Inflammasomes in Alzheimer’s Progression: Nrf2 as a Preventive Target
Abstract
1. Introduction
1.1. Alzheimer’s Disease
1.2. Aβ Proteins
Molecular Mechanisms of Aβ Formation
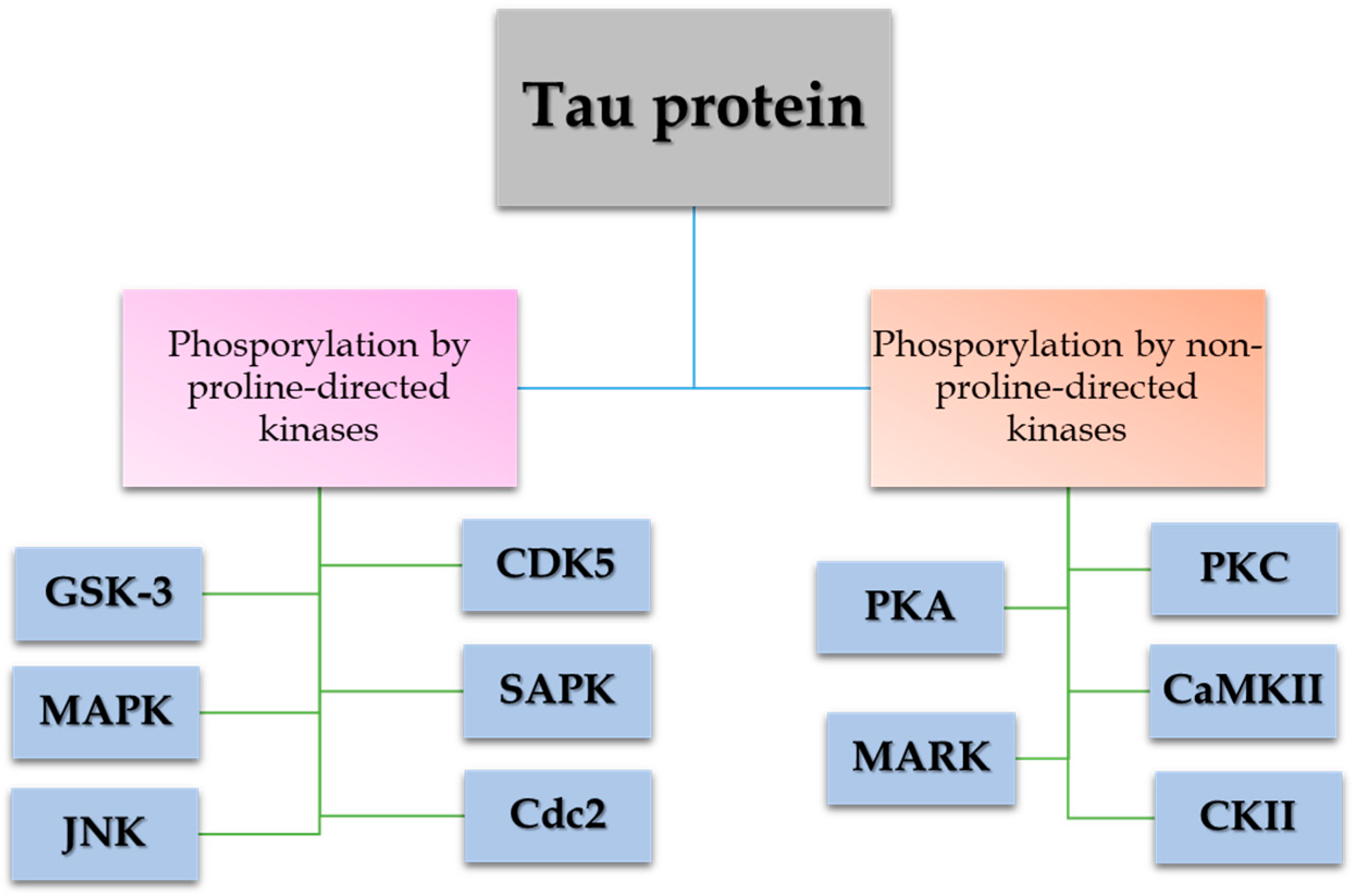
1.3. Tau Proteins
1.4. Cooperation Between Aggregates of Aβ and Hyperphosphorylated Tau Give Rise to Alzheimer’s Disease
1.5. Current Therapies and Rationale for the Work
| Drug | Mechanisms of Action | Current Use/State |
|---|---|---|
| Tacrine | AChE inhibitor. | In disuse due to hepatotoxicity [27,28]. |
| Donepezil | AChE inhibitor. | Commonly prescribed to increase ACh levels [29]. |
| Rivastigmine | AChE inhibitor. | Commonly prescribed to increase ACh levels [30]. |
| Galantamine | AChE inhibitor. | Commonly prescribed to increase ACh levels [31]. |
| Memantine | Weak non-competitive NMDA receptor antagonist; decreases elevated glutamate levels. | Used for moderate to severe Alzheimer’s disease [33]. |
| Aducanumab | Monoclonal antibody that immobilizes Aβ clumps, allowing increased phagocytosis by the immune system. | Approved by the US Food and Drug Administration (FDA) for the treatment of early Alzheimer’s disease [34]. However, its use is controversial [40]. |
| Caprylidene | Improves cytoplasmic energy capacity. | In reference [35]. |
| Flurbiprofen | NSAID inhibiting APP-splitting secretases. | In reference [37]. |
| Valproic acid | Blocks microtubule dissociation. | In reference [38]. |
| Rosiglitazone | Increases insulin sensitivity and glucose utilization. | In reference [40]. |
2. Alzheimer’s Disease and the Immune System
2.1. A Role of the Immune System in Alzheimer’s Disease, the Inflammasome
2.2. Pyroptosis Is Induced by Aβ Accumulation
2.3. ASC Specks and Their Link to Alzheimer’s Disease
2.4. Post-Translational Modifications Regulate NLRP3 Activation and ASC Agglomeration
3. Potential Inhibitory Treatments for Inflammasome Activity in AD
3.1. Antibodies Directed Against ASC Specks
3.2. NLRP3 Inhibitors in AD
3.2.1. The Ketone Body β-Hydroxybutyrate Inhibits NLRP3 Inflammasome-Mediated Inflammation
3.2.2. Protective Effect of MCC50 in AD
3.2.3. JC-124, an Effective Inhibitor of the NLRP3 Inflammasome in AD
4. Nrf2: A Crucial Inhibitory Mechanism in AD
Nrf2 Activators as Therapeutics in Neuronal Inflammation
| Compound | Mechanism of Action | Limitations | Efficacy |
|---|---|---|---|
| DJ-1 | Acts as an oxidative stress sensor and regulates Nrf2 transcription, protecting against oxidative stress [115]. | Pathogenic mutations associated with Parkinson’s disease [136]. | Preclinical [136] |
| Compound B (DJ-1 inhibitor) | Potentiates Nrf2 activation through the PI3K/Akt pathway by binding to the sulfinated form of DJ-1 [116]. | Requires further studies to validate efficacy and safety [137]. | Preclinical [137] |
| ND-13 | Peptide derived from DJ-1, activates the Nrf2 pathway and protects against neurotoxins in Parkinson’s and other diseases [118]. | Limited studies in vitro and in vivo [117]. | Preclinical [117] |
