Naringin vs. Citrus x paradisi L. Peel Extract: An In Vivo Journey into Oxidative Stress Modulation
Abstract
:1. Introduction
1.1. Naringin and Its Pharmacological Effects
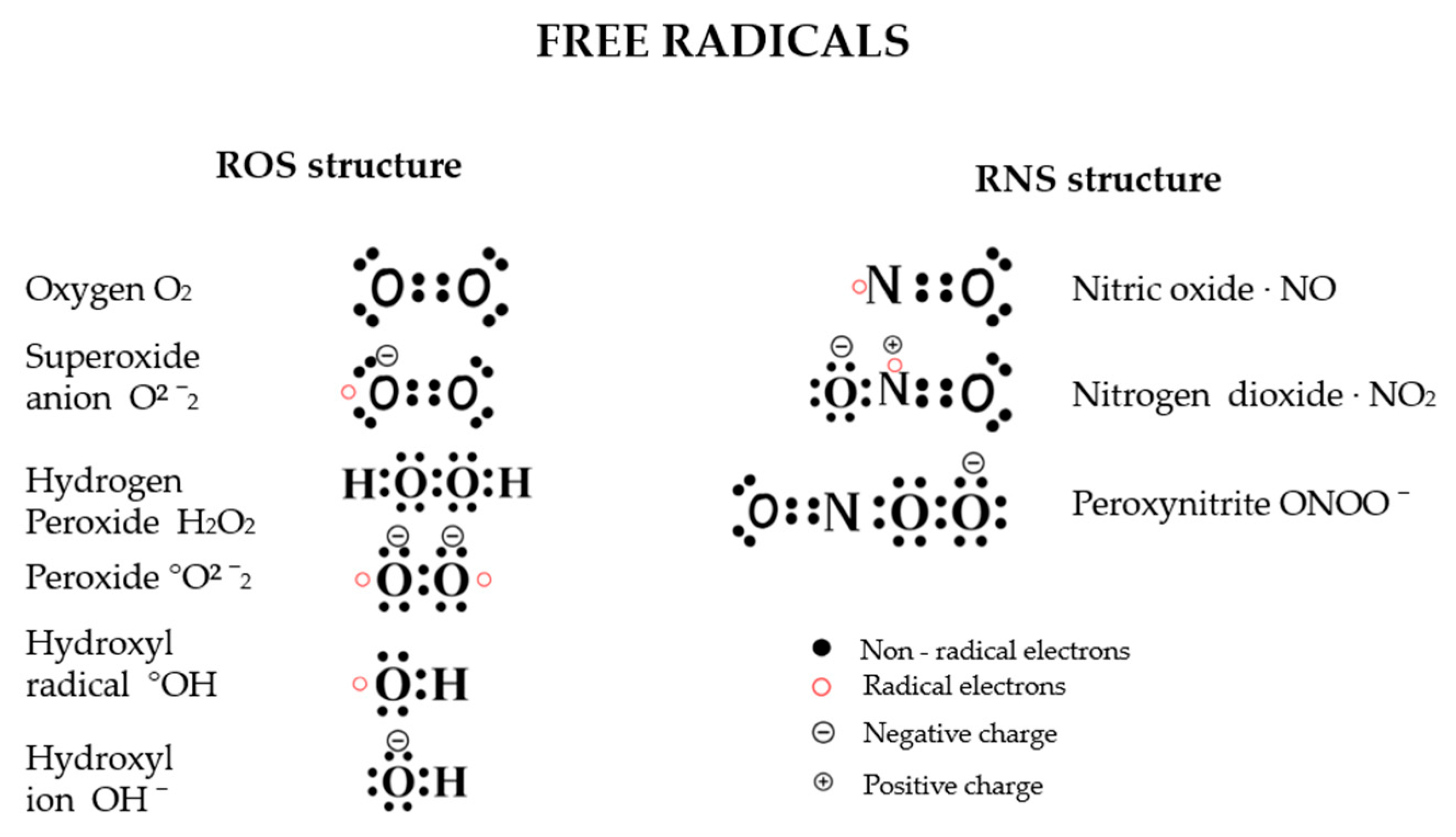
