Protective Effects of Atractylodis Rhizoma Extracts on Lung Injury Induced by Particulate Matter 2.5 in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
- Intact (vehicle) control: Mice received 10 mL/kg of distilled water orally and 0.1 mL/kg of saline intranasally.
- PM2.5 (vehicle) control: Mice received 10 mL/kg of distilled water orally and 1 mg/kg of PM2.5 intranasally.
- DEXA: Mice received 0.75 mg/kg of DEXA (equivalent to 11.40 mg/kg DEXA-water soluble) orally and 1 mg/kg of PM2.5 intranasally.
- AJ400: Mice received 400 mg/kg of AJ orally and 1 mg/kg of PM2.5 intranasally.
- AJ200: Mice received 200 mg/kg of AJ orally and 1 mg/kg of PM2.5 intranasally.
- AJ100: Mice received 100 mg/kg of AJ orally and 1 mg/kg of PM2.5 intranasally.
2.2. Induction of Lung Injuries in Mice Using Particulate Matter 2.5
2.3. Preparation and Administration of Test Substances
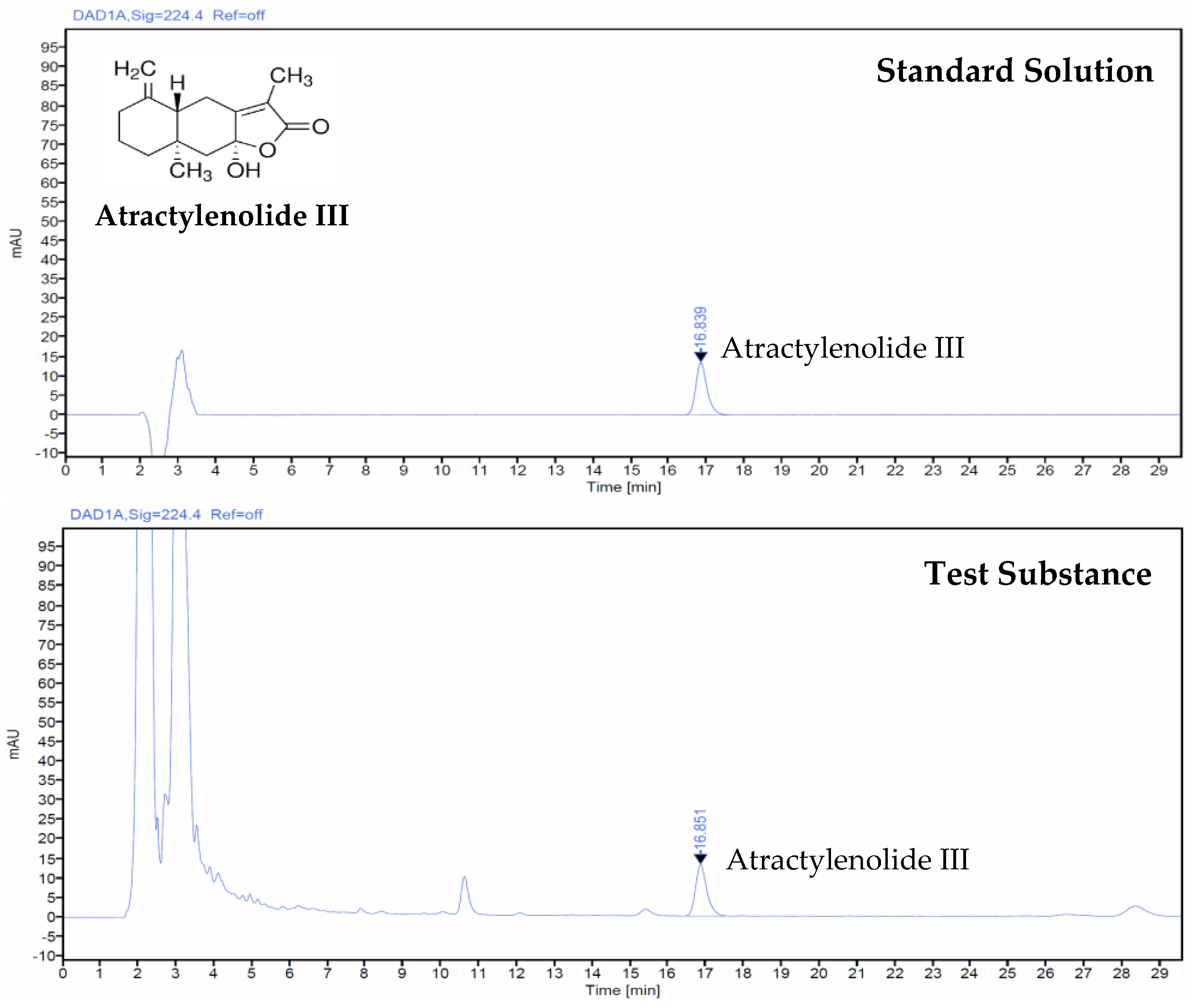
2.4. Analysis of Test Substance Using High-Performance Liquid Chromatography
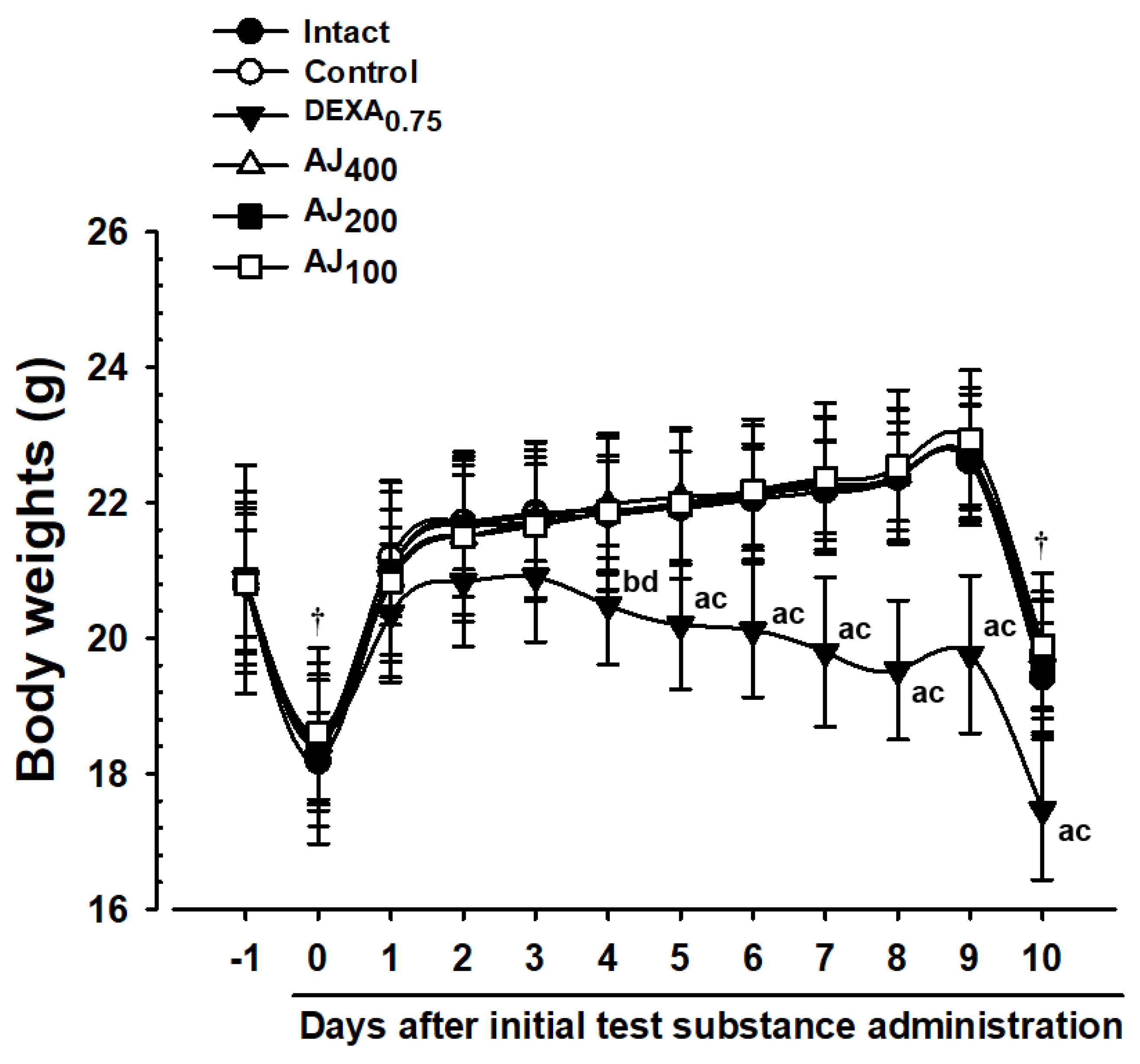
2.5. Monitoring Body Weight Changes
weight at the beginning of administration
2.6. Assessment of Serum Aspartate Aminotransferase and Alanine Aminotransferase Levels
2.7. Measurements of Lung Weights
(24 h post-final administration)] × 100
2.8. Lung Sampling and Gross Inspections
2.9. Bronchoalveolar Lavage Fluid Collection and Cytological Analysis
2.10. Measurement of Lung Lipid Peroxidation
2.11. Measurement of Lung Reactive Oxygen Species (ROS) Levels
2.12. Measurement of Lung Antioxidant Defense Systems
2.13. Real-Time RT-PCR
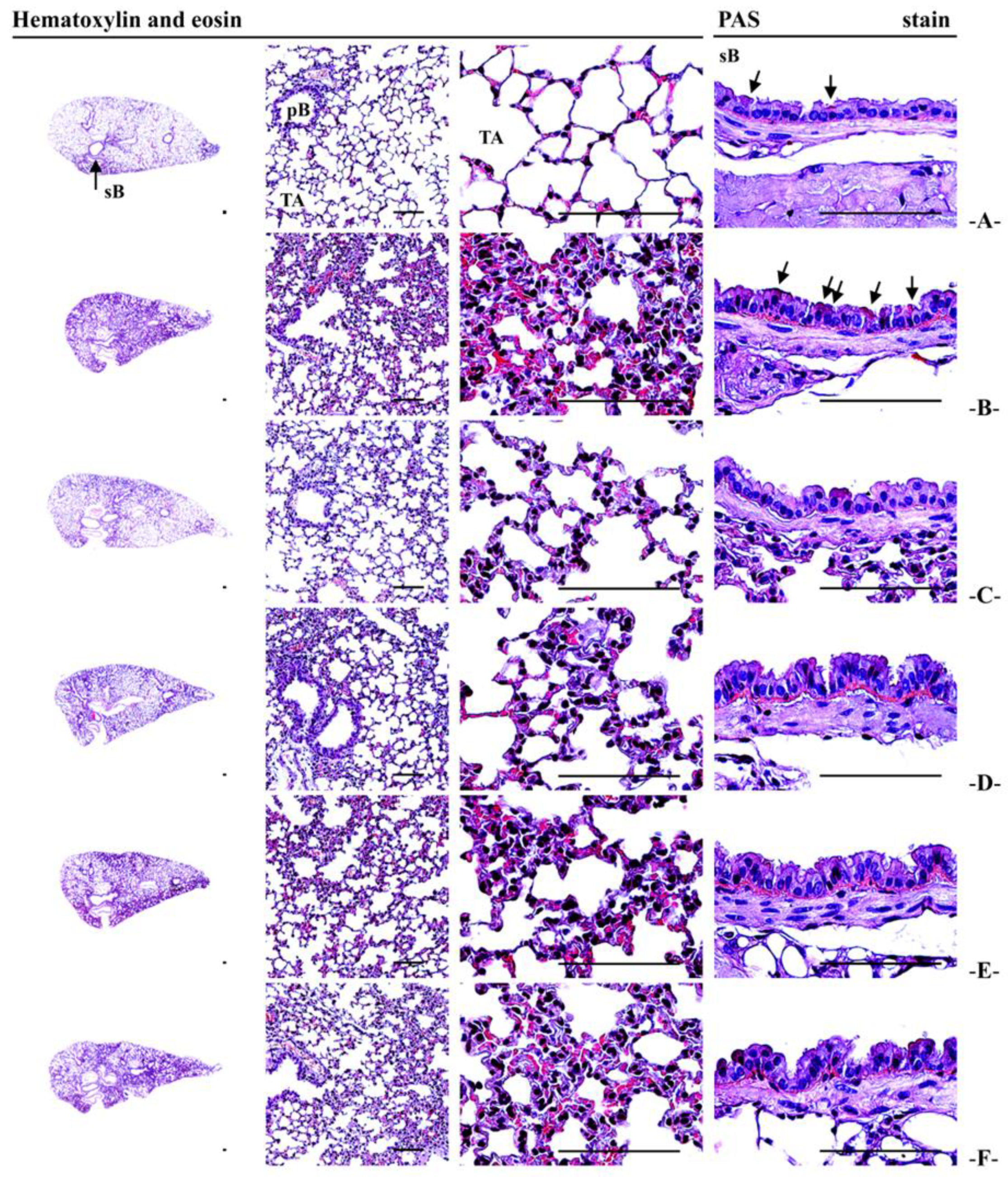
2.14. Histopathological Analysis
2.15. Statistical Analysis
value for intact vehicle control)/value for intact vehicle control] × 100
reference-treated group − values for PM2.5 control)/value for PM2.5 control] × 100
3. Results
3.1. Atractylenolide III Concentration in AJ Extract
3.2. Changes in Body Weight and Weight Gain
3.3. Changes in Gross Observations and Lung Weights
3.4. Bronchoalveolar Lavage Fluid (BALF) Cytology
3.5. Serum Serum Aspartate Aminotransferase and Alanine Aminotransferase Levels
3.6. Lung Cytokine Levels: IL-6, TNF-α, CXCL1, and CXCL2
3.7. Lung Tissue MMP-9 and MMP-12 Content
3.8. Lung Tissue Levels of ACh and Substance P
3.9. Effects on Lung Lipid Peroxidation and Antioxidant Defense Systems
3.10. mRNA Expression of Lung Tissue Genes Involved in Mucus Production
3.11. mRNA Expression of Lung Tissue Genes Involved in Oxidative Stress and Inflammatory Processes: p38 MAPK, NF-κB, PI3K, PTEN, and Akt
3.12. mRNA Expression of Lung Tissue Genes Involved in Cell Apoptosis: Bcl-2 and Bax
