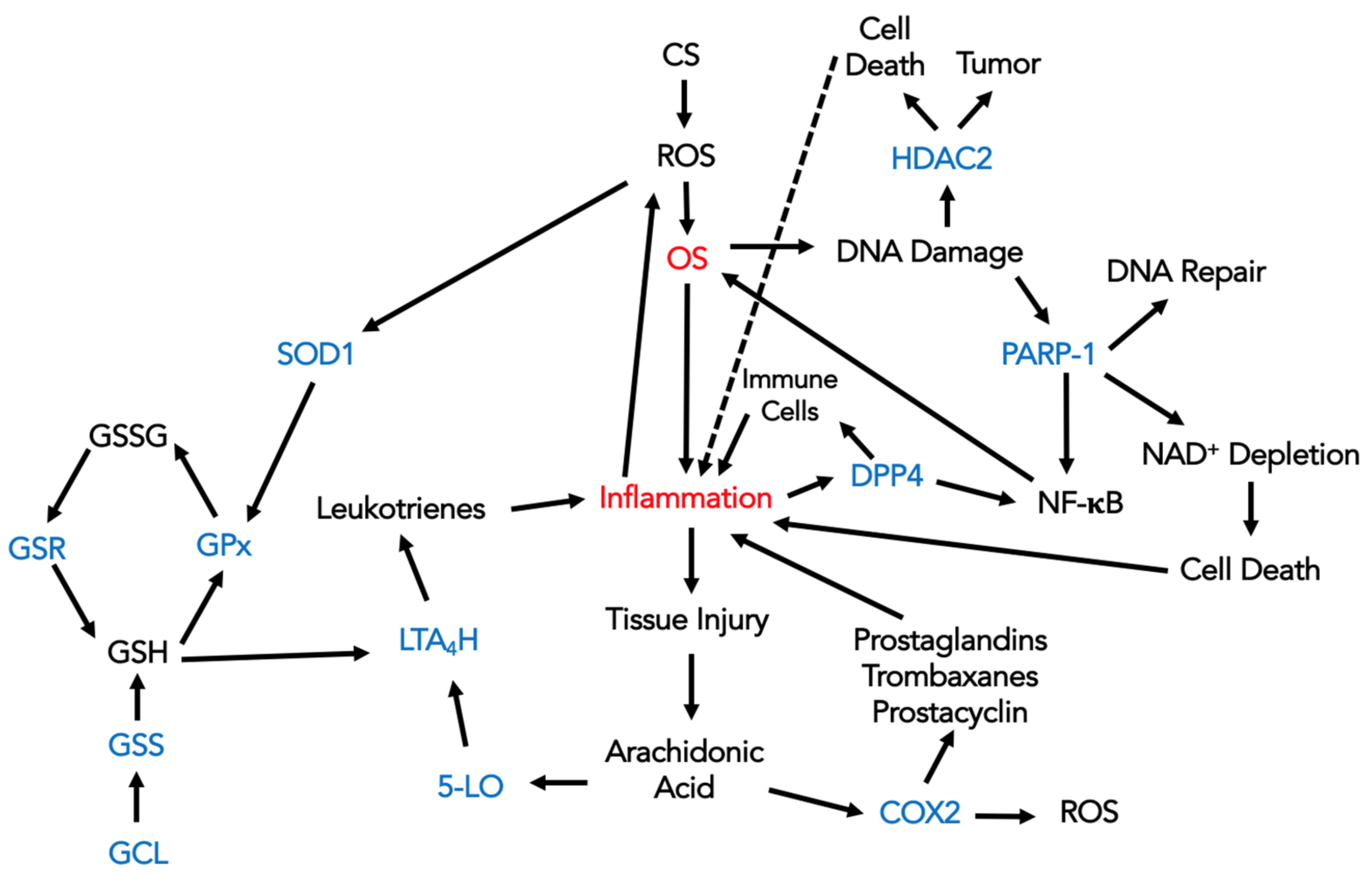
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabbri, L.M.; Rabe, K.F. From COPD to chronic systemic inflammatory syndrome? Lancet 2007, 370, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Oit-Wiscombe, I.; Virag, L.; Soomets, U.; Altraja, A. Increased DNA damage in progression of COPD: A response by poly(ADP-ribose) polymerase-1. PLoS ONE 2013, 8, e70333. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2022. Available online: https://goldcopd.org/ (accessed on 11 July 2022).