1.2. Free Radicals
1.3. Oxidative Stress
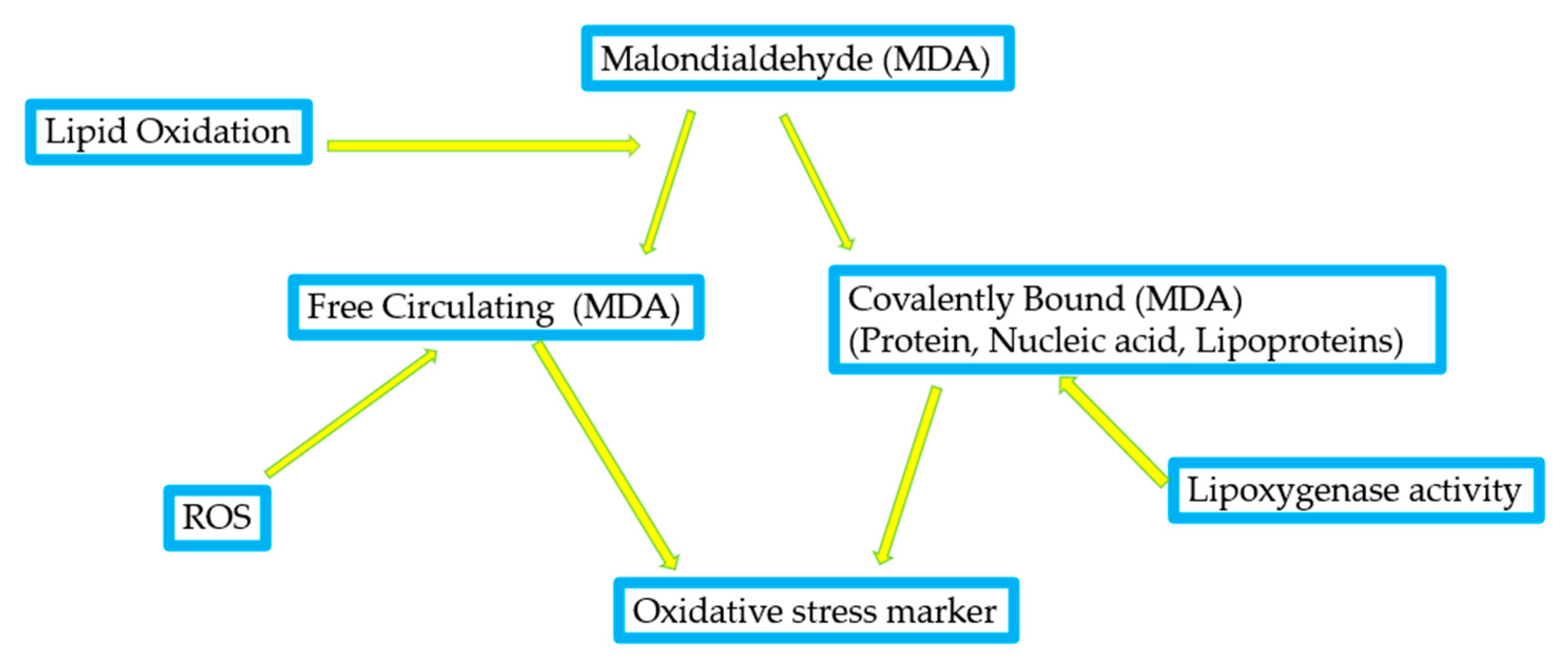