3.13. Lung Histopathological Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.-K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of protopanaxatriol type ginsenoside fraction (Rgx365) on particulate matter-induced pulmonary injury. J. Toxicol. Environ. Health Part A 2019, 82, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.-K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of black ginseng on particulate matter-induced pulmonary injury. Am. J. Chin. Med. 2019, 47, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Guo, J.; Yuan, H.; Zhao, C. The compositions, sources, and size distribution of the dust storm from China in spring of 2000 and its impact on the global environment. Chin. Sci. Bull. 2001, 46, 895–900. [Google Scholar] [CrossRef]
- Wang, W.; Primbs, T.; Tao, S.; Simonich, S.L. Atmospheric particulate matter pollution during the 2008 Beijing Olympics. Environ. Sci. Technol. 2009, 43, 5314–5320. [Google Scholar] [CrossRef]
- Huang, X.F.; He, L.Y.; Hu, M.; Zhang, Y.H. Annual variation of particulate organic compounds in PM2.5 in the urban atmosphere of Beijing. Atmos. Environ. 2006, 40, 2449–2458. [Google Scholar] [CrossRef]
- Chen, C.C.; Yang, C.Y. Association between fine particulate air pollution and hospital admissions for chest pain in a subtropical city: Taipei, Taiwan. J. Toxicol. Environ. Health A 2017, 80, 1269–1275. [Google Scholar] [CrossRef]
- Chiu, H.F.; Tsai, S.S.; Yang, C.Y. Short-term effects of fine particulate air pollution on hospital admissions for hypertension: A time-stratified case-crossover study in Taipei. J. Toxicol. Environ. Health A 2017, 80, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, C.; Ji, G.; Liu, H.; Shao, W.; Zhang, C.; Gu, A.; Zhao, P. Effect of exposure to ambient PM2.5 pollution on the risk of respiratory tract diseases: A meta-analysis of cohort studies. J. Biomed. Res. 2017, 31, 130–142. [Google Scholar]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [PubMed]
- Tsai, S.S.; Tsai, C.Y.; Yang, C.Y. Fine particulate air pollution associated with increased risk of hospital admissions for hypertension in a tropical city, Kaohsiung, Taiwan. J. Toxicol. Environ. Health A 2018, 81, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, composition, and lung diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, D.; Gowri, S.; Nideesh, T.R. Hepatoprotective effect of Pterocarpus marsupium against carbon tetrachloride induced damage in albino rats. Anc. Sci. Life 2007, 27, 19–25. [Google Scholar]
- Kim, H.S.; Park, S.I.; Choi, S.H.; Song, C.H.; Park, S.J.; Shin, Y.K.; Han, C.H.; Lee, Y.J.; Ku, S.-K. Single oral dose toxicity test of blue honeysuckle concentrate in mice. Toxicol. Res. 2015, 31, 61–68. [Google Scholar] [CrossRef]
- Hong, M.H.; Kim, J.H.; Bae, H.; Lee, N.Y.; Shin, Y.C.; Kim, S.H.; Ko, S.G. Atractylodes japonica Koidzumi inhibits the production of proinflammatory cytokines through inhibition of the NF-kappaB/IkappaB signal pathway in HMC-1 human mast cells. Arch. Pharm. Res. 2010, 33, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Chen, L.G.; Chou, D.S.; Liang, W.L.; Wang, C.C. Anti-Oxidative abilities of essential oils from Atractylodes ovata rhizome. Evid.-Based Complement. Altern. Med. 2011, 2011, 204892. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jung, H.W.; Park, Y.K. The roots of Atractylodes japonica Koidzumi promote adipogenic differentiation via activation of the insulin signaling pathway in 3T3-L1 cells. BMC Complement. Altern. Med. 2012, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mansoor, S.; Lee, J.; Chung, H.; Kwon, Y.-S.; Bashir, K.M.I.; Choi, J.-S.; Ku, S.-K. Expectorant effects of Atractylodis Rhizoma extracts on the particulate matter-induced pulmonary injury in mice. Appl. Sci. 2024, 15, 99. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Fan, X.; Zhu, Z.; Zhu, Y. Effect of San’ao decoction on aggravated asthma mice model induced by PM2.5 and TRPA1/TRPV1 expressions. J. Ethnopharmacol. 2019, 236, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Min, B.G.; Park, S.M.; Choi, Y.W.; Ku, S.-K.; Cho, I.J.; Kim, Y.W.; Byun, S.H.; Park, C.A.; Park, S.J.; Na, M.; et al. Effects of Pelargonium sidoides and Coptis Rhizoma 2 : 1 mixed formula (PS + CR) on ovalbumin-induced asthma in mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 9135637. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.H.; Fan, Y.J.; Nguyen, T.V.; Song, C.H.; Chai, O.H. Mangiferin alleviates ovalbumin-induced allergic rhinitis via Nrf2/HO-1/NF-κB signaling pathways. Int. J. Mol. Sci. 2020, 21, 3415. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.-K.; Kim, J.W.; Cho, H.R.; Kim, K.Y.; Min, Y.H.; Park, J.H.; Kim, J.S.; Park, J.H.; Seo, B.I.; Roh, S.S. Effect of β-glucan originated from Aureobasidium pullulans on asthma induced by ovalbumin in mouse. Arch. Pharm. Res. 2012, 35, 1073–1081. [Google Scholar] [CrossRef]
- Kavutcu, M.; Canbolat, O.; Oztürk, S.; Olcay, E.; Ulutepe, S.; Ekinci, C.; Gökhun, I.H.; Durak, I. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin-treated guinea pigs: Effects of vitamins E and C. Nephron 1996, 72, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Jamall, I.S.; Smith, J.C. Effects of cadmium on glutathione peroxidase, superoxidase dismutase and lipid peroxidation in the rat heart: A possible mechanism of cadmium cardiotoxicity. Toxicol. Appl. Pharmacol. 1985, 80, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- He, J.; Xu, Q.; Jing, Y.; Agani, F.; Qian, X.; Carpenter, R.; Li, Q.; Wang, X.R.; Peiper, S.S.; Lu, Z.; et al. Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep. 2012, 13, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods in Enzymatic Analysis; Bergmeyer, H.V., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–686. [Google Scholar]
- Sun, Y.; Larry, W.O.; Ying, L. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Deng, X.; Rui, W.; Zhang, F.; Ding, W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol. Toxicol. 2013, 29, 143–157. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, W.; Zhang, W.; Zhao, Y.; Wu, Y.; Ge, G.; Ba, Y.; Guo, Q.; Gao, T.; Chi, X.; et al. Involvement of EGF receptor signaling and NLRP12 inflammasome in fine particulate matter-induced lung inflammation in mice. Environ. Toxicol. 