Evaluation of Residues of Amazonian Fruit Piquia (Caryocar villosum) as Sustainable Ingredient for Sunscreen and Cosmetic Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sunscreen Formulations
2.3. Photostability Study for Formulations
2.4. Toxicity Evaluation
2.4.1. Phototoxicity Assay (3T3 PT NRU)
2.4.2. Ocular Irritation Potential
2.5. Efficacy Evaluation
2.5.1. UVB Photoprotection Assay
2.5.2. Photoprotection Against UVA-Induced ROS Production
2.5.3. Evaluation of Radical Protection Factor (RPF)
2.5.4. Quantification of Cumulative Radical Production Induced by VIS + NIR Irradiation on Porcine Ear Skin by EPR Spectroscopy
2.5.5. Confocal Raman Microspectroscopy (CRM)
2.6. Statistical Analysis
3. Results
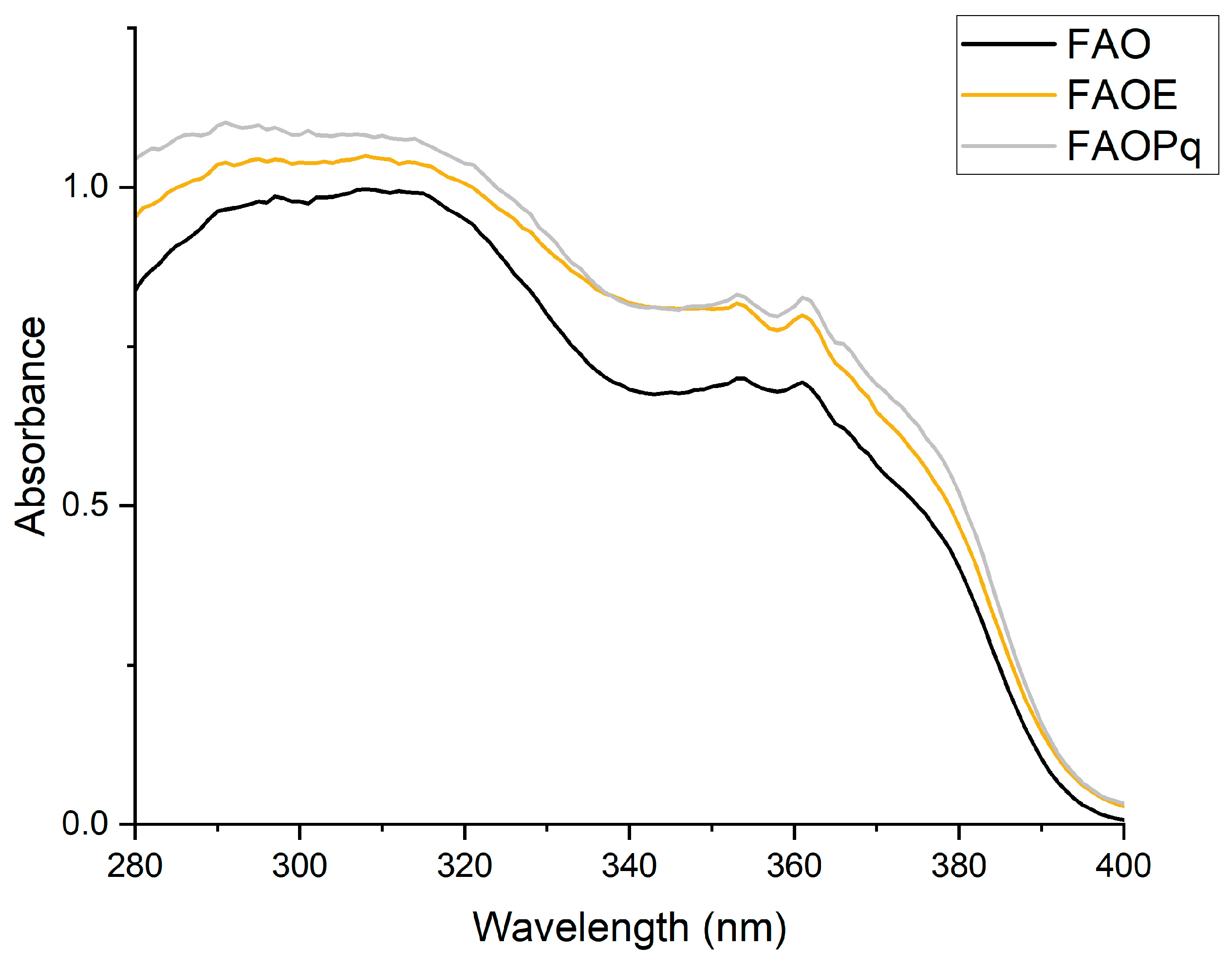
3.1. Photostability of Formulations
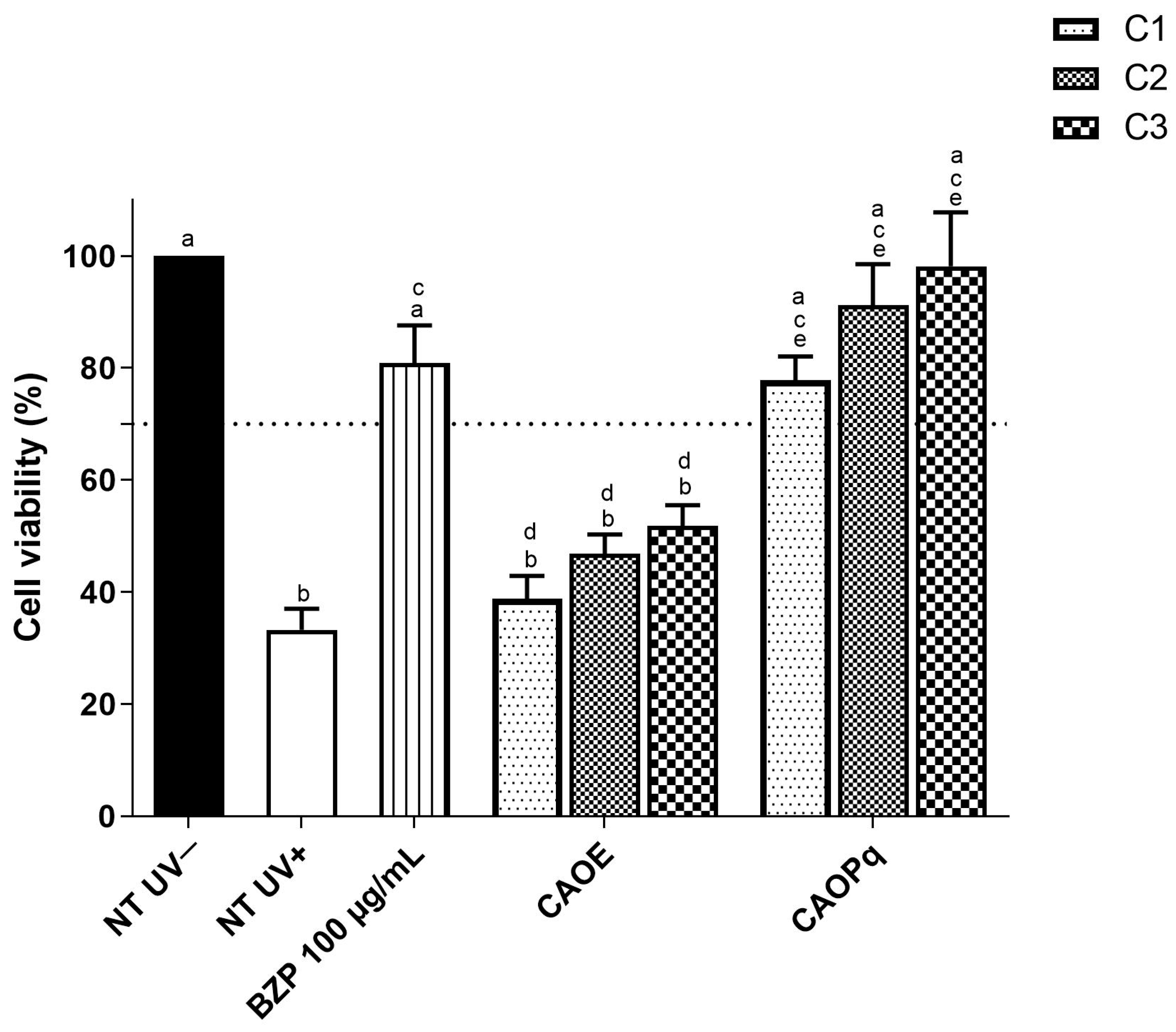
3.2. Phototoxicity
3.3. HET-CAM
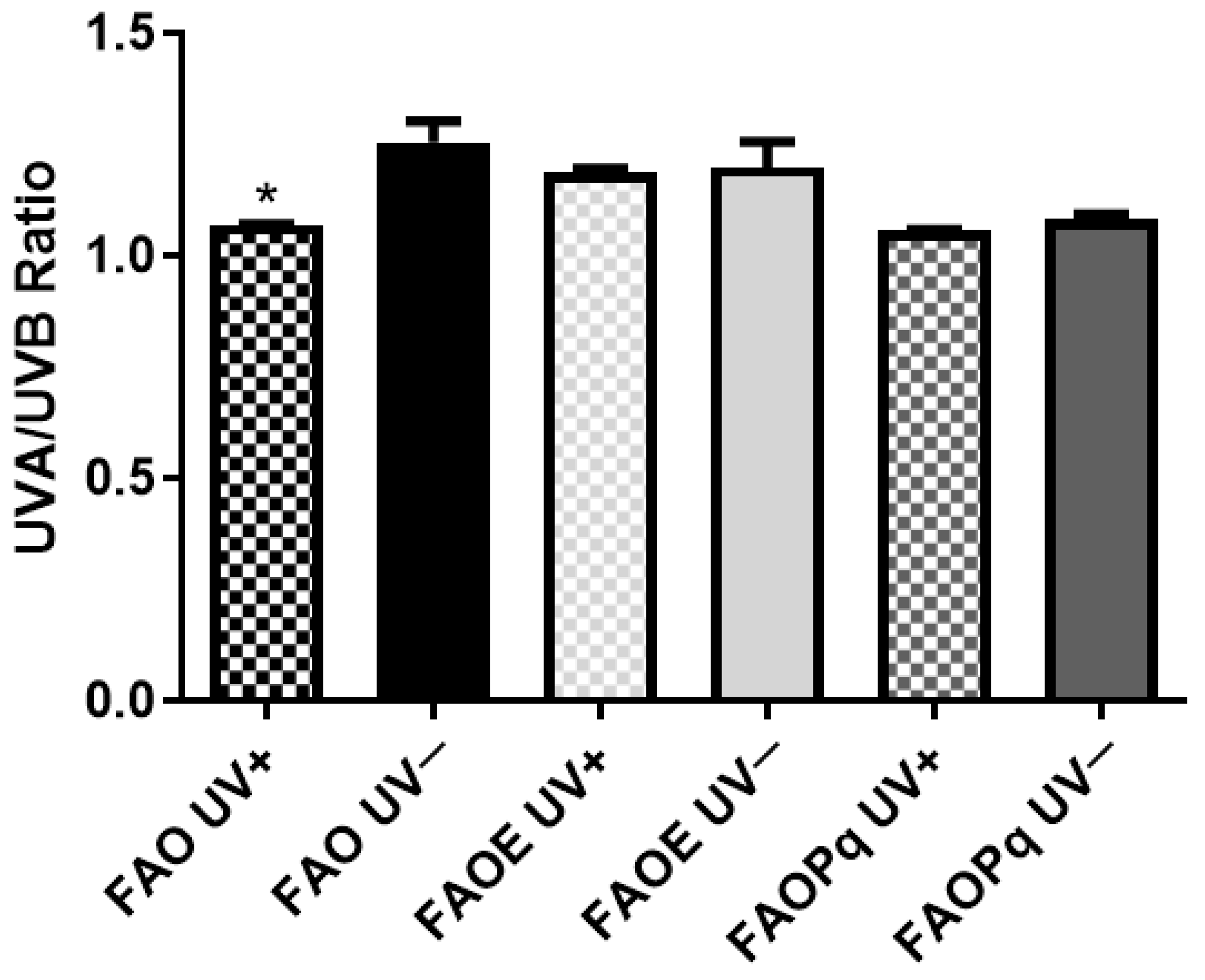
3.4. UVB Photoprotection Assay
3.5. Photoprotection Against UVA-Induced ROS Production
3.6. Radical Protection Factor (RPF)
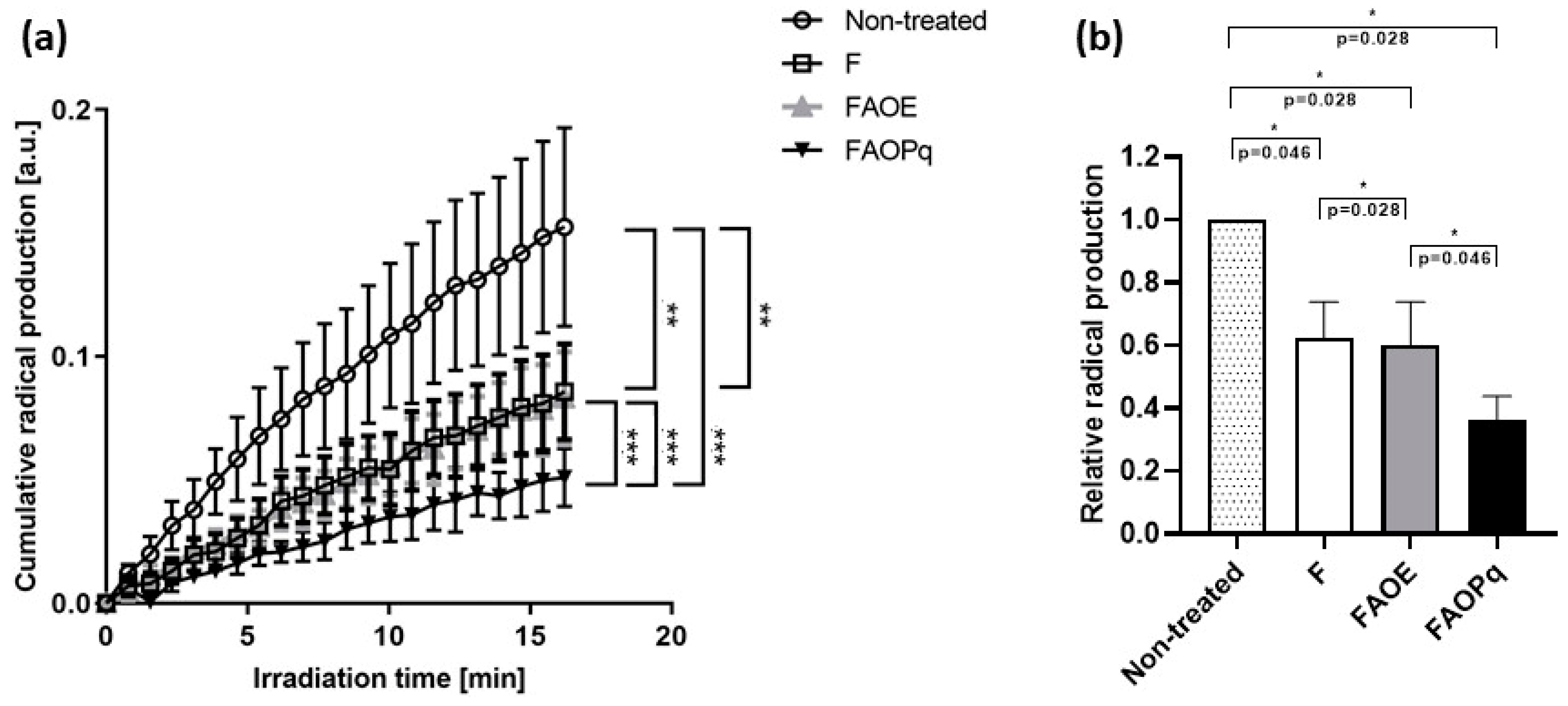
3.7. Quantification of Cumulative Radical Production Induced by VIS + NIR Irradiation on Porcine Ear Skin by EPR Spectroscopy
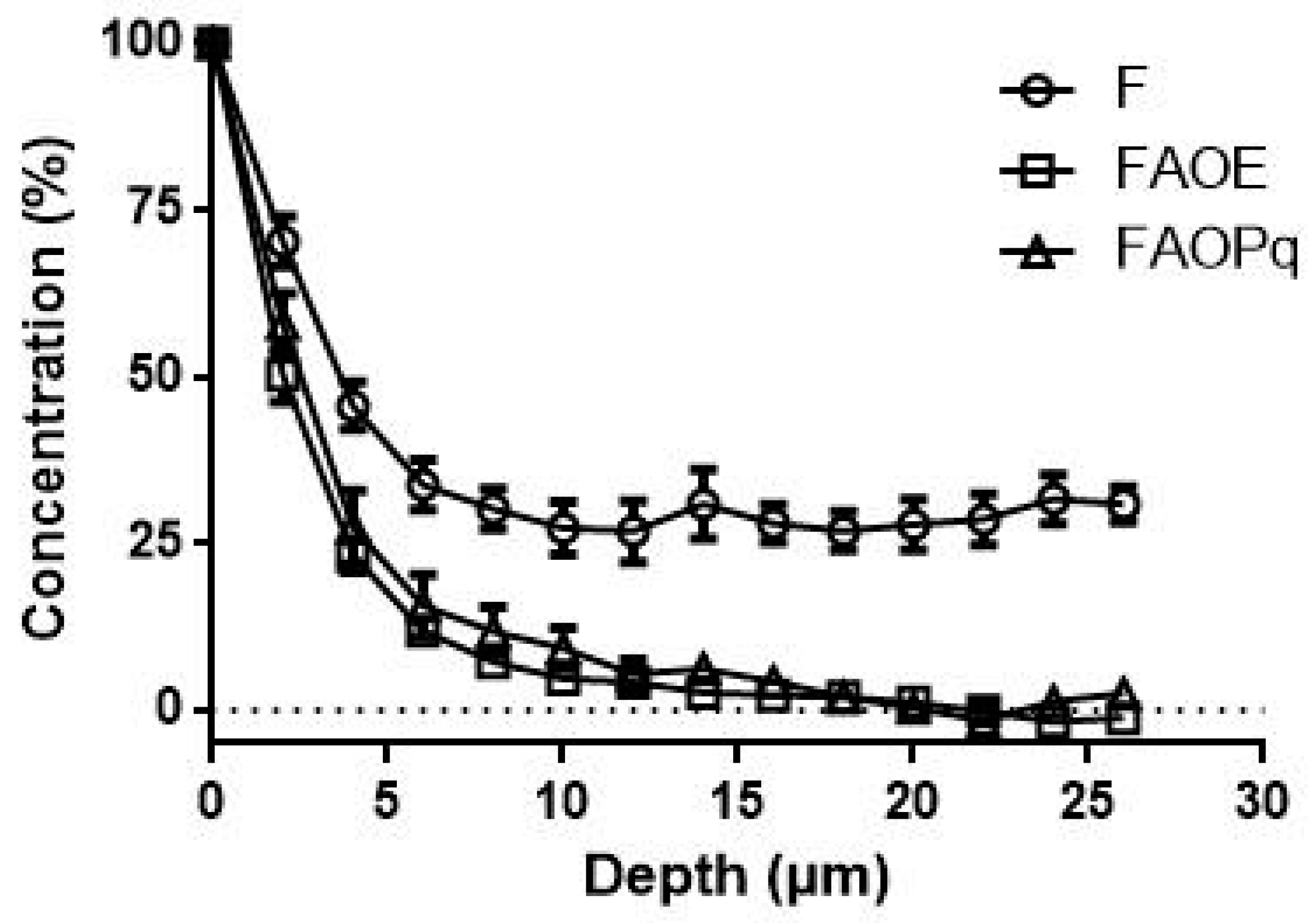
3.8. Confocal Raman Microspectroscopy (CRM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet Radiation and the Skin: Photobiology and Sunscreen Photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef] [PubMed]
- Scarpin, M.S.; Kawakami, C.M.; Rangel, K.C.; de Castro Pereira, K.; Benevenuto, C.G.; Gaspar, L.R. Effects of UV-Filter Photostabilizers in the Photostability and Phototoxicity of Vitamin A Palmitate Combined with Avobenzone and Octyl Methoxycinnamate. Photochem. Photobiol. 2021, 97, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.; Amorós-Galicia, L.; Sohn, M.; Hofer, M.; Quass, K.; Giesinger, J. Analysis of Photokinetics of 2′-Ethylhexyl-4-Methoxycinnamate in Sunscreens. Photochem. Photobiol. Sci. 2019, 18, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. Filter-Filter Interactions. Photostabilization, Triplet Quenching and Reactivity with Singlet Oxygen. Photochem. Photobiol. Sci. 2010, 9, 552–558. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Ferreira, P.J.O.; Duarte, D.J.R.; Miranda, M.S.; Silva, J.C.G.E. Da Structural, Energetic, and UV-Vis Spectral Analysis of UVA Filter 4-Tert-Butyl-4′-Methoxydibenzoylmethane. J. Phys. Chem. A 2014, 118, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Lorigo, M.; Cairrao, E. Antioxidants as Stabilizers of UV Filters: An Example for the UV-B Filter Octylmethoxycinnamate. Biomed. Dermatol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Scalia, S.; Mezzena, M. Incorporation in Lipid Microparticles of the UVA Filter, Butyl Methoxydibenzoylmethane Combined with the UVB Filter, Octocrylene: Effect on Photostability. AAPS PharmSciTech 2009, 10, 384–390. [Google Scholar] [CrossRef]
- Scalia, S.; Mezzena, M. Photostabilization Effect of Quercetin on the UV Filter Combination, Butyl Methoxydibenzoylmethane–Octyl Methoxycinnamate. Photochem. Photobiol. 2010, 86, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Horita, K.; e Silva, J.P.S.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; da Silva, J.C.G.E.; Lobo, J.M.S. Photodegradation of Avobenzone: Stabilization Effect of Antioxidants. J. Photochem. Photobiol. B 2014, 140, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Daher, C.C.; Fontes, I.S.; Rodrigues, R.D.O.; Damasceno, G.A.D.B.; Soares, D.D.S.; AragãO, C.F.S.; Gomes, A.P.B.; Ferrari, M. Development of O/W Emulsions Containing Euterpe oleracea Extract and Evaluation of Photoprotective Efficacy. Braz. J. Pharm. Sci. Biol. 2014, 50, 639–652. [Google Scholar] [CrossRef]
- Tavares, R.S.N.; Kawakami, C.M.; de Castro Pereira, K.; do Amaral, G.T.; Benevenuto, C.G.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Fucoxanthin for Topical Administration, a Phototoxic vs. Photoprotective Potential in a Tiered Strategy Assessed by In Vitro Methods. Antioxidants 2020, 9, 328. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M. A Sustainable Life Cycle for Cosmetics: From Design and Development to Post-Use Phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Almeida, M.R.; Darin, J.D.C.; Hernandes, L.C.; Aissa, A.F.; Chisté, R.C.; Mercadante, A.Z.; Antunes, L.M.G.; Bianchi, M.L.P. Antigenotoxic Effects of Piquiá (Caryocar villosum) in Multiple Rat Organs. Plant Foods Hum. Nutr. 2012, 67, 171–177. [Google Scholar] [CrossRef]