1.4. Oxidative Stress Markers
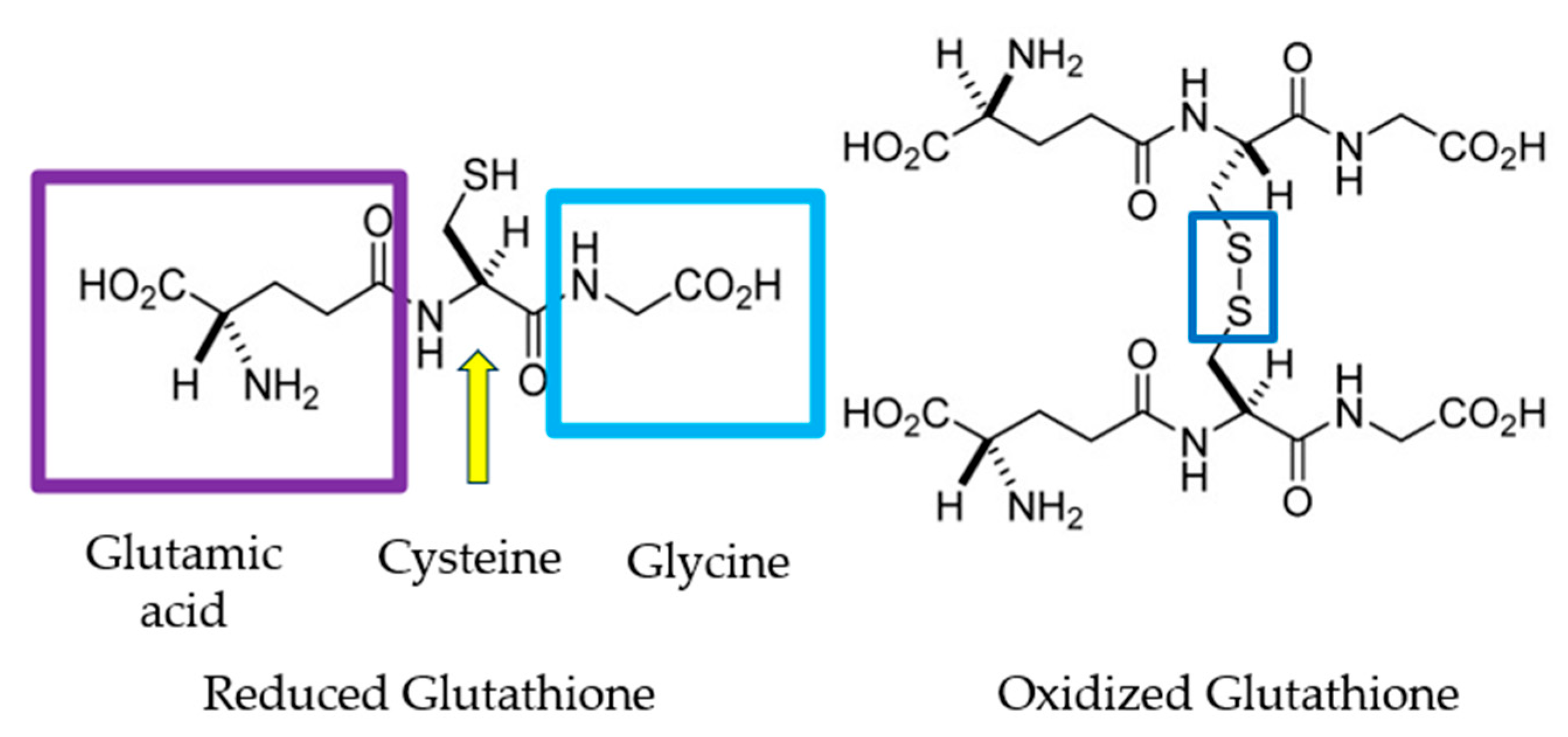
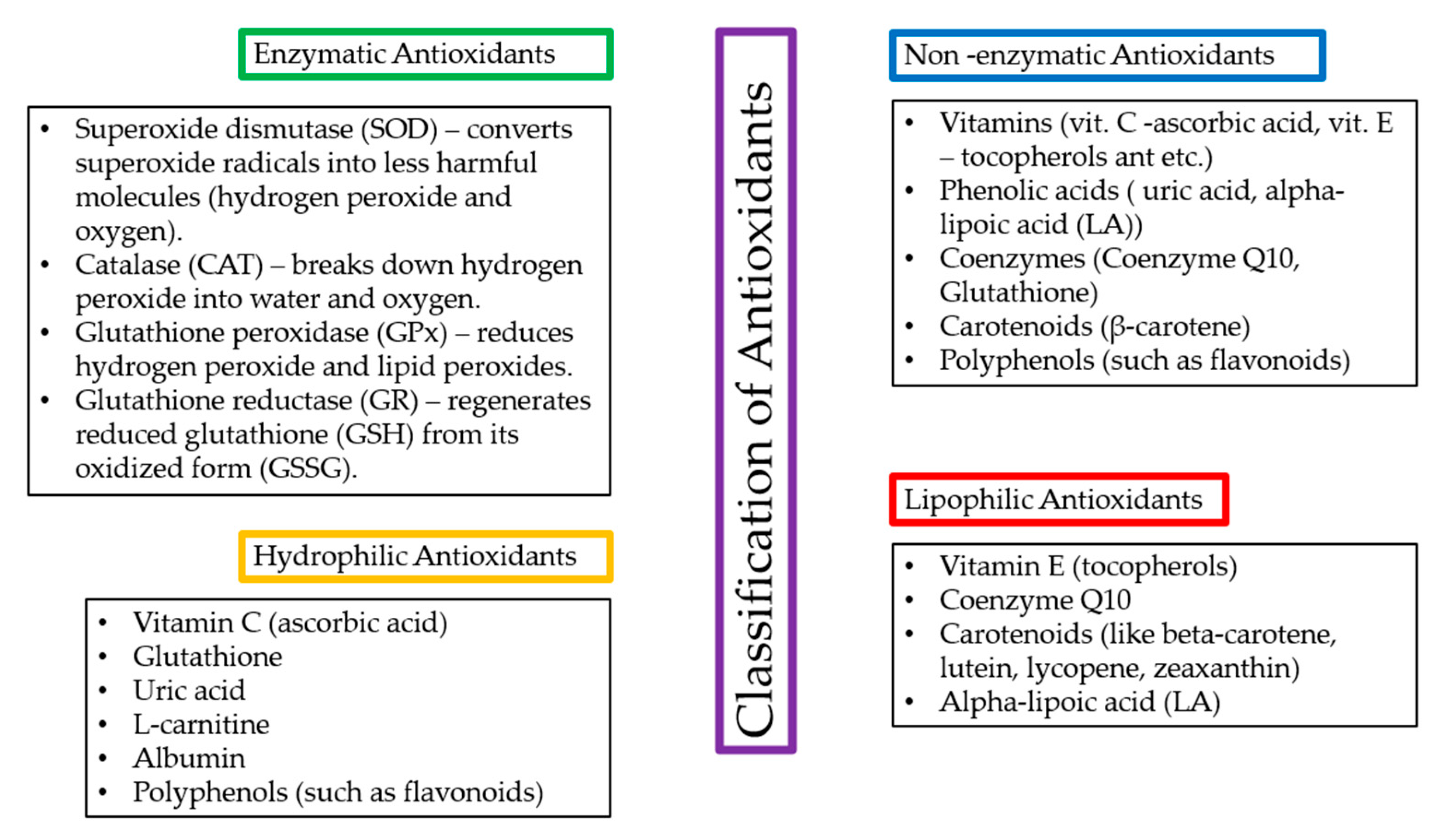
