Investigation of Phytochemical Composition, Antioxidant and Antibacterial Activity of Five Red Flower Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Antibacterial Activity
2.2. Methods
2.2.1. Determination of Total Polyphenols and Antioxidant Activity
2.2.2. Determination of Total Anthocyanins
2.2.3. Identification of Phenolic Compounds by HPLC-DAD Method
2.2.4. Determination of Antimicrobial Activity: Diffusion Method
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentrations (MBC)
2.2.5. Statistical Analysis
3. Results
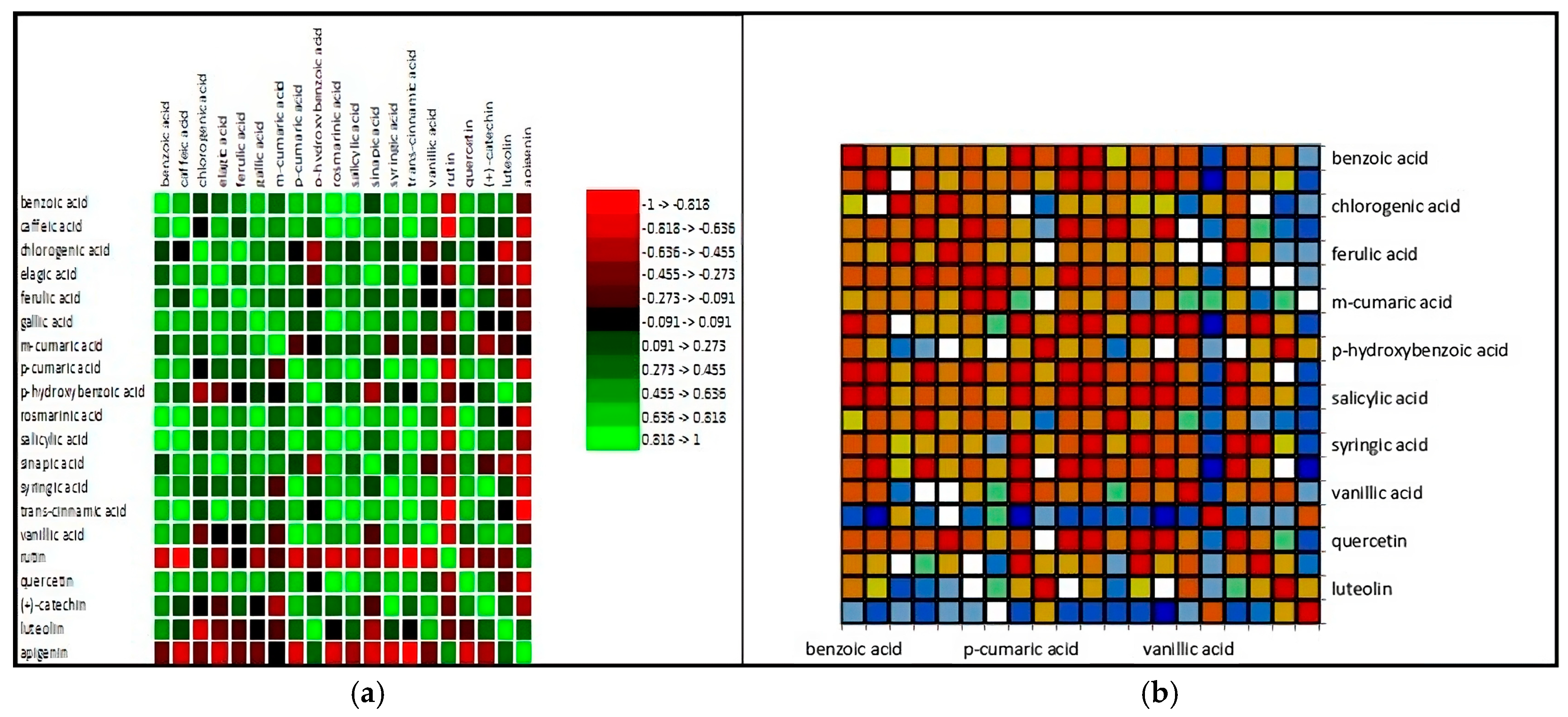
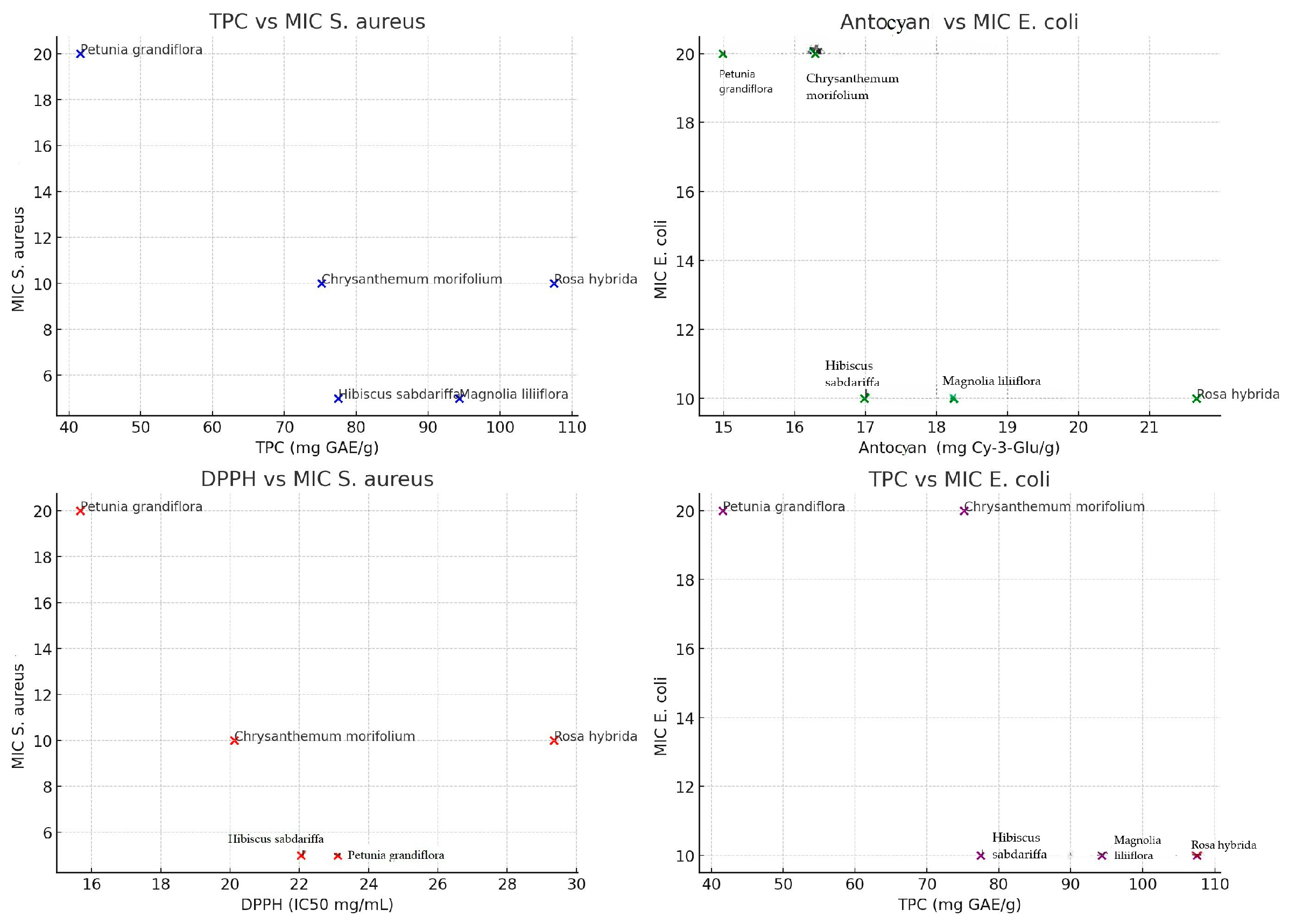
4. Discussion
- TPC vs. MIC S. aureus:
- ○
- r = −0.73, p = 0.16
- ○
- Moderate to strong negative correlation, but not statistically significant.
- TPC vs. MIC E. coli:
- ○
- r = −0.77, p = 0.13
- ○
- Moderate to strong negative correlation.
- Anthocyanins vs. MIC S. aureus:
- ○
- r = −0.42, p = 0.48
- ○
- Weak negative correlation.