- Chisté, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. The Potential of Extracts of Caryocar villosum Pulp to Scavenge Reactive Oxygen and Nitrogen Species. Food Chem. 2012, 135, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Kist, B.B.; Santos, C.E.; de Carvalho, C.; Beling, R.R. Anuário Brasileiro de Horti & Fruti; Editora Gazeta Santa Cruz: Santa Cruz do Sul, Brazil, 2019. [Google Scholar]
- Filho, N.; Franco, W.B. Potential Assessment of Waste Produced Through the Agro-Industrial Processing in Brazil. Rev. Virtual Química 2015, 7, 1968–1987. [Google Scholar] [CrossRef]
- de Moura, L.B. Gerenciamento de Resíduos Em Empresas Do Setor Hortifrúti Localizadas Na Região Do Cariri—Ceará. Rev. Verde Agroecol. Desenvolv. Sustentável 2013, 8, 21–24. [Google Scholar]
- Martins, Q.S.A.; de Barros, H.E.A.; Silva, S.L.D.C.E.; Gualberto, S.A.; Silva, M.V. Da Resíduos da Indústria Processadora de Polpas de Frutas: Capacidade Antioxidante e Fatores Antinutricionais. Rev. Agronegócio Meio Ambiente 2019, 12, 591–608. [Google Scholar] [CrossRef]
- Li, H.; Zhou, M.; Mohammed, A.E.G.A.Y.; Chen, L.; Zhou, C. From Fruit and Vegetable Waste to Degradable Bioplastic Films and Advanced Materials: A Review. Sustain. Chem. Pharm. 2022, 30, 100859. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.D.L.; Dos, L.; Santarém, S.; Lamarão, C.V.; Lima, E.S.; Veiga-Junior, V.F. Da Avaliação in Vitro Da Atividade Fotoprotetora de Resíduos de Frutas Amazônicas 1. Sci. Amazon. 2016, 1, 109–116. [Google Scholar]
- Yamaguchi, K.K.L.; Lamarão, C.V.; Aranha, E.S.P.; Souza, R.S.O.; Oliveira, P.A.D.; Vasconcellos, M.C.; Lima, E.S.; Veiga-Junior, V.F. HPLC-DAD profile of phenolic compounds, cytotoxicity, antioxidant and anti-inflammatory activities of the amazon fruit Caryocar villosum. Quimica Nova 2017, 40, 483–490. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z. Identification and Quantification, by HPLC-DAD-MS/MS, of Carotenoids and Phenolic Compounds from the Amazonian Fruit Caryocar villosum. J. Agric. Food Chem. 2012, 60, 5884–5892. [Google Scholar] [CrossRef]