1.5. Antioxidants and Their Effects
2. Results and Discussion
2.1. Quantitative Analysis of Phenolic Compounds and Selection of Extraction Sample
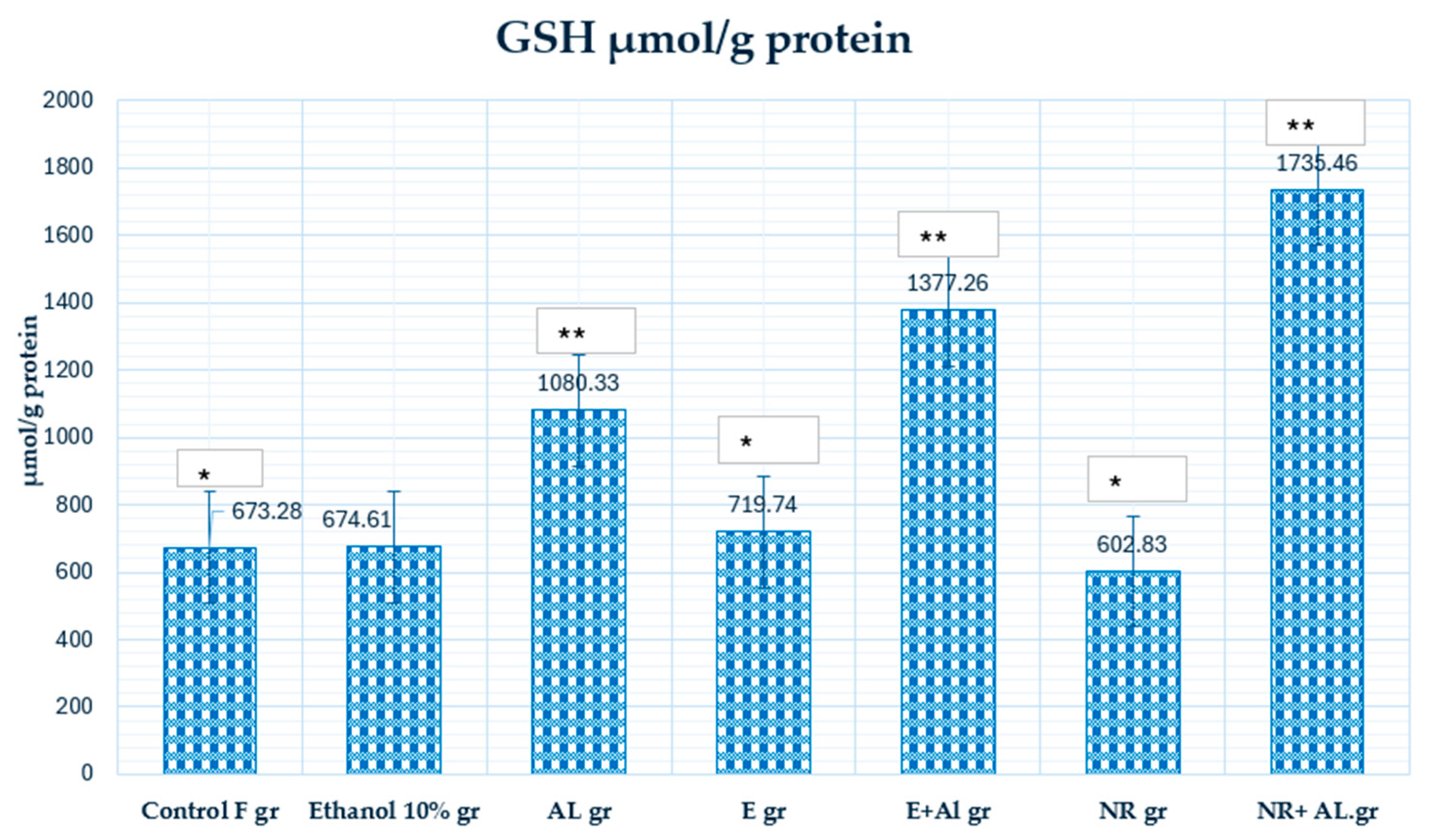