- Anthocyanins vs. MIC E. coli:
- ○
- r = −0.72, p = 0.17
- ○
- Moderate negative correlation.
- DPPH vs. MIC S. aureus:
- ○
- r = −0.57, p = 0.32
- ○
- Moderate negative correlation, not statistically significant.
- DPPH vs. MIC E. coli:
- ○
- r = −0.76, p = 0.13
- ○
- Moderate to strong negative correlation.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Şandru, D. Antimicrobial Effect of E. coli on Essential Oils Derived from Romanian Aromatic Plants. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 87–92. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial Effect of Different Herbal Plant Extracts Against Different Microbial Populations. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.A.; Muddathir, A.M.; Osman, M.A. Antimicrobial Activity, Phytochemical Screening of Crude Extracts, and Essential Oils Constituents of Two Pulicaria spp. Growing in Sudan. Sci. Rep. 2020, 10, 17148. [Google Scholar] [CrossRef]
- Rocha, R.; Pinela, J.; Abreu, R.; Añibarro Ortega, M.; Pires, T.; Saldanha, A.; Alves, M.; Nogueira, A.; Ferreira, I.; Barros, L. Extraction of Anthocyanins from Red Raspberry for Natural Food Colorants Development: Processes Optimization and In Vitro Bioactivity. Processes 2020, 8, 1447. [Google Scholar] [CrossRef]
- Qi, W.; Chen, Y.; Sun, S.; Xu, X.; Zhan, J.; Yan, Z.; Shang, P.; Pan, X.; Liu, H. Inhibiting TLR4 Signaling by Linarin for Preventing Inflammatory Response in Osteoarthritis. Aging 2021, 13, 5369–5382. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Singh, A.; Tripathi, P.; Yadawa, A.K.; Singh, S. Promising Polyphenols in Parkinson’s Disease Therapeutics. Neurochem. Res. 2020, 45, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Vicaş, S.I.; Purcărea, C.; Ruszkai, L.; Laslo, V. Separation of Pigments from Petunia’s Petals Using Thin Layer Chromatography. Analele Univ. Din Oradea Fasc. Protecţia Mediu. 2008, 13, 229–233. [Google Scholar]
- Wang, N.; Zhang, C.; Bian, S.; Chang, P.; Xuan, L.; Fan, L.; Yu, Q.; Liu, Z.; Gu, C.; Zhang, S.; et al. Flavonoid Components of Different Color Magnolia Flowers and Their Relationship to Cultivar Selections. HortScience 2019, 54, 404–408. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Wang, Y.; Wang, Y.; Song, A.; Jiang, J.; Chen, S.; Guan, Z.; Chen, F. CmMYB3-like Negatively Regulates Anthocyanin Biosynthesis and Flower Color Formation During the Post-Flowering Stage in Chrysanthemum morifolium. Hortic. Plant J. 2024, 10, 194–204. [Google Scholar] [CrossRef]
- Wheeler, L.C.; Dunbar-Wallis, A.; Schutz, K.; Smith, S.D. Evolutionary Walks Through Flower Colour Space Driven by Gene Expression in Petunia and Allies (Petunieae). Proc. R. Soc. B 2023, 290, 20230275. [Google Scholar] [CrossRef] [PubMed]
- Almășan, A.L.; Stroe, M.M.; Ianovici, N. Considerations Regarding the Anthocyanins in Plant and Human Life. Biostudent 2021, 4, 5–24. [Google Scholar]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, C.; Zhong, W.; Shu, Y.; Zhang, Y.; Yang, D. Antibacterial Effect and Mechanism of Anthocyanin from Lycium ruthenicum Murr. Front. Microbiol. 2022, 13, 974602. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Antioxidant, Cytotoxic, and Antibacterial Activities of Clitoria ternatea Flower Extracts and Anthocyanin-Rich Fraction. Sci. Rep. 2022, 12, 14890. [Google Scholar] [CrossRef] [PubMed]