| Omaveloxolone (RTA408) | Activates Nrf2 and increases NQO1 expression, restoring damaged neurons and reducing Aβ aggregates in AD models [120]. | Still under clinical investigation with limited specific applications [119]. | Clinical (approved for Friedreich’s Ataxia) [119] |
| Dl-3-N-Butylphthalide | Inhibits NLRP3 by suppressing TXNIP-NLRP3 interaction, reducing inflammation [121]. | Clinical efficacy not fully established in AD [122]. | Preclinical [122] |
| Dimethyl Fumarate | Blocks Keap1-Nrf2 interaction, activating the antioxidant response [123]. | Approved for multiple sclerosis, but its use in AD is still under evaluation [123]. | Clinical (approved for MS) [138] |
| Sulforaphane | Increases Nrf2 expression, reducing oxidative stress-induced cytotoxicity [124]. | Needs more clinical evidence for use in AD [124]. | Preclinical [124] |
| Resveratrol | Inhibits Aβ protein aggregation and modulates intracellular effectors involved in neuronal survival/death [125]. | Limited clinical studies in AD [125]. | Preclinical [125] |
| Ellagic Acid | Inhibits Keap1, accumulating Nrf2 in the nucleus and activating target genes to alleviate ROS effects in neurodegeneration [126]. | Requires more studies to validate efficacy in neurodegenerative diseases [126]. | Preclinical [126] |
| Epigallocatechin Gallate (EGCG) | Activates Deap-1/Nrf2 pathway to reduce oxidative stress and minimize ROS [127]. | Few clinical studies in AD [127]. | Preclinical [127] |
| Curcumin | Stimulates the Nrf2 pathway through Keap1 inhibition, improving Nrf2 nuclear translocation [127]. | Low bioavailability and need for improved formulations [127]. | Preclinical [127] |
| Allicin | Promotes Nrf2, suppresses oxidative stress, inhibits caspase-3, and prevents apoptosis [129]. | Limited clinical efficacy in neurodegenerative diseases [130]. | Preclinical [130] |
| β-Carotene | Suppresses oxidative stress and inflammation, restoring Nrf2 and HO-1 protein expressions [131]. | Limited efficacy and possible adverse effects at high doses [139]. | Preclinical [139] |
| Moringa (M. oleifera) | Enhances antioxidant defenses in the brain, reduces inflammation, and regulates neurotransmitter levels, potentiating Nrf2 [132]. | Needs more clinical trials to confirm effectiveness [132]. | Preclinical [132] |
| N-Acetyl-Cysteine (NAC) | Modulates Nrf2 activity by modifying critical cysteines in Keap1, inhibiting its ubiquitination and activating the antioxidant response [133]. | Risks associated with high doses, such as renal toxicity [133]. | Clinical (in other conditions) [133] |
5. Discussion
5.1. Potential of Nrf2 in AD
5.2. Potencial of NLRP3 in AD
6. Conclusions and Future Perspectives
- Reduction in oxidative damage and neuroinflammation: Activating Nrf2 can lower ROS levels, inhibit inflammasome-mediated inflammation, and promote neuronal survival.