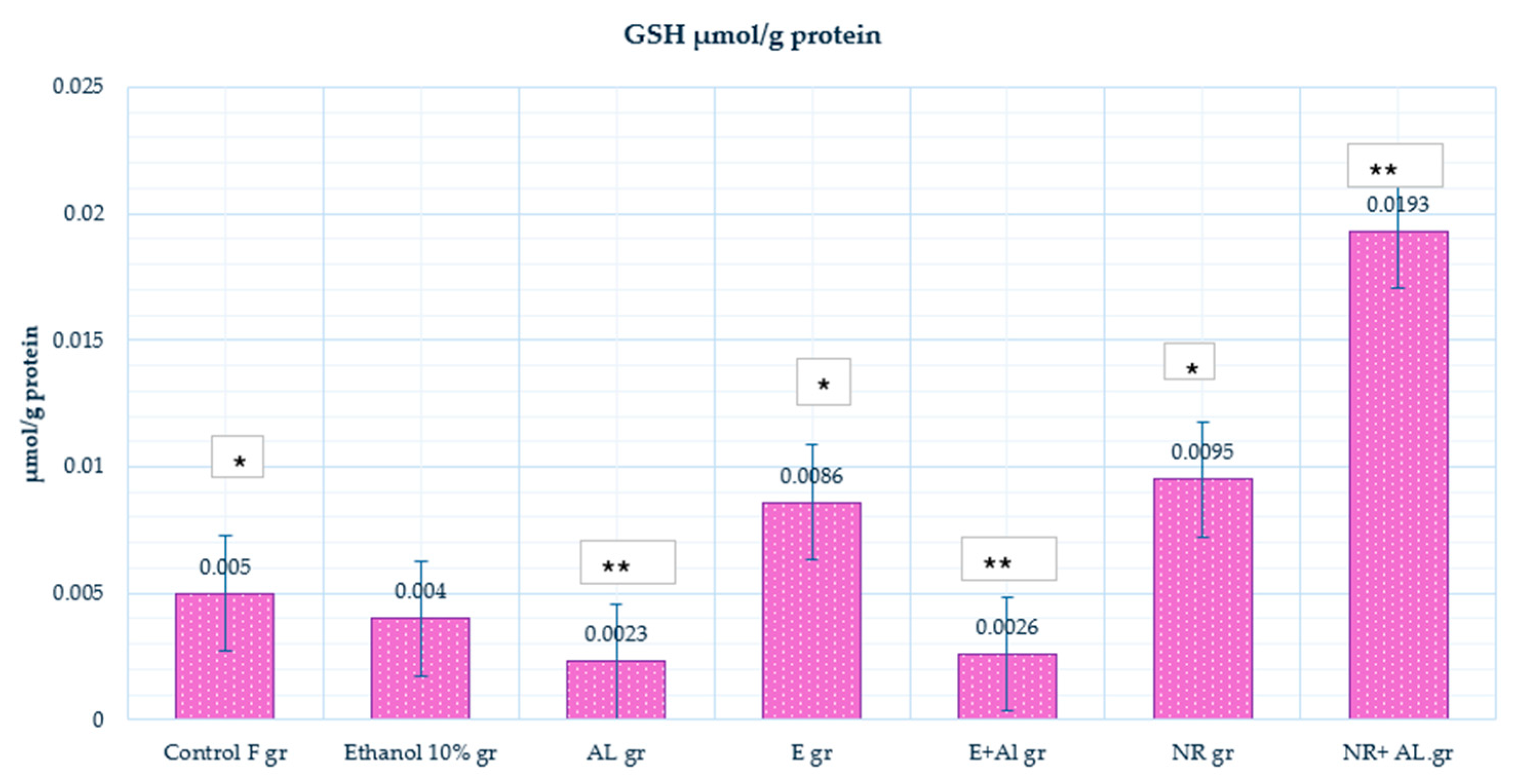
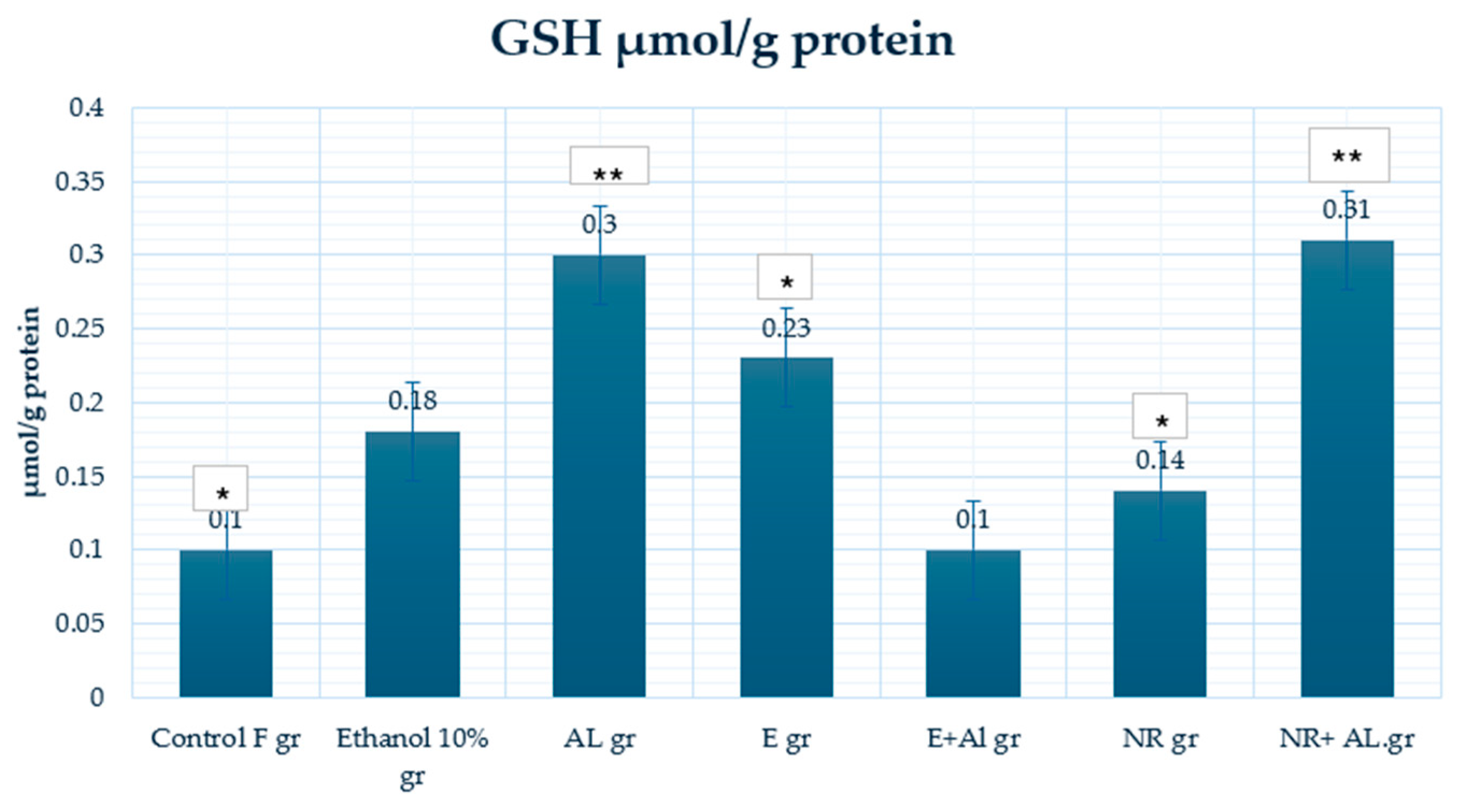
2.2. Result of GSH