- do Nascimento-Silva, N.R.R.; Naves, M.M.V. Potential of Whole Pequi (Caryocar Spp.) Fruit—Pulp, Almond, Oil, and Shell—As a Medicinal Food. J. Med. Food 2019, 22, 952–962. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Damiani, E.; Baschong, W.; Greci, L. UV-Filter Combinations under UV-A Exposure: Concomitant Quantification of over-All Spectral Stability and Molecular Integrity. J. Photochem. Photobiol. B Biol. 2007, 87, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Lopes, N.P.; Gaspar, L.R. Photostability Evaluation of Five UV-Filters, Trans-Resveratrol and Beta-Carotene in Sunscreens. Eur. J. Pharm. Sci. 2015, 78, 79–89. [Google Scholar] [CrossRef]
- Gaspar, L.R.; Campos, P.M.B.G.M. Evaluation of the Photostability of Different UV Filter Combinations in a Sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L. A Method for Broad Spectrum Classification of Sunscreens. Int. J. Cosmet. Sci. 1994, 16, 47–52. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 432: In Vitro 3T3 NRU Phototoxicity Test; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Gaspar, L.R.; Tharmann, J.; Campos, P.M.B.G.M.; Liebsch, M. Skin Phototoxicity of Cosmetic Formulations Containing Photounstable and Photostable UV-Filters and Vitamin A Palmitate. Toxicol. Vitr. 2013, 27, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, H. DB-ALM Protocol n° 78: 3T3 Neutral Red Uptake (NRU) Phototoxicity Assay; Federal Institute for Risk Assessment (BfR): Berlin, Germany, 2008. [Google Scholar]
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Rangel, K.C.; Villela, L.Z.; de Castro Pereira, K.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Assessment of the Photoprotective Potential and Toxicity of Antarctic Red Macroalgae Extracts from Curdiea Racovitzae and Iridaea Cordata for Cosmetic Use. Algal Res. 2020, 50, 101984. [Google Scholar] [CrossRef]
- Meinke, M.C.; Haag, S.F.; Schanzer, S.; Groth, N.; Gersonde, I.; Lademann, J. Radical Protection by Sunscreens in the Infrared Spectral Range. Photochem. Photobiol. 2011, 87, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Lohan, S.B.; Ivanov, D.; Schüler, N.; Berger, B.; Albrecht, S.; Meinke, M.C. EPR Spectroscopy as a Method for ROS Quantification in the Skin. Methods Mol. Biol. 2021, 2202, 137–148. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Lohan, S.B.; Schanzer, S.; Campos, P.M.B.G.M.; Lademann, J.; Meinke, M.C. Eco-Friendly Sunscreen Formulation Based on Starches and PEG-75 Lanolin Increases the Antioxidant Capacity and the Light Scattering Activity in the Visible Light. J. Photochem. Photobiol. B Biol. 2021, 222, 112264. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Campos, P.M.; Schanzer, S.; Albrecht, S.; Lohan, S.B.; Lademann, J.; Darvin, M.E.; Meinke, M.C. Radical-Scavenging Activity of a Sunscreen Enriched by Antioxidants Providing Protection in the Whole Solar Spectral Range. Ski. Pharmacol. Physiol. 2017, 30, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Infante, V.H.P.; Campos, P.M.B.G.M.; Gaspar, L.R.; Darvin, M.E.; Schleusener, J.; Rangel, K.C.; Meinke, M.C.; Lademann, J. Safety and Efficacy of Combined Essential Oils for the Skin Barrier Properties: In Vitro, Ex Vivo and Clinical Studies. Int. J. Cosmet. Sci. 2022, 44, 118–130. [Google Scholar] [CrossRef]
- de Miranda, L.L.R.; Harvey, K.E.; Ahmed, A.; Harvey, S.C. UV-Filter Pollution: Current Concerns and Future Prospects. Environ. Monit. Assess. 2021, 193, 840. [Google Scholar] [CrossRef]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Occurrence and Environmental Hazard of Organic UV Filters in Seawater and Wastewater from Gran Canaria Island (Canary Islands, Spain). Environ. Pollut. 2022, 300, 118843. [Google Scholar] [CrossRef]
- Bonda, C.; Hu, R.; Jockusch, S. Avobenzone Photochemistry:Improving Photoprotection by Stabilizing Avobenzone with Ethylhexyl Methoxycrylene. HPC Today 2014, 9, 6–10. [Google Scholar]
- Freitas, J.V.; Praça, F.S.G.; Bentley, M.V.L.B.; Gaspar, L.R. Trans-Resveratrol and Beta-Carotene from Sunscreens Penetrate Viable Skin Layers and Reduce Cutaneous Penetration of UV-Filters. Int. J. Pharm. 2015, 484, 131–137. [Google Scholar] [CrossRef] [PubMed]
- de Mayo, P. Photochemical syntheses. 37. Enone Photoannelation. Acc. Chem. Res. 1971, 4, 41–47. [Google Scholar] [CrossRef]
- Bonda, C.; Zhang, J. Photostabilization of Retinol and Retinyl Palmitate by Ethylhexyl Methoxycrylene. Cosmet. Toilet. 2011, 126, 40–48. [Google Scholar]
- Bonda, C.; Zhang, J.; Pavlovic, A. The Photostability and Photostabilization of Trans-Resveratrol. Cosmet. Toilet. 2011, 126, 652. [Google Scholar]