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, M.; Rovati, G.E.; Cavanillas, A.B. The leukotriene receptor antagonist montelukast and its possible role in the cardiovascular field. Eur. J. Clin. Pharmacol 2017, 73, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ito, M.; Elliott, W.M.; Cosio, B.; Caramori, G.; Kon, O.M.; Barczyk, A.; Hayashi, S.; Adcock, I.M.; Hogg, J.C.; et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005, 352, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Seys, L.J.M.; Widagdo, W.; Verhamme, F.M.; Kleinjan, A.; Janssens, W.; Joos, G.F.; Bracke, K.R.; Haagmans, B.L.; Brusselle, G.G. DPP4, the Middle East Respiratory Syndrome Coronavirus Receptor, is Upregulated in Lungs of Smokers and Chronic Obstructive Pulmonary Disease Patients. Clin. Infect. Dis. 2018, 66, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.; Touma, J.; Davoudi, B.; Benard, M.; Sauer, T.; Geisler, J.; Vetvik, K.; Rahbar, A.; Soderberg-Naucler, C. Human cytomegalovirus infection is correlated with enhanced cyclooxygenase-2 and 5-lipoxygenase protein expression in breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Haeggstrom, J.Z. Structure, function, and regulation of leukotriene A4 hydrolase. Am. J. Respir. Crit. Care Med. 2000, 161 Pt 2, S25–S31. [Google Scholar] [CrossRef]
- Boonacker, E.; Van Noorden, C.J. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 2003, 82, 53–73. [Google Scholar] [CrossRef]
- Sethi, G.S.; Dharwal, V.; Naura, A.S. Poly(ADP-Ribose)Polymerase-1 in Lung Inflammatory Disorders: A Review. Front. Immunol. 2017, 8, 1172. [Google Scholar] [CrossRef]
- Bernardo, I.; Bozinovski, S.; Vlahos, R. Targeting oxidant-dependent mechanisms for the treatment of COPD and its comorbidities. Pharmacol. Ther. 2015, 155, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. New frontiers in the treatment of comorbid cardiovascular disease in chronic obstructive pulmonary disease. Clin. Sci. 2019, 133, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Kadushkin, A.; Tahanovich, A.; Movchan, L.; Levandovskaya, O.; Shman, T. Nortriptyline enhances corticosteroid sensitivity of blood T cells from patients with chronic obstructive pulmonary disease. J. Physiol. Pharmacol. 2021, 72, 793–805. [Google Scholar]
- Tager, M.; Piecyk, A.; Kohnlein, T.; Thiel, U.; Ansorge, S.; Welte, T. Evidence of a defective thiol status of alveolar macrophages from COPD patients and smokers. Chronic obstructive pulmonary disease. Free Radic. Biol. Med. 2000, 29, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res. 2014, 122, 69–101. [Google Scholar] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Piirila, P.; Seikkula, T.; Valimaki, P. Differences between Finnish and European reference values for pulmonary diffusing capacity. Int. J. Circumpolar Health 2007, 66, 449–457. [Google Scholar] [CrossRef]