- Łysiak, G.P. Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties. Foods 2022, 11, 948. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.J.; Su, S.L.; Yan, H.; Guo, S.; Qian, D.W.; Duan, J.A. Evaluation of Anti-Inflammatory and Antioxidant Effects of Chrysanthemum Stem and Leaf Extract on Zebrafish Inflammatory Bowel Disease Model. Molecules 2022, 27, 2114. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Valdés, R.; Chacón-Hernández, J.C.; Reyes-Zepeda, F.; Hernández-Castillo, F.D.; Anguiano-Cabello, J.C.; Heinz-Castro, R.T.Q.; Mora-Ravelo, S.G. In Vitro Antibacterial Activity of Magnolia tamaulipana Against Tomato Phytopathogenic Bacteria. Plant Protect. Sci. 2020, 56, 268–274. [Google Scholar] [CrossRef]
- Bajpai, V.; Yoon, J.; Chul, S. Antioxidant and Antidermatophytic Activities of Essential Oil and Extracts of Magnolia liliflora Desr. Food Chem. Toxicol. 2009, 47, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rocha, J.V.; Vásquez-Morales, S.G. The Potential of Magnolia spp. in the Production of Alternative Pest Control Substances. Molecules 2023, 28, 4681. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Lim, S.-C.; Choi, J.; Park, H.-J. Identification and Quantification of the Sedative and Anticonvulsant Flavone Glycoside from Chrysanthemum boreale. Arch. Pharmacal Res. 2013, 36, 51–60. [Google Scholar] [CrossRef]
- Kim, J.-W.; Han, J.-Y.; Hong, J.T.; Li, R.; Eun, J.S.; Oh, K.-W. Ethanol Extract of the Flower Chrysanthemum morifolium Augments Pentobarbital-Induced Sleep Behaviors: Involvement of Cl− Channel Activation. Evid.-Based Complement. Altern. Med. 2011, 2011, 109164. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Lee, H.W.; Jung, J.-C.; Oh, S. Chrysanthemum morifolium and Its Bioactive Substance Enhanced the Sleep Quality in Rodent Models via Cl− Channel Activation. Nutrients 2023, 15, 1309. [Google Scholar] [CrossRef]
- Pandey, J.; Bastola, T.; Dhakal, B.; Poudel, A.; Devkota, H.P. Chrysanthemum morifolium Ramat.: A Medicinal Plant with Diverse Traditional Uses, Bioactive Constituents, and Pharmacological Activities. In Medicinal Plants of the Asteraceae Family: Traditional Uses, Phytochemistry and Pharmacological Activities; Devkota, H.P., Aftab, T., Eds.; Springer Nature: Singapore, 2022; pp. 125–143. [Google Scholar]
- Zhan, G.; Long, M.; Shan, K.; Xie, C.; Yang, R. Antioxidant Effect of Chrysanthemum morifolium (Chuju) Extract on H2O2-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling. Antioxidants 2022, 11, 1068. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, Y.J.; Fu, X.Q.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Bai, J.X.; Wu, Y.; Wang, X.Q.; Li, A.S.; et al. Chrysoeriol Suppresses Hyperproliferation of Rheumatoid Arthritis Fibroblast-like Synoviocytes and Inhibits JAK2/STAT3 Signaling. BMC Complement. Med. Ther. 2022, 22, 73. [Google Scholar] [CrossRef]
- Yeasmin, D.; Swarna, R.J.; Nasrin, M.S.; Parvez, S.; Alam, M.F. Evaluation of Antibacterial Activity of Three Flower Colours of Chrysanthemum morifolium Ramat. Against Multi-Drug Resistant Human. Pathogenic Bacteria. Int. J. Biosci. 2016, 9, 78–87. [Google Scholar]