- Prevention of ASC speck formation: By limiting NLRP3 inflammasome activation, Nrf2 inducers can disrupt the positive feedback loop that worsens inflammation and encourages Aβ aggregation.
- Improvement in preclinical models: Compounds such as dimethyl fumarate, β-hydroxybutyrate, and omaveloxolone have shown protective effects in animal models of AD, reducing both amyloid burden and inflammation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santa-Maria, I. Estudios Sobre la Fosforilación y Agregación de la Proteína Tau y su Posible Relación con la Enfermedad del Alzheimer; Universidad Autonoma de Madrid: Madrid, Spain, 2008. [Google Scholar]
- Fact Sheets of Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 20 October 2024).
- Petra-Lidenberg, M.A. Alois Alzheimer und die Entdeckung der Krankheit. Available online: https://www.alzheimer-forschung.de/alzheimer/wasistalzheimer/geschichte-der-alzheimer-krankheit/ (accessed on 2 April 2024).
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Allegmine Z. Psychiatr. Psych.-Gerichtl. Med. 1907, 64, 146–148. [Google Scholar]
- Minati, L.; Edginton, T.; Bruzzone, M.G.; Giaccone, G. Current concepts in Alzheimer’s disease: A multidisciplinary review. Am. J. Alzheimers Dis. Other Dement. 2009, 24, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Huang, C.R.; Lin, K.J.; Hsieh, Y.H.; Chen, S.D.; Lin, Y.C.; Chao, A.C.; Yang, D.I. Potential Roles of Hypoxia-Inducible Factor-1 in Alzheimer’s Disease: Beneficial or Detrimental? Antioxidants 2024, 13, 1378. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Gong, J.S.; Yanagisawa, K.; Michikawa, M. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J. Neurosci. 2002, 22, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.X.; Papadopoulos, V. Function of beta-amyloid in cholesterol transport: A lead to neurotoxicity. FASEB J. 2002, 16, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Soscia, S.J.; Kirby, J.E.; Washicosky, K.J.; Tucker, S.M.; Ingelsson, M.; Hyman, B.; Burton, M.A.; Goldstein, L.E.; Duong, S.; Tanzi, R.E.; et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS ONE 2010, 5, e9505. [Google Scholar] [CrossRef]
- Matsui, T.; Ingelsson, M.; Fukumoto, H.; Ramasamy, K.; Kowa, H.; Frosch, M.P.; Irizarry, M.C.; Hyman, B.T. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 2007, 1161, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Tharp, W.G.; Sarkar, I.N. Origins of amyloid-beta. BMC Genom. 2013, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Yoshikai, S.; Sasaki, H.; Doh-ura, K.; Furuya, H.; Sakaki, Y. Genomic organization of the human amyloid beta-protein precursor gene. Gene 1990, 87, 257–263. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Koo, E.H. The amyloid precursor protein: Beyond amyloid. Mol. Neurodegener. 2006, 1, 5. [Google Scholar] [CrossRef][Green Version]
- Rogaeva, E.; Meng, Y.; Lee, J.H.; Gu, Y.; Kawarai, T.; Zou, F.; Katayama, T.; Baldwin, C.T.; Cheng, R.; Hasegawa, H.; et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007, 39, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Blomqvist, M.E.; Chalmers, K.; Andreasen, N.; Bogdanovic, N.; Wilcock, G.K.; Cairns, N.J.; Feuk, L.; Brookes, A.J.; Love, S.; Blennow, K.; et al. Sequence variants of IDE are associated with the extent of beta-amyloid deposition in the Alzheimer’s disease brain. Neurobiol. Aging 2005, 26, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Dreses-Werringloer, U.; Lambert, J.C.; Vingtdeux, V.; Zhao, H.; Vais, H.; Siebert, A.; Jain, A.; Koppel, J.; Rovelet-Lecrux, A.; Hannequin, D.; et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell 2008, 133, 1149–1161. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Polanco, J.C.; Ittner, L.M.; Gotz, J. Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 2015, 129, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lindwall, G.; Cole, R.D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 1984, 259, 5301–5305. [Google Scholar] [CrossRef] [PubMed]
- Shiurba, R.A.; Ishiguro, K.; Takahashi, M.; Sato, K.; Spooner, E.T.; Mercken, M.; Yoshida, R.; Wheelock, T.R.; Yanagawa, H.; Imahori, K.; et al. Immunocytochemistry of tau phosphoserine 413 and tau protein kinase I in Alzheimer pathology. Brain Res. 1996, 737, 119–132. [Google Scholar] [CrossRef]