2017, 32, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abdelaziz, R.R.; Elmahdy, M.K.; Suddek, G.M. Flavocoxid attenuates airway inflammation in ovalbumin-induced mouse asthma model. Chem. Biol. Interact. 2018, 292, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Jung, C.J.; Ku, S.M.; Jung, D.H.; Ku, S.-K.; Choi, J.-S. Antitussive, expectorant, and anti-inflammatory effects of Adenophorae Radix powder in ICR mice. J. Ethnopharmacol. 2019, 239, 111915. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Jung, T.Y.; Ku, S.-K.; Yang, H.B.; Lee, H.S. Toxico-pathological study of p,p-DDE after experimental aerosol exposed to ICR Mouse. Toxicol. Res. 2005, 21, 151–160. [Google Scholar]
- André, D.M.; Horimoto, C.M.; Calixto, M.C.; Alexandre, E.C.; Antunes, E. Epigallocatechin-3-gallate protects against the exacerbation of allergic eosinophilic inflammation associated with obesity in mice. Int. Immunopharmacol. 2018, 62, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Sauder, D.C.; DeMars, C.E. An updated recommendation for multiple comparisons. Adv. Methods Pract. Psychol. Sci. 2019, 2, 26–44. [Google Scholar] [CrossRef]
- Choi, B.R.; Kim, H.J.; Lee, Y.J.; Ku, S.-K. Anti-diabetic obesity effects of Wasabia Japonica Matsum leaf extract on 45% Kcal high-fat diet-fed mice. Nutrients 2020, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Chun, Y.S.; Kim, J.K.; Ku, S.-K.; Jeon, S.W.; Park, T.S.; Shim, S.M. Modulating lipid and glucose metabolism by glycosylated kaempferol rich roasted leaves of Lycium chinense via upregulating adiponectin and AMPK activation in obese mice-induced type 2 diabetes. J. Funct. Foods 2020, 72, 104072. [Google Scholar] [CrossRef]
- Filippopoulou, F.; Habeos, G.I.; Rinotas, V.; Sophocleous, A.; Sykiotis, G.P.; Douni, E.; Chartoumpekis, D.V. Dexamethasone administration in mice leads to less body weight gain over time, lower serum glucose, and higher insulin levels independently of NRF2. Antioxidants 2021, 11, 4. [Google Scholar] [CrossRef]
- Tajima, Y. Biological Reference Data Book on Experimental Animals; Soft Science Inc.: Tokyo, Japan, 1989. [Google Scholar]
- Tumes, D.J.; Cormie, J.; Calvert, M.G.; Stewart, K.; Nassenstein, C.; Braun, A.; Foster, P.S.; Dent, L.A. Strain-dependent resistance to allergen-induced lung pathophysiology in mice correlates with rate of apoptosis of lung-derived eosinophils. J. Leukoc. Biol. 2007, 1, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Sodikoff, C.H. Laboratory Profiles of Small Animal Diseases: A Guide to Laboratory Diagnosis; Mosby: St. Louise, MO, USA, 1995; pp. 1–36. [Google Scholar]
- Groneberg, D.A.; Eynott, P.R.; Oates, T.; Lim, S.; Wu, R.; Carlstedt, I.; Nicholson, A.G.; Chung, K.F. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir. Med. 2002, 96, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.O.; Renner, A.; Huber, R.M.; Seeds, M.C.; Rubin, B.K. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 86–91. [Google Scholar] [CrossRef]