- Owoade, O.; Adetutu, A.; Olorunnisola, O. A Review of Chemical Constituents and Pharmacological Properties of H. sabdariffa L. Int. J. Curr. Res. Biosci. Plant Biol. 2019, 6, 42–51. [Google Scholar] [CrossRef]
- Panaitescu, M.; Lengyel, E. Monitoring the Antibacterial Activity of H. sabdariffa Extracts. Manag. Sustain. Dev. 2017, 9, 31–34. [Google Scholar] [CrossRef]
- Zhang, W.; Abdel-Rahman, F.H.; Saleh, M.A. Natural Resistance of Rose Petals to Microbial Attack. J. Environ. Sci. Health B 2011, 46, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Koes, R.; Shang, H.; Fu, Z.; Wang, L.; Dong, X.; Zhang, J.; Passeri, V.; Li, Y.; Jiang, H.; et al. Identification and Functional Analysis of Three New Anthocyanin R2R3-MYB Genes in Petunia. Plant Direct 2019, 3, e00114. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Fazal-ur-Rehman; Adeel, S.; Habib, N.; Batool, F.; Usama, M.; Iqbal, F.; Fatima, A. Extraction of Anthocyanin from Rose Petals for Coloration of Biomordanted Wool Fabric. Coatings 2023, 13, 623. [Google Scholar] [CrossRef]
- Kwon, E.-K.; Lee, D.-Y.; Lee, H.; Kim, D.-O.; Baek, N.-I.; Kim, Y.-E.; Kim, H.-Y. Flavonoids from the Buds of Rosa damascena Inhibit the Activity of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase and Angiotensin I-Converting Enzyme. J. Agric. Food Chem. 2010, 58, 882–886. [Google Scholar] [CrossRef]
- Ştegarus, D.I.; Lengyel, E.; Apostolescu, G.F.; Botoran, O.R.; Tanase, C. Phytochemical Analysis and Biological Activity of Three Stachys Species (Lamiaceae) from Romania. Plants 2021, 10, 2710. [Google Scholar] [CrossRef] [PubMed]
- Cristea, R.M.; Sava, C.; Căpătână, C.; Kanellou, A. Phytochemical Analysis and Specific Activities of Bark and Flower Extracts from Four Magnolia Plant Species. Horticulturae 2024, 10, 141. [Google Scholar] [CrossRef]
- Popescu, D.I.; Frum, A.; Dobrea, C.M.; Cristea, R.; Gligor, F.G.; Vicas, L.G.; Ionete, R.E.; Sutan, N.A.; Georgescu, C. Comparative Antioxidant and Antimicrobial Activities of Several Conifer Needles and Bark Extracts. Pharmaceutics 2024, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.; Wrolstad, R. AOAC Official Method. 2005.02: Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method. In Official Methods of Analysis of AOAC International; Horowitz, H., Ed.; AOAC: Washington, DC, USA, 2005; Volume 2. [Google Scholar]
- Ibrahim, N.; Kebede, A. In Vitro Antibacterial Activities of Methanol and Aqueous Leaf Extracts of Selected Medicinal Plants Against Human Pathogenic Bacteria. Saudi J. Biol. Sci. 2020, 27, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S.; Perju, M. Influence of Enzymatic and Ultrasonic Extraction on Phenolics Content and Antioxidant Activity of H. sabdariffa, L. Flowers. Bulg. Chem. Commun. 2020, 52, 25–29. [Google Scholar]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Granata, G.; Napoli, E. Effect of Petal Color, Water Status, and Extraction Method on Qualitative Characteristics of Rosa rugosa Liqueur. Plants 2022, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Ferreira, R.d.M.; Coelho, L.A.B.; Barreto, D.W. Production of Anthocyanin-Rich Red Rose Petal Extract by Enzymatic Maceration. Biomass 2024, 4, 429–441. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Characterization of Phenolic Compounds, Anthocyanidin, Antioxidant and Antimicrobial Activity of 25 Varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crops Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Park, C.H.; Park, S.-Y.; Lee, S.Y.; Kim, J.K.; Park, S.U. Analysis of Metabolites in White Flowers of Magnolia denudata Desr. and Violet Flowers of Hibiscus liliiflora Desr. Molecules 2018, 23, 1558. [Google Scholar] [CrossRef]
- Ogata, J.; Kanno, Y.; Itoh, Y.; Tsugawa, H.; Suzuki, M. Plant Biochemistry: Anthocyanin Biosynthesis in Roses. Nature 2005, 435, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Saati, E.A.; Wahyudi, V.A.; Dyah, A.; Andriawan, S. Anthocyanin Extract of Rosa sp. as a Natural Preservative in Euthynnus affinis. AACL Bioflux 2022, 15, 136–146. [Google Scholar]
- Griesbach, R.J.; Stehmann, J.R.; Meyer, F. Anthocyanins in the “Red” Flowers of Petunia exserta. Phytochemistry 1999, 51, 525–528. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Zhang, Q.; Yu, P.; Zhou, Y.; Jia, G. The Composition of Anthocyanins and Carotenoids Influenced the Flower Color Heredity in Asiatic Hybrid Lilies. Horticulturae 2022, 8, 1206. [Google Scholar] [CrossRef]
- Hoang, T.N.N.; Nguyen, N.P.M.; Dong, T.A.D.; Le, T.H.A. Anthocyanin Isolation from Hibiscus sabdariffa L. Flowers by Extraction, Macroporous D101 Resin Purification, and Biological Evaluation. J. Agric. Food Res. 2023, 14, 100848. [Google Scholar] [CrossRef]
- Lestyan, M.; Teusdea, A.; Gabor, G.; Muresan, M.; Bodog, F.; Vicas, S. The Total Anthocyanins Content of Hibiscus Species. Analele Univ. Din Oradea Fasc. Protecţia Mediu. 2014, 22, 75–80. [Google Scholar]
- Ademiluyi, A.O.; Oboh, G.; Agbebi, O.J.; Akinyemi, A.J. Anthocyanin-Rich Red Dye of Hibiscus sabdariffa Calyx Modulates Cisplatin-Induced Nephrotoxicity and Oxidative Stress in Rats. Int. J. Biomed. Sci. 2013, 9, 243–248. [Google Scholar]
- Amer, S.A.; Al-Khalaifah, H.S.; Gouda, A.; Osman, A.; Goda, N.I.A.; Mohammed, H.A.; Darwish, M.I.M.; Hassan, A.M.; Mohamed, S.K.A. Potential Effects of Anthocyanin-Rich Roselle (Hibiscus sabdariffa L.) Extract on the Growth, Intestinal Histomorphology, Blood Biochemical Parameters, and the Immune Status of Broiler Chickens. Antioxidants 2022, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Baréa, B.; Fernandes, A.; de Freitas, V.; Salas, E. Isolation and Characterization of Anthocyanins from Hibiscus sabdariffa Flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [CrossRef]
- Shi, S.G.; Li, S.J.; Kang, Y.X.; Liu, J.J. Molecular Characterization and Expression Analyses of an Anthocyanin Synthase Gene from Magnolia sprengeri Pamp. Appl. Biochem. Biotechnol. 2015, 175, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Vankar, P.S.; Srivastava, J. Evaluation of Anthocyanin Content in Red and Blue Flowers. Int. J. Food Eng. 2010, 6. [Google Scholar] [CrossRef]