- Lange, P.; Marott, J.L.; Vestbo, J.; Olsen, K.R.; Ingebrigtsen, T.S.; Dahl, M.; Nordestgaard, B.G. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: A study of the general population. Am. J. Respir Crit. Care Med. 2012, 186, 975–981. [Google Scholar] [CrossRef]
- Oit-Wiscombe, I.; Soomets, U.; Altraja, A. Antioxidant Glutathione Analogues UPF1 and UPF17 Modulate the Expression of Enzymes Involved in the Pathophysiology of Chronic Obstructive Pulmonary Disease. Curr. Issues Mol. Biol. 2024, 46, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Mendez, K.M.; Broadhurst, D.I.; Reinke, S.N. Migrating from partial least squares discriminant analysis to artificial neural networks: A comparison of functionally equivalent visualisation and feature contribution tools using jupyter notebooks. Metabolomics 2020, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Pizent, A.; Lazarus, M.; Kovacic, J.; Tariba Lovakovic, B.; Brcic Karaconji, I.; Zivkovic Semren, T.; Sekovanic, A.; Orct, T.; Branovic-Cakanic, K.; Brajenovic, N.; et al. Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs. Biomolecules 2020, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Tokac, D.; Anlar, H.G.; Bacanli, M.; Dilsiz, S.A.; Iritas, S.; Basaran, N. Oxidative stress status of Turkish welders. Toxicol. Ind. Health 2020, 36, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.R.; Emrani, P.; Cassano, P.A. Genetic variation and gene expression in antioxidant related enzymes and risk of COPD: A systematic review. Thorax 2008, 63, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Hackett, N.R.; Heguy, A.; Harvey, B.G.; O’Connor, T.P.; Luettich, K.; Flieder, D.B.; Kaplan, R.; Crystal, R.G. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am. J. Respir. Cell Mol. Biol. 2003, 29 Pt 1, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Pierrou, S.; Broberg, P.; O’Donnell, R.A.; Pawlowski, K.; Virtala, R.; Lindqvist, E.; Richter, A.; Wilson, S.J.; Angco, G.; Moller, S.; et al. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Kang, J.; Stipanuk, M.H. Differential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation. Biochem. J. 2006, 393 Pt 1, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, O.; Wu, X.; Hwang, S.T.; Akilov, O.E. The synergistic proapoptotic effect of PARP-1 and HDAC inhibition in cutaneous T-cell lymphoma is mediated via Blimp-1. Blood Adv. 2020, 4, 4788–4797. [Google Scholar] [CrossRef]