- Kim, D.H.; Chu, H.S.; Lee, J.Y.; Hwang, S.J.; Lee, S.H.; Lee, H.M. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Na, H.G.; Kim, Y.D.; Choi, Y.S.; Bae, C.H.; Song, S.Y. Diesel exhaust particles elevate MUC5AC and MUC5B expression via the TLR4-mediated activation of ERK1/2, p38 MAPK, and NF-κB signaling pathways in human airway epithelial cells. Biochem. Biophys. Res. Commun. 2019, 512, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Zhao, P.; Tian, Y.; Liu, X.; He, H.; Jia, R.; Oliver, B.G.; Li, J. Exposure to air pollution exacerbates inflammation in rats with preexisting COPD. Mediators Inflamm. 2020, 2020, 4260204. [Google Scholar] [CrossRef]
- Schaumann, F.; Borm, P.J.; Herbrich, A.; Knoch, J.; Pitz, M.; Schins, R.P.; Luettig, B.; Hohlfeld, J.M.; Heinrich, J.; Krug, N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am. J. Respir. Crit. Care Med. 2004, 170, 898–903. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell Mol. Life Sci. 2006, 63, 414–434. [Google Scholar] [CrossRef]
- Subudhi, U.; Das, K.; Paital, B.; Bhanja, S.; Chainy, G.B. Alleviation of enhanced oxidative stress and oxygen consumption of L-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem. Biol. Interact. 2008, 173, 105–114. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, R.; Liu, H. The acute pulmonary toxicity in mice induced by Staphylococcus aureus, particulate matter, and their combination. Exp. Anim. 2019, 68, 159–168. [Google Scholar] [CrossRef]
- Carini, M.; Aldini, G.; Piccone, M.; Facino, R.M. Fluorescent probes as markers of oxidative stress in keratinocyte cell lines following UVB exposure. Il Farm. 2000, 55, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.L.; Kabir, S.M.; Lee, E.S.; Son, D.S. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-κB activation via EGFR-transactivated Akt signaling. PLoS ONE 2013, 8, e83789. [Google Scholar] [CrossRef]
- Chen, S.; Li, D.; Zhang, H.; Yu, D.; Chen, R.; Zhang, B.; Tan, Y.; Niu, Y.; Duan, H.; Mai, B.; et al. The development of a cell-based model for the assessment of carcinogenic potential upon long-term PM2.5 exposure. Environ. Int. 2019, 131, 104943. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, H.J.; Heo, H.J. Ecklonia cava attenuates PM2.5-induced cognitive decline through mitochondrial activation and anti-inflammatory effect. Mar. Drugs 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.J.; Kim, E.J.; Oh, I.K.; Kim, Y.K.; Park, C.H.; Chung, J.H. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J. Korean Med. Sci. 2010, 25, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guo, J.; Xiao, C. Effect of PM2.5 environmental pollution on rat lung. Environ. Sci. Pollut. Res. Int. 2018, 25, 36136–36146. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Yi, L.; Lan, L.L.; Wei, H.Y.; Wei, D. Long-term PM2.5 exposure increases the risk of non-small cell lung cancer (NSCLC) progression by enhancing interleukin-17a (IL-17a)-regulated proliferation and metastasis. Aging 2020, 12, 11579–11602. [Google Scholar] [CrossRef]