- Magfiroh, A.; Hastuti, E.; Nurchayati, Y.; Setiari, N. Anthocyanin Content and Antioxidant Activity of Red Chrysanthemum (Chrysanthemum morifolium Ramat.) at Different Flower Ages. Borneo J. Resour. Sci. Technol. 2023, 13, 72–80. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Taghrir, H.; Boveiri Dehsheikh, A.; Zomorodian, K.; Irajie, C.; Mahmoodi Sourestani, M.; Iraji, A. Linarin, a Glycosylated Flavonoid, with Potential Therapeutic Attributes: A Comprehensive Review. Pharmaceuticals 2021, 14, 1104. [Google Scholar] [CrossRef]
- Chang, Y.; Xing, M.; Hu, X.; Feng, H.; Wang, Y.; Guo, B.; Sun, M.; Ma, L.; Fei, P. Antibacterial Activity of Chrysanthemum buds Crude Extract Against Cronobacter sakazakii and Its Application as a Natural Disinfectant. Front. Microbiol. 2021, 11, 632177. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Salcedo, M.D.R.; Gonzalez-Espindola, L.A.; Alonso-Castro, A.J.; Gonzalez-Martinez, M.D.R.; Domínguez, F.; Garcia-Carranca, A. Antimicrobial Activity and Cytotoxic Effects of Magnolia dealbata and Its Active Compounds. Nat. Prod. Commun. 2011, 6, 1121–1124. [Google Scholar]
- Hodaei, M.; Rahimmalek, M.; Arzani, A. Variation in Bioactive Compounds, Antioxidant and Antibacterial Activity of Iranian C. morifolium Cultivars and Determination of Major Polyphenolic Compounds Based on HPLC Analysis. J. Food Sci. Technol. 2021, 58, 1538–1548. [Google Scholar] [CrossRef] [PubMed]

| Sample | Sample 1 | Sample 2 | Sample 3 | Average | |
|---|---|---|---|---|---|
| M. liliiflora | Dry Extract (mg) | 54.13 | 61.27 | 49.87 | 55.09 |
| Extract Concentration (mg/mL) | 5.41 | 6.12 | 4.98 | 5.50 | |
| C. morifolium | Dry Extract (mg) | 37.99 | 43.23 | 40.14 | 40.45 |
| Extract Concentration (mg/mL) | 3.79 | 4.32 | 4.01 | 4.04 | |
| H. sabdariffa | Dry Extract (mg) | 44.16 | 45.57 | 45.81 | 45.18 |
| Extract Concentration (mg/mL) | 4.41 | 4.55 | 4.58 | 4.51 | |
| P. grandiflora | Dry Extract (mg) | 26.29 | 32.22 | 29.11 | 29.20 |
| Extract Concentration (mg/mL) | 2.62 | 3.22 | 2.91 | 2.92 | |
| R. hybrida L. | Dry Extract (mg) | 68.25 | 70.19 | 74.85 | 71.09 |
| Extract Concentration (mg/mL) | 6.82 | 7.01 | 7.48 | 7.10 | |
| TPC mg GAE/g d.w. | Total Anthocyanins mg Cy-3-Glu/g d.w. | DPPH IC50 mg/mL | ABTS μmol TE/g d.w. | FRAP μmol TE/g d.w. | |
|---|---|---|---|---|---|
| M. liliiflora | 94.34 ± 0.21 | 18.24 ± 0.11 | 23.11 ± 0.17 | 42.19 ± 0.11 | 45.71 ± 0.22 |
| C. morifolium | 75.17 ± 0.16 | 16.29 ± 0.11 | 20.12 ± 0.11 | 38.11 ± 0.12 | 41.23 ± 0.11 |
| H. sabdariffa | 77.44 ± 0.16 | 16.98 ± 0.13 | 22.05 ± 0.12 | 40.19 ± 0.19 | 39.32 ± 0.17 |
| P. grandiflora | 41.61 ± 0.11 | 14.99 ± 0.13 | 15.68 ± 0.10 | 21.17 ± 0.12 | 22.18 ± 0.15 |
| R. hybrida L. | 107.49 ± 0.19 | 21.66 ± 0.18 | 29.35 ± 0.17 | 49.89 ± 0.24 | 48.68 ± 0.23 |
| Ascorbic acid | - | 21.33 ± 1.13 mmol TE/g d.w. | 22.27 ± 1.21 mmol TE/g d.w. |
| Compound | M. liliiflora | C. morifolium | H. sabdariffa | P. grandiflora | R. hybrida L. |
|---|---|---|---|---|---|
| Benzoic Acid | 10,873.22 ± 25.04 | 11,209.12 ± 55.19 | 12,009.37 ± 50.57 | 10,568.93 ± 43.22 | 13,421.74 ± 56.26 |
| Caffeic Acid | 21.13 ± 1.31 | 87.26 ± 2.05 | 234.59 ± 15.27 | 99.98 ± 10.13 | 207.43 ± 11.54 |
| Chlorogenic Acid | 5683.35 ± 26.01 | 3794.48 ± 29.67 | 5544.34 ± 27.55 | 2272.32 ± 11.18 | 3896.51 ± 12.01 |
| Ellagic Acid | nd | 20.22 ± 1.21 | 234.77 ± 15.71 | nd | 54.45 ± 1.11 |
| Ferulic Acid | 1065.77 ± 19.58 | 643.88 ± 19.22 | 983.26 ± 17.04 | 197.48 ± 5.21 | 1012.66 ± 18.92 |