- Kikuchi, A.; Hata, Y.; Kumasaka, R.; Nanbu, Y.; Yagi, M. Photoexcited Singlet and Triplet States of a UV Absorber Ethylhexyl Methoxycrylene. Photochem. Photobiol. 2013, 89, 523–528. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Bispo, M.; Morocho-Jácome, A.L.; Escudeiro, C.C.; Martinez, R.M.; de Oliveira Pinto, C.A.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Photoprotective Efficacy of the Association of Rosmarinic Acid 0.1% with Ethylhexyl Methoxycinnamate and Avobenzone. Cosmetics 2023, 10, 11. [Google Scholar] [CrossRef]
- Freitas, J.V.; Gaspar, L.R. In Vitro Photosafety and Efficacy Screening of Apigenin, Chrysin and Beta-Carotene for UVA and VIS Protection. Eur. J. Pharm. Sci. 2016, 89, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Roxo, M.; Peixoto, H.; Wetterauer, P.; Lima, E.; Wink, M.; Soković, M. Piquiá Shells (Caryocar villosum): A Fruit by-Product with Antioxidant and Antiaging Properties in Caenorhabditis Elegans. Oxidative Med. Cell. Longev. 2020, 2020, 7590707. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Properties and Anti-Ultraviolet Activity of Gallic Acid-Chitosan-Gelatin Mixed Gel. J. Ocean Univ. China 2022, 21, 204–212. [Google Scholar] [CrossRef]
- Milani, L.P.G.; Garcia, N.O.S.; Morais, M.C.; Dias, A.L.S.; Oliveira, N.L.; Conceição, E.C. Extract from Byproduct Psidium Guajava Standardized in Ellagic Acid: Additivation of the in Vitro Photoprotective Efficacy of a Cosmetic Formulation. Rev. Bras. Farmacogn. 2018, 28, 692–696. [Google Scholar] [CrossRef]
- Spasojević, I. Free Radicals and Antioxidants at a Glance Using EPR Spectroscopy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 114–142. [Google Scholar] [CrossRef]
- Roessler, M.M.; Salvadori, E. Principles and Applications of EPR Spectroscopy in the Chemical Sciences. Chem. Soc. Rev. 2018, 47, 2534–2553. [Google Scholar] [CrossRef] [PubMed]
- Thbayh, D.K.; Fiser, B. Computational Study of Synthetic and Natural Polymer Additives—Antioxidant Potential of BHA, TBHQ, BHT, and Curcumin. Polym. Degrad. Stab. 2022, 201, 109979. [Google Scholar] [CrossRef]
- Theofanous, A.; Sarli, I.; Fragou, F.; Bletsa, E.; Deligiannakis, Y.; Louloudi, M. Antioxidant Hydrogen-Atom-Transfer to DPPH Radicals by Hybrids of {Hyaluronic-Acid Components}@SiO2. Langmuir 2022, 38, 12333–12345. [Google Scholar] [CrossRef]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective Characteristics of Natural Antioxidant Polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Heinemann, N.; Rademacher, F.; Darvin, M.E.; Raab, C.; Keck, C.M.; Vollert, H.; Fluhr, J.W.; Gläser, R.; Harder, J.; et