- Monian, P.; Jiang, X. Clearing the final hurdles to mitochondrial apoptosis: Regulation post cytochrome C release. Exp. Oncol. 2012, 34, 185–191. [Google Scholar] [PubMed]
- Wang, A.S.; Xu, Y.; Zhang, Z.W.; Lu, B.B.; Yin, X.; Yao, A.J.; Han, L.Y.; Zou, Z.Q.; Li, Z.; Zhang, X.H. Sulforaphane protects MLE-12 lung epithelial cells against oxidative damage caused by ambient air particulate matter. Food Funct. 2017, 8, 4555–4562. [Google Scholar] [CrossRef]
- Li, X.; Ding, Z.; Zhang, C.; Zhang, X.; Meng, Q.; Wu, S.; Wang, S.; Yin, L.; Pu, Y.; Chen, R. MicroRNA-1228(*) inhibit apoptosis in A549 cells exposed to fine particulate matter. Environ. Sci. Pollut. Res. Int. 2016, 23, 10103–10113. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Fujimoto, M.; Miyamoto, S.; Ishikawa, N.; Serada, S.; Hattori, N.; Nomura, S.; Kohno, N.; Yokoyama, A.; Naka, T. Sputum Leucine-rich alpha-2 glycoprotein as a marker of airway inflammation in asthma. PLoS ONE 2016, 11, e0162672. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Chen, X.; Xu, X.; Zhu, J.; Nie, L.; Long, X. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 2012, 139, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, C. Preparative isolation and purification of atractylon and atractylenolide III from the Chinese medicinal plant Atractylodes macrocephala by high-speed counter-current chromatography. J. Sep. Sci. 2006, 29, 1630–1636. [Google Scholar] [CrossRef]
- Lee, H.; Im, H.J.; Lim, H.; Kim, H.P.; Kim, J.; Kim, J.S. Anti-allergic effects of the rhizomes of Atractylodes japonica and the main constituents. In Proceedings of the 2012 International Conference on Biomedical Engineering and Biotechnology, Macau, China, 28–30 May 2012; pp. 57–59. [Google Scholar]
- Kim, J.-H. Pharmacokinetic analysis of atractylenolide III in rat plasma after oral administration of Atractylodes japonica rhizome extract by ultra-performance liquid chromatography-ion trap mass spectrometry. Acta Chromatogr. 2019, 31, 266–271. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.; Lee, G.; Doh, E.-J.; Hong, S. Quantitative interrelation between atractylenolide I, II, and III in Atractylodes japonica Koidzumi rhizomes, and evaluation of their oxidative transformation using a biomimetic kinetic model. ACS Omega 2018, 3, 14833–14840. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-S.; Kim, S.Y.; Pel, P.; Huh, J.; Joo, S.-W.; Lim, Y.Y.; Park, S.J.; Lim, J.L.; Chin, Y.-W. Standardized extract of Atractylodis Rhizoma Alba and Fructus Schisandrae ameliorates coughing and increases expectoration of phlegm. Molecules 2020, 25, 3064. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Administration. Atractylodes Rhizoma, Cheongju, Republic of Korea. Available online: https://www.mfds.go.kr/files/upload/herbmed/photo_data/KP_1411.pdf (accessed on 5 January 2025).
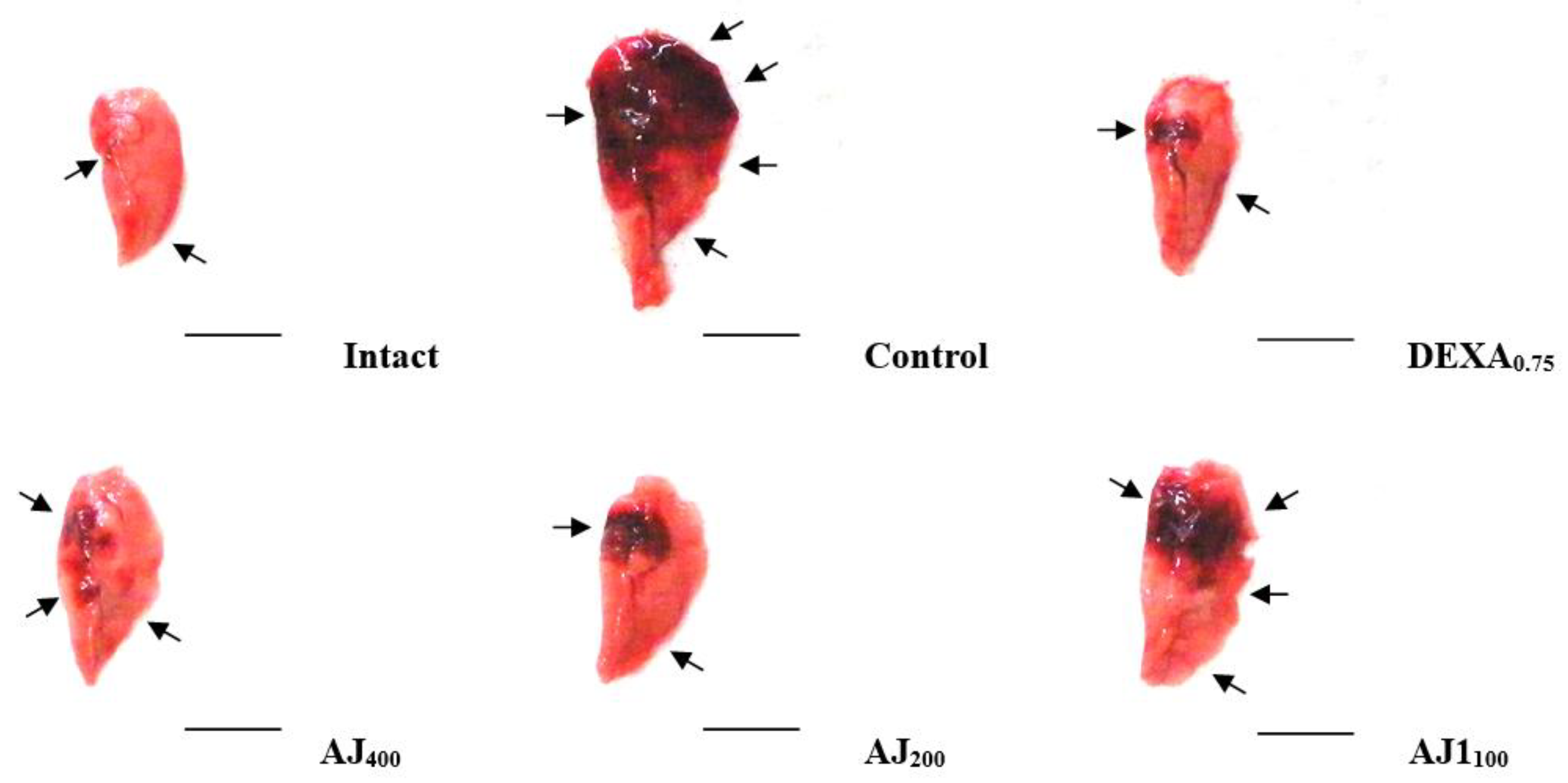

| Groups | Lung Weights | Congestional Regions (%)—Gross Findings | |
|---|---|---|---|
| Absolute (g) | Relative (%) | ||
| Controls | |||
| Intact vehicle | 0.122 ± 0.005 | 0.627 ± 0.030 | 2.41 ± 1.68 |