- Xie, L.; Helmerhorst, E.; Taddei, K.; Plewright, B.; Van Bronswijk, W.; Martins, R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J. Neurosci. 2002, 22, RC221. [Google Scholar] [CrossRef]
- Salvadores, N.; Geronimo-Olvera, C.; Court, F.A. Axonal Degeneration in AD: The Contribution of Abeta and Tau. Front. Aging Neurosci. 2020, 12, 581767. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, M.; Dawson, H.N.; Binder, L.I.; Vitek, M.P.; Ferreira, A. Tau is essential to beta-amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 6364–6369. [Google Scholar] [CrossRef] [PubMed]
- Manning, F.C. Tacrine therapy for the dementia of Alzheimer’s disease. Am. Fam. Physician 1994, 50, 819–826. [Google Scholar]
- Qizilbash, N.; Whitehead, A.; Higgins, J.; Wilcock, G.; Schneider, L.; Farlow, M. Cholinesterase inhibition for Alzheimer disease: A meta-analysis of the tacrine trials. Dementia Trialists’ Collaboration. JAMA 1998, 280, 1777–1782. [Google Scholar] [CrossRef]
- Cui, X.; Guo, Y.E.; Fang, J.H.; Shi, C.J.; Suo, N.; Zhang, R.; Xie, X. Donepezil, a drug for Alzheimer’s disease, promotes oligodendrocyte generation and remyelination. Acta Pharmacol. Sin. 2019, 40, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.H.; Lane, R.; Study, G. Rivastigmine: A placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.; Kershaw, P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: Effects on the course of Alzheimer’s disease. Biol. Psychiatry 2001, 49, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine: A review of its use in Alzheimer’s disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Torosyan, N.; Sethanandha, C.; Grill, J.D.; Dilley, M.L.; Lee, J.; Cummings, J.L.; Ossinalde, C.; Silverman, D.H. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: Results of a randomized, double-blinded, pilot study. Exp. Gerontol. 2018, 111, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Toda, A.; Kimoto, A.; Takebayashi, Y.; Higashiyama, R.; Tagata, Y.; Ito, M.; Ota, T.; Shibata, N.; Arai, H. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: A prospective, open-label pilot study. Clin. Interv. Aging 2016, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Meister, S.; Zlatev, I.; Stab, J.; Docter, D.; Baches, S.; Stauber, R.H.; Deutsch, M.; Schmidt, R.; Ropele, S.; Windisch, M.; et al. Nanoparticulate flurbiprofen reduces amyloid-beta42 generation in an in vitro blood-brain barrier model. Alzheimers Res. Ther. 2013, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Long, Z.; Feng, M.; Zhao, Y.; Luo, S.; Wang, K.; Wang, Y.; Yang, G.; He, G. Valproic acid stimulates hippocampal neurogenesis via activating the Wnt/β-Catenin signaling pathway in the APP/PS1/Nestin-GFP triple transgenic mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2019, 11, 62. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Orzel-Sajdlowska, A. Aducanumab-Hope or Disappointment for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4367. [Google Scholar] [CrossRef]
- Nelson, M.L.; Pfeifer, J.A.; Hickey, J.P.; Collins, A.E.; Kalisch, B.E. Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1042. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladacenco, O.; Roza, E.; Costachescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Mahroo, A.; Konstandin, S.; Gunther, M. Blood-Brain Barrier Permeability to Water Measured Using Multiple Echo Time Arterial Spin Labeling MRI in the Aging Human Brain. J. Magn. Reson. Imaging 2024, 59, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B. Alzheimer’s disease: Innate immunity gone awry? Metabolism 2017, 69S, S41–S49. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.D.; Holtzman, D.M. TREM2 Function in Alzheimer’s Disease and Neurodegeneration. ACS Chem. Neurosci. 2016, 7, 420–427. [Google Scholar] [CrossRef]
- Rajesh, Y.; Kanneganti, T.D. Innate Immune Cell Death in Neuroinflammation and Alzheimer’s Disease. Cells 2022, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.; Buelvas, N. Inflammasome: Activation mechanisms. Invest. Clin. 2015, 56, 74–99. [Google Scholar] [PubMed]