al. Skin Care Product Rich in Antioxidants and Anti-Inflammatory Natural Compounds Reduces Itching and Inflammation in the Skin of Atopic Dermatitis Patients. Antioxidants 2022, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Gabard, B.; Surber, C. Skin Penetration and Sun Protection Factor of Five UV Filters: Effect of the Vehicle. Ski. Pharmacol. Physiol. 2003, 16, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ekstein, S.F.; Hylwa, S. Sunscreens: A Review of UV Filters and Their Allergic Potential. Dermatitis 2023, 34, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, D.Y.; Mell, H.; Perceval, O.; Burga, K.; Domart-Coulon, I.; Hédouin, L.; Delaunay, M.; Guillaume, M.M.M.; Castelin, M.; Calvayrac, C.; et al. What Are the Toxicity Thresholds of Chemical Pollutants for Tropical Reef-Building Corals? A Systematic Review. Environ. Evid. 2023, 12, 4. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Final Administrative Order (OTC000006). In Over-the-Counter Monograph M020: Sunscreen Drug Products for Over-the-Counter Human Use; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021. [Google Scholar]
- Nicolai, M.; Mota, J.; Fernandes, A.S.; Pereira, F.; Pereira, P.; Reis, C.P.; Velasco, M.V.R.; Baby, A.R.; Rosado, C.; Rijo, P. Assessment of the Potential Skin Application of Plectranthus Ecklonii Benth. Pharmaceuticals 2020, 13, 120. [Google Scholar] [CrossRef]


| Ingredients | F | FAO | FAOE | FAOPq |
|---|---|---|---|---|
| Cetearyl Alcohol and Cetearyl Glucoside | 3.0 | 3.0 | 3.0 | 3.0 |
| C12-15 Alkyl Benzoate | 5.0 | 5.0 | 5.0 | 5.0 |
| Isopropyl myristate | 3.0 | 3.0 | 3.0 | 3.0 |
| butylated hydroxytoluene | 0.05 | 0.05 | 0.05 | 0.05 |
| Glycerol | 2.0 | 2.0 | 2.0 | 2.0 |
| Hydroxyethyl Acrylate/Sodium acryloyldimethyl taurate copolymer and squalane and polysorbate 60 | 1.0 | 1.0 | 1.0 | 1.0 |
| Propylene glycol | 2.0 | 2.0 | 2.0 | 2.0 |
| Phenoxyethanol and parabens | 0.8 | 0.8 | 0.8 | 0.8 |
| Cyclopentasiloxane | 2.0 | 2.0 | 2.0 | 2.0 |
| Avobenzone (AVO) | - | 4.0 | 4.0 | 4.0 |
| Octyl methoxycinnamate (OMC) | - | 8.0 | 8.0 | 8.0 |
| Ethylhexyl methoxycrylene (EHMCR) | - | - | 5.0 | - |
| Piquia shells hydroalcoholic extract (PqSE) | - | - | - | 1.0 or 5.0 * |