| PM2.5 | 0.179 ± 0.006 a | 0.908 ± 0.050 c | 62.02 ± 10.13 c |
| Reference | |||
| DEXA | 0.123 ± 0.007 b | 0.706 ± 0.020 cd | 8.52 ± 3.28 cd |
| Test substance—AJ | |||
| 400 mg/kg | 0.144 ± 0.010 ab | 0.727 ± 0.032 cd | 14.68 ± 3.75 cd |
| 200 mg/kg | 0.152 ± 0.007 ab | 0.774 ± 0.049 cd | 24.79 ± 10.55 cd |
| 100 mg/kg | 0.158 ± 0.006 ab | 0.797 ± 0.054 cd | 37.11 ± 10.26 cd |
| Groups | Total Cells | Total Leukocytes | Differential Counts | |||
|---|---|---|---|---|---|---|
| Lymphocytes | Neutrophils | Eosinophils | Monocytes | |||
| Controls | ||||||
| Intact vehicle | 10.10 ± 2.56 | 6.60 ± 1.35 | 3.90 ± 1.20 | 1.05 ± 0.35 | 0.02 ± 0.02 | 1.12 ± 0.53 |
| PM2.5 | 94.80 ± 14.90 c | 61.10 ± 11.84 c | 39.50 ± 11.68 c | 12.05 ± 1.57 a | 1.50 ± 0.28 c | 6.57 ± 1.16 c |
| Reference | ||||||
| DEXA | 19.80 ± 3.16 ce | 11.90 ± 1.66 ce | 7.00 ± 1.25 ce | 2.26 ± 0.88 b | 0.06 ± 0.03 de | 1.85 ± 0.66 e |
| Test substance—AJ | ||||||
| 400 mg/kg | 41.30 ± 10.24 ce | 24.30 ± 4.90 ce | 15.10 ± 4.36 ce | 4.86 ± 0.88 ab | 0.30 ± 0.21 ce | 2.69 ± 0.80 ce |
| 200 mg/kg | 58.40 ± 7.82 ce | 32.00 ± 4.83 ce | 20.10 ± 4.68 ce | 6.53 ± 1.13 ab | 0.53 ± 0.23 ce | 3.63 ± 0.63 ce |
| 100 mg/kg | 66.50 ± 8.81 ce | 41.50 ± 4.72 ce | 27.30 ± 4.40 c | 7.60 ± 1.05 ab | 0.81 ± 0.17 ce | 4.39 ± 0.42 ce |
| Groups | Lung Contents (pg/mL) | |||
|---|---|---|---|---|
| TNF-α | IL-6 | CXCL1 | CXCL2 | |
| Controls | ||||
| Intact vehicle | 30.03 ± 10.58 | 30.70 ± 11.43 | 37.06 ± 11.37 | 17.09 ± 3.88 |
| PM2.5 | 227.93 ± 65.55 c | 412.00 ± 56.09 c | 375.20 ± 117.27 c | 190.09 ± 24.98 a |
| Reference | ||||
| DEXA | 70.28 ± 12.42 cd | 73.70 ± 18.55 cd | 114.97 ± 28.13 cd | 58.79 ± 18.94 ab |
| Test substance—AJ | ||||
| 400 mg/kg | 89.84 ± 16.89 cd | 137.64 ± 31.04 cd | 152.93 ± 36.92 cd | 76.21 ± 16.46 ab |
| 200 mg/kg | 118.62 ± 22.21 cd | 206.01 ± 60.02 cd | 184.85 ± 22.35 cd | 102.45 ± 17.37 ab |
| 100 mg/kg | 144.26 ± 15.80 ce | 266.09 ± 59.85 cd | 224.65 ± 21.31 ce | 129.45 ± 23.04 ab |
| Groups | Lung Contents (nM/mg Protein) | Lung Enzyme Activity (U/mg Protein) | |||
|---|---|---|---|---|---|
| MDA | ROS | GSH | SOD | CAT | |
| Controls | |||||
| Intact vehicle | 4.15 ± 1.14 | 27.23 ± 10.36 | 48.74 ± 13.90 | 331.90 ± 56.94 | 78.20 ± 16.16 |
| PM2.5 | 20.55 ± 4.14 a | 90.03 ± 11.76 a | 6.39 ± 1.03 d | 72.00 ± 17.37 d | 9.90 ± 1.79 d |
| Reference | |||||
| DEXA | 6.36 ± 1.86 c | 44.05 ± 13.75 bc | 17.64 ± 4.24 de | 192.30 ± 45.78 de | 35.80 ± 11.51 de |
| Test substance—AJ | |||||
| 400 mg/kg | 10.24 ± 1.24 ac | 52.79 ± 11.47 ac | 14.65 ± 3.44 de | 159.90 ± 23.25 de | 28.20 ± 12.35 df |
| 200 mg/kg | 12.57 ± 2.23 ac | 59.04 ± 10.50 ac | 12.01 ± 2.30 de | 138.10 ± 21.37 de | 23.70 ± 5.58 de |
| 100 mg/kg | 15.51 ± 1.69 ac | 65.52 ± 11.17 ac | 9.96 ± 1.42 de | 110.80 ± 12.33 de | 17.60 ± 5.02 df |
| Groups | Controls | Reference | Test Substance—AJ | |||
|---|---|---|---|---|---|---|
| Intact Vehicle | PM2.5 | DEXA | 400 mg/kg | 200 mg/kg | 100 mg/kg | |