| Gallic Acid | 298.29 ± 8.81 | 562.55 ± 15.51 | 884.44 ± 17.11 | 109.99 ± 5.33 | 709.99 ± 16.13 |
| m-Coumaric Acid | 5.66 ± 0.59 | 29.09 ± 2.72 | 31.31 ± 2.12 | nd | 12.12 ± 0.94 |
| p-Coumaric Acid | 562.55 ± 10.16 | 389.27 ± 11.23 | 788.33 ± 11.63 | 656.72 ± 15.01 | 1022.95 ± 18.55 |
| p-Hydroxybenzoic Acid | nd | 41.13 ± 1.54 | nd | 18.96 ± 0.59 | 66.18 ± 1.01 |
| Rosmarinic Acid | nd | 2.33 ± 0.30 | 109.99 ± 3.34 | nd | 99.88 ± 3.45 |
| Salicylic Acid | nd | 1.22 ± 0.03 | 19.72 ± 0.59 | nd | 27.91 ± 1.17 |
| Sinapic Acid | nd | nd | 0.27 ± 0.00 | nd | nd |
| Syringic Acid | 893.27 ± 15.16 | 562.29 ± 14.14 | 1001.10 ± 17.34 | 873.88 ± 15.11 | 1231.55 ± 17.22 |
| Trans-Cinnamic Acid | 0.26 ± 0.00 | nd | 45.62 ± 0.01 | 12.97 ± 0.01 | 34.52 ± 0.01 |
| Vanillic Acid | 129.92 ± 2.52 | 197.88 ± 3.25 | 243.45 ± 5.95 | 308.75 ± 5.55 | 527.87 ± 6.23 |
| Rutin | 3456.32 ± 19.15 | 2996.48 ± 22.07 | 1999.57 ± 17.31 | 2341.73 ± 19.44 | 1912.31 ± 15.16 |
| Quercetin | 892.88 ± 5.01 | 784.59 ± 6.28 | 1001.72 ± 6.26 | 777.15 ± 5.71 | 999.15 ± 2.37 |
| (+)-Catechin | 108.95 ± 4.54 | 77.94 ± 2.23 | 93.26 ± 4.58 | 102.21 ± 5.18 | 128.98 ± 5.33 |
| Luteolin | 67.97 ± 2.31 | 112.07 ± 7.61 | 65.46 ± 2.19 | 99.17 ± 3.27 | 134.83 ± 5.22 |
| Apigenin | 105.03 ± 4.35 | 198.45 ± 9.11 | 19.19 ± 0.06 | 97.79 ± 4.22 | 77.03 ± 3.19 |
| Total | 23,291.35 | 21,710.25 | 25,309.49 | 18,435.82 | 25,578.06 |
| Bacterial Strains | Activity | M. liliiflora | C. morifolium | H. sabdariffa | P. grandiflora | Rosa hybrida L. | Ampicillin |
|---|---|---|---|---|---|---|---|
| S. aureus ATCC 33862 | AA | ++++ | +++ | ++++ | ++ | +++ | +++++ |
| MIC | 5 | 10 | 5 | 20 | 10 | <1.5 | |
| MBC | 10 | 20 | 10 | 40 | 20 | 1.5 | |
| E. coli ATCC 25922 | AA | +++ | ++ | +++ | ++ | +++ | +++++ |
| MIC | 10 | 20 | 10 | 20 | 10 | <1.5 | |
| MBC | 20 | 40 | 20 | 40 | 20 | 1.5 | |
| P. fluorescens ATCC 13525 | AA | + | + | + | − | − | ++++ |
| MIC | 40 | 40 | 40 | − | − | <1.5 | |
| MBC | >40 | >40 | >40 | − | − | 1.5 | |
| P. mirabilis ATCC 12453 | AA | ++ | + | ++ | + | + | ++++ |
| MIC | 20 | 40 | 20 | 40 | 40 | <1.5 | |
| MBC | 40 | >40 | 40 | >40 | >40 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.I.; Botoran, O.R.; Cristea, R.M. Investigation of Phytochemical Composition, Antioxidant and Antibacterial Activity of Five Red Flower Extracts. Antioxidants 2025, 14, 151. https://doi.org/10.3390/antiox14020151
Popescu DI, Botoran OR, Cristea RM. Investigation of Phytochemical Composition, Antioxidant and Antibacterial Activity of Five Red Flower Extracts. Antioxidants. 2025; 14(2):151. https://doi.org/10.3390/antiox14020151
Chicago/Turabian StylePopescu (Stegarus), Diana Ionela, Oana Romina Botoran, and Ramona Maria (Iancu) Cristea. 2025. "Investigation of Phytochemical Composition, Antioxidant and Antibacterial Activity of Five Red Flower Extracts" Antioxidants 14, no. 2: 151. https://doi.org/10.3390/antiox14020151
APA StylePopescu, D. I., Botoran, O. R., & Cristea, R. M. (2025). Investigation of Phytochemical Composition, Antioxidant and Antibacterial Activity of Five Red Flower Extracts. Antioxidants, 14(2), 151. https://doi.org/10.3390/antiox14020151