| Water | 81.15 | 69.15 | 64.15 | 64.15 or 68.15 |
| Samples | MPE | IC50 (−UV) (µg/mL) | Probability of Toxicity |
|---|---|---|---|
| Norfloxacin | 0.501 0.434 | - - | Phototoxic/ noncytotoxic |
| CAO (4:8) | 0.035 0.026 | 20.373 12.36 | Nonphototoxic/cytotoxic |
| CAO* (5:7) | 0.233 0.269 | 36.714 - | Phototoxic/ cytotoxic |
| CAOE* (5:7:5) | −0.046 0.007 | 15.532 23.982 | Nonphototoxic/cytotoxic |
| CAOPq* (5:7:5) | 0.005 0.073 | 14.254 69.307 | Nonphototoxic/cytotoxic |
| Samples | Irritation Score | Classification |
|---|---|---|
| NaCl 0.9% | 0.0 ± 0.0 | Non-irritating |
| SDS 1% | 12.0 ± 0.0 | Irritating |
| FAO | 0.0 ± 0.0 | Non-irritating |
| FAOE | 0.0 ± 0.0 | Non-irritating |
| FAOPq | 0.0 ± 0.0 | Non-irritating |
| Formulation | RPF (× 1014 radicals/mg) |
|---|---|
| F | 64 ± 6 |
| FAO | 90 ± 5 |
| FAOE | 93 ± 5 |
| FAOPq 1% | 857 ± 94 |
| FAOPq 5% | 3258 ± 244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, I.; Grimmelprez, G.C.P.; Yamaguchi, K.K.L.; Schleusener, J.; Lohan, S.B.; Meinke, M.C.; Gaspar, L.R. Evaluation of Residues of Amazonian Fruit Piquia (Caryocar villosum) as Sustainable Ingredient for Sunscreen and Cosmetic Formulations. Antioxidants 2025, 14, 122. https://doi.org/10.3390/antiox14020122
de Souza I, Grimmelprez GCP, Yamaguchi KKL, Schleusener J, Lohan SB, Meinke MC, Gaspar LR. Evaluation of Residues of Amazonian Fruit Piquia (Caryocar villosum) as Sustainable Ingredient for Sunscreen and Cosmetic Formulations. Antioxidants. 2025; 14(2):122. https://doi.org/10.3390/antiox14020122
Chicago/Turabian Stylede Souza, Izadora, Gabriella C. P. Grimmelprez, Klenicy K. L. Yamaguchi, Johannes Schleusener, Silke B. Lohan, Martina C. Meinke, and Lorena R. Gaspar. 2025. "Evaluation of Residues of Amazonian Fruit Piquia (Caryocar villosum) as Sustainable Ingredient for Sunscreen and Cosmetic Formulations" Antioxidants 14, no. 2: 122. https://doi.org/10.3390/antiox14020122
APA Stylede Souza, I., Grimmelprez, G. C. P., Yamaguchi, K. K. L., Schleusener, J., Lohan, S. B., Meinke, M. C., & Gaspar, L. R. (2025). Evaluation of Residues of Amazonian Fruit Piquia (Caryocar villosum) as Sustainable Ingredient for Sunscreen and Cosmetic Formulations. Antioxidants, 14(2), 122. https://doi.org/10.3390/antiox14020122