- Bukowska, B.; Sicinska, P. Influence of Benzo(a)pyrene on Different Epigenetic Processes. Int. J. Mol. Sci. 2021, 22, 13453. [Google Scholar] [CrossRef]
- Tomovic, K.; Lazarevic, J.; Kocic, G.; Deljanin-Ilic, M.; Anderluh, M.; Smelcerovic, A. Mechanisms and pathways of anti-inflammatory activity of DPP-4 inhibitors in cardiovascular and renal protection. Med. Res. Rev. 2019, 39, 404–422. [Google Scholar] [CrossRef]
- Ni, Y.; Yu, Y.; Dai, R.; Shi, G. Diffusing capacity in chronic obstructive pulmonary disease assessment: A meta-analysis. Chron. Respir. Dis. 2021, 18, 14799731211056340. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2011. Available online: https://goldcopd.org/ (accessed on 11 July 2022).
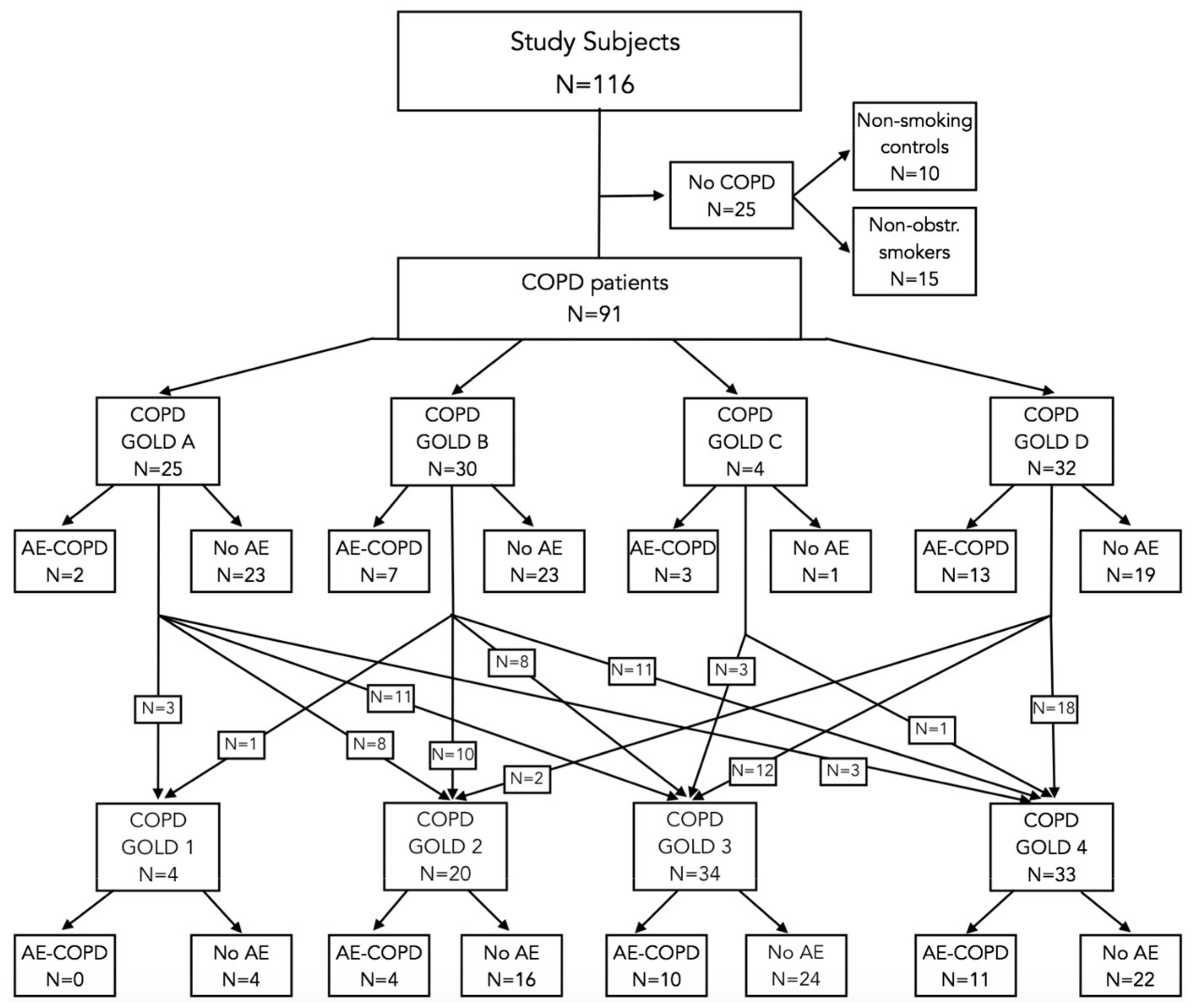



| Characteristics | Non-Smoking Controls (n = 10) | Non-Obstructive Smokers (n = 15) | COPD | ||||
|---|---|---|---|---|---|---|---|
| GOLD A (n = 25) | GOLD B (n = 30) | GOLD C (n = 4) | GOLD D (n = 32) | p-Values ** | |||
| Age | 64.0 ± 3.4 | 61.1 ± 2.9 | 69.0 ± 2.5 | 67.1 ± 2.0 | 80.0 ± 2.7 | 71.7 ± 1.6 | 0.008 |
| Male | 7 (70%) | 9 (60%) | 21 (84%) | 29 (97%) | 4 (100%) | 31 (97%) | 0.004 |
| BMI | 25.7 ± 1.6 | 27.0 ± 1.6 | 25.8 ± 1.0 | 23.4 ± 0.8 | 22.1 ± 0.6 | 24.0 ± 0.9 | 0.138 |
| Smoking (pack-years) | - | 35.7 ± 2.6 | 40.4 ± 3.8 | 39.5 ± 3.6 | 45.0 ± 5.0 | 43.5 ± 4.4 | 0.799 |
| Current smoker | - | 10 (67%) | 17 (68%) | 16 (53%) | 0 (0%) | 13 (41%) | 0.038 |
| Smoking cessation amongst ex-smokers (years ago) | - | 6.8 ± 3.5 | 12.1 ± 3.4 | 9.0 ± 2.5 | 7.6 ± 3.1 | 7.9 ± 1.9 | 0.061 |
| FEV1 % predicted | 97.6 ± 4.5 | 82 ± 4.5 | 53.6 ± 4.7 | 41.1 ± 3.4 | 34.8 ± 3.0 | 30.0 ± 1.8 | <0.001 |
| Absolute decline in FEV1 % over years (%/year) * | 0.1 ± 0.1 | 0.6 ± 0.1 | 1.4 ± 0.4 | 1.6 ± 0.2 | 1.2 ± 0.1 | 1.6 ± 0.1 | <0.001 |
| Current exacerbation | - | - | 2 (8%) | 7 (23%) | 3 (75%) | 13 (41%) | 0.006 |
| PEF % predicted | 99.5 ± 6.8 | 80.6 ± 4.1 | 43.7 ± 4.3 | 35.1 ± 2.9 | 27.8 ± 5.5 | 27.3 ± 1.6 | <0.001 |
| FVC % predicted | 96.8 ± 4.7 | 79.9 ± 4.7 | 70.8 ± 4.8 | 59.2 ± 3.8 | 53.8 ± 7.8 | 46.7 ± 2.7 | <0.001 |
| FEV1/FVC % | 81.9 ± 1.2 | 82.3 ± 1.6 | 62.7 ± 1.9 | 60.9 ± 1.6 | 55.8 ± 2.6 | 52.5 ± 1.5 | <0.001 |
| KCO | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.020 |
| KCO % | 93.4 ± 4.8 | 80.3 ± 8.9 | 76.4 ± 7.5 | 56.6 ± 5.6 | 48.8 ± 0.0 | 40.5 ± 9.5 | 0.005 |
| DLCO | 6.3 ± 0.6 | 5.6 ± 0.8 | 4.8 ± 0.6 | 3.5 ± 0.4 | 2.8 ± 0.0 | 2.8 ± 0.6 | 0.014 |
| DLCO % | 77.3 ± 9.0 | 61.6 ± 7.4 | 54.4 ± 5.3 | 38.7 ± 3.8 | 30 ± 0.0 | 31.3 ± 6.1 | 0.002 |
| TLC | 5.3 ± 0.3 | 4.82 ± 0.3 | 4.8 ± 0.3 | 4.6 ± 0.2 | 4.8 ± 0.0 | 4.6 ± 0.3 | 0.716 |