- de Alba, E. Structure, interactions and self-assembly of ASC-dependent inflammasomes. Arch. Biochem. Biophys. 2019, 670, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Boucher, D.; Monteleone, M.; Coll, R.C.; Chen, K.W.; Ross, C.M.; Teo, J.L.; Gomez, G.A.; Holley, C.L.; Bierschenk, D.; Stacey, K.J.; et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 2018, 215, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Rathkey, J.K.; Yang, J.; Dubyak, G.R.; Abbott, D.W.; Xiao, T.S. Structures of the Gasdermin D C-Terminal Domains Reveal Mechanisms of Autoinhibition. Structure 2018, 26, 778–784.e773. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Wu, H. Structural mechanisms of inflammasome assembly. FEBS J. 2015, 282, 435–444. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.A.; Dixit, V.M.; Power, C. Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosci. 2020, 43, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Han, Y.H.; Liu, X.D.; Jin, M.H.; Sun, H.N.; Kwon, T. Role of NLRP3 inflammasome-mediated neuronal pyroptosis and neuroinflammation in neurodegenerative diseases. Inflamm. Res. 2023, 72, 1839–1859. [Google Scholar] [CrossRef]
- Feng, Y.S.; Tan, Z.X.; Wu, L.Y.; Dong, F.; Zhang, F. The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Res. Rev. 2020, 64, 101192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Z.; Song, W. NLRP3 inflammasome as a novel therapeutic target for Alzheimer’s disease. Signal Transduct. Target. Ther. 2020, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, Y.; Guan, Q.; Zhang, X.; Shen, H.; Sheng, Y.; Wang, J.; Zhou, X.; Li, W.; Guo, L.; et al. New mechanism of nerve injury in Alzheimer’s disease: Beta-amyloid-induced neuronal pyroptosis. J. Cell Mol. Med. 2020, 24, 8078–8090. [Google Scholar] [CrossRef] [PubMed]
- Si, X.-L.; Fang, Y.-J.; Li, L.-F.; Gu, L.-Y.; Yin, X.-Z.; Yan, Y.-P.; Pu, J.-L.; Zhang, B.-R. From inflammasome to Parkinson’s disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson’s disease? Exp. Neurol. 2021, 336, 113525. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front. Immunol. 2013, 4, 109. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Tan, L.; Jiang, T.; Zhu, X.C.; Wang, H.F.; Jia, C.D.; Yu, J.T. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis 2014, 5, e1382. [Google Scholar] [CrossRef] [PubMed]
- Udan, M.L.; Ajit, D.; Crouse, N.R.; Nichols, M.R. Toll-like receptors 2 and 4 mediate Abeta(1–42) activation of the innate immune response in a human monocytic cell line. J. Neurochem. 2008, 104, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, Y.; Li, Q.; Chen, C.; Chen, H.; Song, Y.; Hua, F.; Zhang, Z. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci. Lett. 2020, 736, 135279. [Google Scholar] [CrossRef]
- Hulse, J.; Bhaskar, K. Crosstalk Between the NLRP3 Inflammasome/ASC Speck and Amyloid Protein Aggregates Drives Disease Progression in Alzheimer’s and Parkinson’s Disease. Front. Mol. Neurosci. 2022, 15, 805169. [Google Scholar] [CrossRef]
- Mueller-Steiner, S.; Zhou, Y.; Arai, H.; Roberson, E.D.; Sun, B.; Chen, J.; Wang, X.; Yu, G.; Esposito, L.; Mucke, L.; et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: Implications for Alzheimer’s disease. Neuron 2006, 51, 703–714. [Google Scholar] [CrossRef]
- Sloane, B.F. Cathepsin B and cystatins: Evidence for a role in cancer progression. Semin. Cancer Biol. 1990, 1, 137–152. [Google Scholar]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.S.; Sborgi, L.; Ruhl, S.; Hiller, S.; Broz, P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 2016, 7, 11929. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, H.; Cui, N. Autophagy Regulation on Pyroptosis: Mechanism and Medical Implication in Sepsis. Mediat. Inflamm. 2021, 2021, 9925059. [Google Scholar] [CrossRef]
- Broderick, L.; Hoffman, H.M. cASCading specks. Nat. Immunol. 2014, 15, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.S.; Bossaller, L.; De Nardo, D.; Ratter, J.M.; Stutz, A.; Engels, G.; Brenker, C.; Nordhoff, M.; Mirandola, S.R.; Al-Amoudi, A.; et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 2014, 15, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Mazo, A.; Martin-Sanchez, F.; Gomez, A.I.; Martinez, C.M.; Amores-Iniesta, J.; Compan, V.; Barbera-Cremades, M.; Yague, J.; Ruiz-Ortiz, E.; Anton, J.; et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef] [PubMed]