| MUC5AC | 1.00 ± 0.06 | 4.80 ± 0.67 a | 2.12 ± 0.71 ab | 2.50 ± 0.50 ab | 2.95 ± 0.44 ab | 3.45 ± 0.69 ab |
| MUC5B | 1.00 ± 0.05 | 2.88 ± 0.25 a | 1.64 ± 0.25 ab | 1.89 ± 0.21 ab | 2.06 ± 0.22 ab | 2.26 ± 0.23 ab |
| NF-κB1 | 1.00 ± 0.04 | 9.18 ± 1.04 a | 2.42 ± 0.79 ab | 4.34 ± 1.39 ab | 5.67 ± 0.95 ab | 6.93 ± 1.05 ab |
| p38 MAPKα | 1.00 ± 0.04 | 7.33 ± 0.93 a | 2.90 ± 0.72 ab | 3.31 ± 0.52 ab | 4.64 ± 0.69 ab | 5.36 ± 0.47 ab |
| PTEN | 1.00 ± 0.05 | 0.31 ± 0.10 a | 0.66 ± 0.15 ab | 0.61 ± 0.11 ab | 0.54 ± 0.07 ab | 0.49 ± 0.03 ab |
| PI3K | 1.00 ± 0.06 | 7.02 ± 1.00 a | 2.30 ± 0.53 ab | 2.83 ± 0.58 ab | 4.00 ± 0.77 ab | 5.11 ± 0.61 ab |
| Akt1 | 1.00 ± 0.05 | 5.09 ± 1.14 a | 1.90 ± 0.36 ab | 2.27 ± 0.31 ab | 2.88 ± 0.46 ab | 3.26 ± 0.18 ab |
| Bcl-2 | 1.00 ± 0.06 | 0.35 ± 0.07 a | 0.70 ± 0.12 ab | 0.63 ± 0.11 ab | 0.57 ± 0.11 ab | 0.49 ± 0.04 ab |
| Bax | 1.00 ± 0.05 | 6.63 ± 0.93 a | 2.49 ± 0.40 ab | 3.10 ± 0.68 ab | 4.08 ± 0.75 ab | 4.67 ± 0.80 ab |
| Groups | Mean ASA (%/mm2) | Mean Alveolar Septal Thickness (μm) | Mean Thickness of SB (μm) | Mean IF Cell Numbers Infiltrated in AR (×10 cells/mm2) | PAS-Positive Cells on the SB (cells/mm2) |
|---|---|---|---|---|---|
| Controls | |||||
| Intact vehicle | 84.85 ± 6.23 | 3.79 ± 0.69 | 13.46 ± 1.28 | 31.60 ± 10.49 | 13.00 ± 4.03 |
| PM2.5 | 40.27 ± 9.52 a | 42.53 ± 4.37 a | 17.25 ± 1.26 a | 527.50 ± 105.72 a | 38.40 ± 6.17 a |
| Reference | |||||
| DEXA | 78.91 ± 3.83 c | 12.68 ± 2.85 ac | 17.03 ± 1.85 a | 235.90 ± 59.60 ac | 36.40 ± 5.48 a |
| Test substance—AJ | |||||
| 400 mg/kg | 72.55 ± 8.71 bc | 14.04 ± 2.19 ac | 28.67 ± 6.71 ac | 278.20 ± 51.00 ac | 83.20 ± 16.34 ac |
| 200 mg/kg | 62.39 ± 6.38 ac | 23.15 ± 4.53 ac | 25.67 ± 4.22 ac | 328.40 ± 42.90 ac | 71.00 ± 16.20 ac |
| 100 mg/kg | 57.65 ± 4.01 ac | 27.20 ± 4.79 ac | 22.79 ± 1.96 ac | 387.20 ± 38.84 ac | 60.60 ± 14.36 ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, E.-H.; Bashir, K.M.I.; Lee, J.; Chung, H.; Kwon, Y.-S.; Choi, J.-S.; Ku, S.-K. Protective Effects of Atractylodis Rhizoma Extracts on Lung Injury Induced by Particulate Matter 2.5 in Mice. Antioxidants 2025, 14, 127. https://doi.org/10.3390/antiox14020127
Yun E-H, Bashir KMI, Lee J, Chung H, Kwon Y-S, Choi J-S, Ku S-K. Protective Effects of Atractylodis Rhizoma Extracts on Lung Injury Induced by Particulate Matter 2.5 in Mice. Antioxidants. 2025; 14(2):127. https://doi.org/10.3390/antiox14020127
Chicago/Turabian StyleYun, Eun-Hee, Khawaja Muhammad Imran Bashir, Jeongjun Lee, Hunsuk Chung, Young-Sam Kwon, Jae-Suk Choi, and Sae-Kwang Ku. 2025. "Protective Effects of Atractylodis Rhizoma Extracts on Lung Injury Induced by Particulate Matter 2.5 in Mice" Antioxidants 14, no. 2: 127. https://doi.org/10.3390/antiox14020127
APA StyleYun, E.-H., Bashir, K. M. I., Lee, J., Chung, H., Kwon, Y.-S., Choi, J.-S., & Ku, S.-K. (2025). Protective Effects of Atractylodis Rhizoma Extracts on Lung Injury Induced by Particulate Matter 2.5 in Mice. Antioxidants, 14(2), 127. https://doi.org/10.3390/antiox14020127