| TLC % | 84.9 ± 5.8 | 79.2 ± 3.4 | 73.9 ± 2.1 | 68.0 ±2.5 | 63.4 ± 0.0 | 67.4 ± 3.2 | 0.021 |
| Characteristics | Non-Smoking Controls (n = 10) | Non-Obstructive Smokers (n = 15) | Severity of Airflow Limitation | ||||
|---|---|---|---|---|---|---|---|
| GOLD 1 (n = 4) | GOLD 2 (n = 20) | GOLD 3 (n = 34) | GOLD 4 (n = 33) | p-Values ** | |||
| Age | 64.0 ± 3.4 | 61.1 ± 2.9 | 76.8 ± 3.0 | 67.9 ± 2.1 | 70.1 ± 2.0 | 70.0 ± 2.0 | 0.029 |
| Male | 7 (70%) | 9 (60%) | 4 (100%) | 19 (95%) | 32 (94%) | 30 (91%) | 0.010 |
| BMI | 25.7 ± 1.6 | 27.0 ± 1.6 | 23.4 ± 1.7 | 23.8 ± 1.1 | 25.5 ± 0.7 | 23.2 ± 0.9 | 0.093 |
| Smoking (pack-years) | - | 35.7 ± 2.6 | 56.3 ± 10.3 | 41.7 ± 3.1 | 38.2 ± 3.5 | 42.8 ± 4.4 | 0.236 |
| Current Smoker | - | 10 (67%) | 2 (50%) | 13 (65%) | 15 (44%) | 16 (48%) | 0.471 |
| Smoking cessation amongst ex-smokers (years ago) | - | 6.8 ± 3.5 | 5.0 ± 1.0 | 8.0 ± 3.8 | 13.1 ± 2.2 | 10.4 ± 2.5 | 0.093 |
| Current exacerbation | - | - | 0 (0%) | 4 (20%) | 10 (29%) | 11 (33%) | 0.439 |
| PEF % predicted | 99.5 ± 6.8 | 80.6 ± 4.1 | 71.0 ± 14.4 | 51.4 ± 3.6 | 32.6 ± 1.5 | 22.4 ± 0.9 | <0.001 |
| FEV1 % predicted | 97.6 ± 4.5 | 82 ± 4.5 | 94.5 ± 9.0 | 60.7 ± 2.1 | 38.7 ± 1.1 | 23.4 ± 0.8 | <0.001 |
| Absolute decline in FEV1 % over years (%/year) * | 0.1 ± 0.1 | 0.6 ± 0.1 | 0.1 ± 0.2 | 1.0 ± 0.1 | 1.7 ± 0.3 | 1.9 ± 0.1 | <0.001 |
| FVC % predicted | 96.8 ± 4.7 | 79.9 ± 4.7 | 110.3 ± 7.7 | 80.0 ± 2.6 | 57.7 ± 1.7 | 38.0 ± 1.4 | <0.001 |
| FEV1/FVC % | 81.9 ± 1.2 | 82.3 ± 1.6 | 64.7 ± 2.5 | 60.7 ± 1.3 | 54.6 ± 1.5 | 51.8 ± 2.2 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oit-Wiscombe, I.; Virág, L.; Kilk, K.; Soomets, U.; Altraja, A. Pattern of Expression of Genes Involved in Systemic Inflammation and Glutathione Metabolism Reveals Exacerbation of COPD. Antioxidants 2024, 13, 953. https://doi.org/10.3390/antiox13080953
Oit-Wiscombe I, Virág L, Kilk K, Soomets U, Altraja A. Pattern of Expression of Genes Involved in Systemic Inflammation and Glutathione Metabolism Reveals Exacerbation of COPD. Antioxidants. 2024; 13(8):953. https://doi.org/10.3390/antiox13080953
Chicago/Turabian StyleOit-Wiscombe, Ingrid, László Virág, Kalle Kilk, Ursel Soomets, and Alan Altraja. 2024. "Pattern of Expression of Genes Involved in Systemic Inflammation and Glutathione Metabolism Reveals Exacerbation of COPD" Antioxidants 13, no. 8: 953. https://doi.org/10.3390/antiox13080953
APA StyleOit-Wiscombe, I., Virág, L., Kilk, K., Soomets, U., & Altraja, A. (2024). Pattern of Expression of Genes Involved in Systemic Inflammation and Glutathione Metabolism Reveals Exacerbation of COPD. Antioxidants, 13(8), 953. https://doi.org/10.3390/antiox13080953