- de Rivero Vaccari, J.P.; Mim, C.; Hadad, R.; Cyr, B.; Stefansdottir, T.A.; Keane, R.W. Mechanism of action of IC 100, a humanized IgG4 monoclonal antibody targeting apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC). Transl. Res. 2023, 251, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Lobanova, E.; Zhang, Y.P.; Emin, D.; Brelstaff, J.; Kahanawita, L.; Malpetti, M.; Quaegebeur, A.; Triantafilou, K.; Triantafilou, M.; Zetterberg, H.; et al. ASC specks as a single-molecule fluid biomarker of inflammation in neurodegenerative diseases. Nat. Commun. 2024, 15, 9690. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Xiao, T.S. Post-translational regulation of inflammasomes. Cell Mol. Immunol. 2017, 14, 65–79. [Google Scholar] [CrossRef]
- Gong, T.; Jiang, W.; Zhou, R. Control of Inflammasome Activation by Phosphorylation. Trends Biochem. Sci. 2018, 43, 685–699. [Google Scholar] [CrossRef]
- Stutz, A.; Kolbe, C.C.; Stahl, R.; Horvath, G.L.; Franklin, B.S.; van Ray, O.; Brinkschulte, R.; Geyer, M.; Meissner, F.; Latz, E. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 2017, 214, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.R.; Kasper, S.; Gottier, C.; Lang, S.; Atrott, K.; Vavricka, S.R.; Scharl, S.; Raselli, T.; Frey-Wagner, I.; Gutte, P.M.; et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Invest. 2016, 126, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, L.; Moreau, F.; MacDonald, J.A.; Chadee, K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat. Immunol. 2016, 17, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y.; et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 944. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Jiang, S.; Dong, S.; Liao, Y.; Zhou, Y. The Role of Post-Translational Modifications in Regulation of NLRP3 Inflammasome Activation. Int. J. Mol. Sci. 2023, 24, 6126. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Planillo, R.; Kuffa, P.; Martinez-Colon, G.; Smith, B.L.; Rajendiran, T.M.; Nunez, G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. beta-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation 2020, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Vontell, R.T.; de Rivero Vaccari, J.P.; Sun, X.; Gultekin, S.H.; Bramlett, H.M.; Dietrich, W.D.; Keane, R.W. Identification of inflammasome signaling proteins in neurons and microglia in early and intermediate stages of Alzheimer’s disease. Brain Pathol. 2023, 33, e13142. [Google Scholar] [CrossRef]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.; Rubio Araiz, A.; Bryson, K.J.; Finucane, O.; Larkin, C.; Mills, E.L.; Robertson, A.A.B.; Cooper, M.A.; O’Neill, L.A.J.; Lynch, M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav. Immun. 2017, 61, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brone, B.; Huaux, F.; Octave, J.N.; et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhao, F.; Chojnacki, J.E.; Fulp, J.; Klein, W.L.; Zhang, S.; Zhu, X. NLRP3 Inflammasome Inhibitor Ameliorates Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Desu, H.L.; Plastini, M.; Illiano, P.; Bramlett, H.M.; Dietrich, W.D.; de Rivero Vaccari, J.P.; Brambilla, R.; Keane, R.W. IC100: A novel anti-ASC monoclonal antibody improves functional outcomes in an animal model of multiple sclerosis. J. Neuroinflammation 2020, 17, 143. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef] [PubMed]
- De Plano, L.M.; Calabrese, G.; Rizzo, M.G.; Oddo, S.; Caccamo, A. The Role of the Transcription Factor Nrf2 in Alzheimer’s Disease: Therapeutic Opportunities. Biomolecules 2023, 13, 549. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Bahn, G.; Park, J.S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, A.; Siegl, S.; Fendt, M.; Jansen, S.; Soppa, U.; Brandenburg, L.O.; Pufe, T.; Weis, J.; Wruck, C.J. Oral administration of methysticin improves cognitive deficits in a mouse model of Alzheimer’s disease. Redox Biol. 2017, 12, 843–853. [Google Scholar] [CrossRef]
- Jimenez-Blasco, D.; Santofimia-Castano, P.; Gonzalez, A.; Almeida, A.; Bolanos, J.P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015, 22, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Khorolskaya, V.G.; Sadovnikova, I.S.; Shaforostova, E.A.; Cherednichenko, V.R.; Burakova, I.Y.; Plotnikov, E.Y.; Popov, V.N. Age-Related Decline in Nrf2/ARE Signaling Is Associated with the Mitochondrial DNA Damage and Cognitive Impairments. Int. J. Mol. Sci. 2022, 23, 5197. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sykiotis, G.P.; Nishimura, M.; Bodmer, R.; Bohmann, D. Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell 2013, 12, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid. Redox Signal 2017, 26, 28–43. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, M.; Inden, M.; Yanagisawa, D.; Kitamura, Y. DJ-1/PARK7: A New Therapeutic Target for Neurodegenerative Disorders. Biol. Pharm. Bull. 2017, 40, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Xu, X.; Ye, X.; Yan, J. DJ-1 upregulates the Nrf2/GPX4 signal pathway to inhibit trophoblast ferroptosis in the pathogenesis of preeclampsia. Sci. Rep. 2022, 12, 2934. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.; Graos, M.; Anjo, S.I.; Manadas, B. Modulation of signaling pathways by DJ-1: An updated overview. Redox Biol. 2022, 51, 102283. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Kraus, A.C.; Saludes, M.A.; Konkalmatt, P.; Ruiz Dominguez, A.; Asico, L.D.; Latham, P.S.; Offen, D.; Jose, P.A.; Cuevas, S. ND-13, a DJ-1-Derived Peptide, Attenuates the Renal Expression of Fibrotic and Inflammatory Markers Associated with Unilateral Ureter Obstruction. Int. J. Mol. Sci. 2020, 21, 7048. [Google Scholar] [CrossRef] [PubMed]
- Lev, N.; Barhum, Y.; Ben-Zur, T.; Aharony, I.; Trifonov, L.; Regev, N.; Melamed, E.; Gruzman, A.; Offen, D. A DJ-1 Based Peptide Attenuates Dopaminergic Degeneration in Mice Models of Parkinson’s Disease via Enhancing Nrf2. PLoS ONE 2015, 10, e0127549. [Google Scholar] [CrossRef]
- Profeta, V.; McIntyre, K.; Wells, M.; Park, C.; Lynch, D.R. Omaveloxolone: An activator of Nrf2 for the treatment of Friedreich ataxia. Expert. Opin. Investig. Drugs 2023, 32, 5–16. [Google Scholar] [CrossRef]
- Cui, X.; Zong, S.; Song, W.; Wang, C.; Liu, Y.; Zhang, L.; Xia, P.; Wang, X.; Zhao, H.; Wang, L.; et al. Omaveloxolone ameliorates cognitive dysfunction in APP/PS1 mice by stabilizing the STAT3 pathway. Life Sci. 2023, 335, 122261. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xu, Y.; Wang, X.; Guo, C.; Wang, T.; Wang, Z.Y. Dl-3-n-Butylphthalide Inhibits NLRP3 Inflammasome and Mitigates Alzheimer’s-Like Pathology via Nrf2-TXNIP-TrX Axis. Antioxid. Redox Signal 2019, 30, 1411–1431. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Pereira, M.; Carrascal, M.A.; Brites, G.; Neves, B.; Moreira, P.; Resende, R.; Silva, M.M.; Santos, A.E.; Pereira, C.; et al. Calcium Modulation, Anti-Oxidant and Anti-Inflammatory Effect of Skin Allergens Targeting the Nrf2 Signaling Pathway in Alzheimer’s Disease Cellular Models. Int. J. Mol. Sci. 2020, 21, 7791. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Falla, D.; Barrera-Ocampo, A.; Hagel, C.; Korwitz, A.; Vinueza-Veloz, M.F.; Zhou, K.; Schonewille, M.; Zhou, H.; Velazquez-Perez, L.; Rodriguez-Labrada, R.; et al. Familial Alzheimer’s disease-associated presenilin-1 alters cerebellar activity and calcium homeostasis. J. Clin. Invest. 2014, 124, 1552–1567. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Menard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef]
- Wang, Q.; Botchway, B.O.A.; Zhang, Y.; Liu, X. Ellagic acid activates the Keap1-Nrf2-ARE signaling pathway in improving Parkinson’s disease: A review. Biomed. Pharmacother. 2022, 156, 113848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, S.; Li, Q.; Lang, W.; Li, W.; Jiang, X.; Wan, Z.; Chen, J.; Wang, H. Epigallocatechin-3-gallate: A phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front. Pharmacol. 2022, 13, 977521. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef]
- Omar, S.H.; Al-Wabel, N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, L.; Lin, S.; Chen, S.; Liu, Y.; Zhou, W.; Wang, X. Protective role of beta-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int. Immunopharmacol. 2018, 61, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Banaclocha, M. N-Acetyl-Cysteine: Modulating the Cysteine Redox Proteome in Neurodegenerative Diseases. Antioxidants 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Enriquez, C.; Gonzalez, C.; Aguirre-Martinez, G.; Buc Calderon, P. The Multifaceted Roles of NRF2 in Cancer: Friend or Foe? Antioxidants 2024, 13, 70. [Google Scholar] [CrossRef]
- Huang, M.; Chen, S. DJ-1 in neurodegenerative diseases: Pathogenesis and clinical application. Prog. Neurobiol. 2021, 204, 102114. [Google Scholar] [CrossRef] [PubMed]
- Niki, T.; Endo, J.; Takahashi-Niki, K.; Yasuda, T.; Okamoto, A.; Saito, Y.; Ariga, H.; Iguchi-Ariga, S.M. DJ-1-binding compound B enhances Nrf2 activity through the PI3-kinase-Akt pathway by DJ-1-dependent inactivation of PTEN. Brain Res. 2020, 1729, 146641. [Google Scholar] [CrossRef] [PubMed]
- Hojsgaard Chow, H.; Talbot, J.; Lundell, H.; Marstrand, L.; Gobel Madsen, C.; Bach Sondergaard, H.; Bredahl Hansen, M.; Solberg Sorensen, P.; Siebner, H.R.; Sellebjerg, F. Dimethyl fumarate treatment of primary progressive multiple sclerosis: Results of an open-label extension study. Mult. Scler. Relat. Disord. 2023, 70, 104458. [Google Scholar] [CrossRef] [PubMed]
- Gandla, K.; Babu, A.K.; Unnisa, A.; Sharma, I.; Singh, L.P.; Haque, M.A.; Dashputre, N.L.; Baig, S.; Siddiqui, F.A.; Khandaker, M.U.; et al. Carotenoids: Role in Neurodegenerative Diseases Remediation. Brain Sci. 2023, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Seo, W.; Silwal, P.; Jo, E.K. Crosstalks between inflammasome and autophagy in cancer. J. Hematol. Oncol. 2020, 13, 100. [Google Scholar] [CrossRef]


| Compound | Mechanisms of Action | Evidence of Positive Effects on AE | Stage of Development |
|---|---|---|---|
| β-hydroxybutyrate | It inhibits caspase-1 cleavage, hinders the decrease in intracellular K+ efflux in macrophages, decreases microgliosis, and prevents oligomerization of ASCs and the appearance of ASC specks [91,92,93]. | Yes, in 5XFAD mice [91] | Preclinical development [91]. |
| IC 100 (IgG4κ) | IL-β decreases, ASC uptake by IC 100 prevents the inflammasome from polymerizing with ASC, and ASC specks are formed [81,94]. | No (used as marker in EA). | Not investigated. |
| MCC950 | It blocks canonical and non-canonical NLRP3 activation, inhibits procaspase-1 cleavage, ASC specks form, and reduces Tau hyperphosphorylation [95,96,97]. | Yes, in APP/PS1 mice [96] and PS19 transgenic mice with the Tau P301S mutation on a C57BL/6J background [97]. | Preclinical Development [96,97]. |
| JC-124 | Blocks NLRP3 formation and caspase-1 activation, inhibits β-secretase [98]. | Yes, in TgCRND8 mice [98]. | Preclinical development [98]. |
| NLRP3 protein kinase A | Phosphorylates at Ser295 attenuating the ATPase activity of NLRP3 and causing its inhibition, leads NLPR3 to degrade via ubiquitination [87,88]. | No. | Not investigated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Hernández, R.; de la Torre-Álamo, M.M.; García-Bueno, B.; Baroja-Mazo, A.; Fenoy, F.J.; Cuevas, S. Inflammasomes in Alzheimer’s Progression: Nrf2 as a Preventive Target. Antioxidants 2025, 14, 121. https://doi.org/10.3390/antiox14020121
López-Hernández R, de la Torre-Álamo MM, García-Bueno B, Baroja-Mazo A, Fenoy FJ, Cuevas S. Inflammasomes in Alzheimer’s Progression: Nrf2 as a Preventive Target. Antioxidants. 2025; 14(2):121. https://doi.org/10.3390/antiox14020121
Chicago/Turabian StyleLópez-Hernández, Rubén, María Magdalena de la Torre-Álamo, Belén García-Bueno, Alberto Baroja-Mazo, Francisco Jose Fenoy, and Santiago Cuevas. 2025. "Inflammasomes in Alzheimer’s Progression: Nrf2 as a Preventive Target" Antioxidants 14, no. 2: 121. https://doi.org/10.3390/antiox14020121
APA StyleLópez-Hernández, R., de la Torre-Álamo, M. M., García-Bueno, B., Baroja-Mazo, A., Fenoy, F. J., & Cuevas, S. (2025). Inflammasomes in Alzheimer’s Progression: Nrf2 as a Preventive Target. Antioxidants, 14(2), 121. https://doi.org/10.3390/antiox14020121