and MDA in Mouse Blood
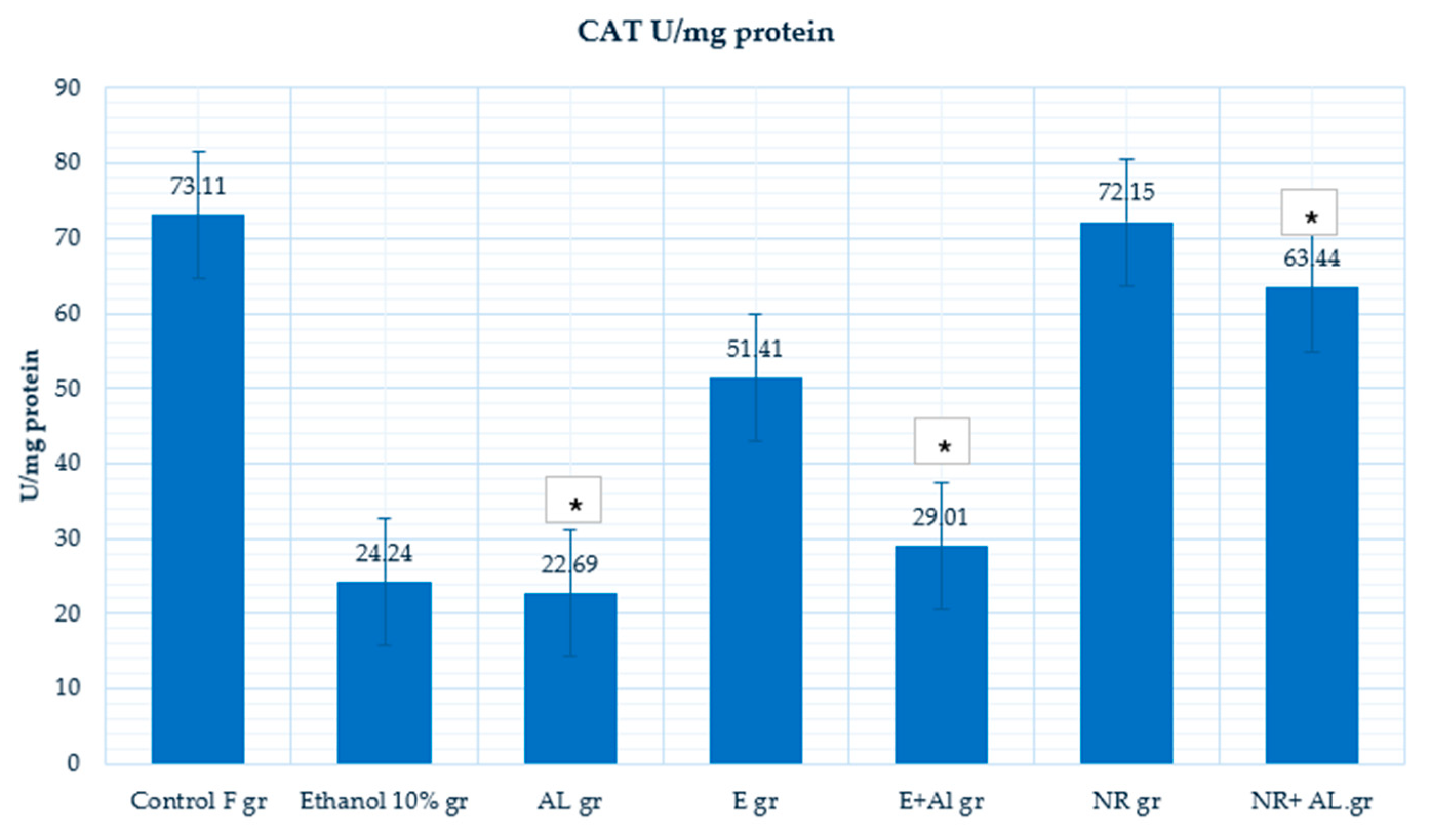
2.3. Result of GSH, MDA and CAT in Mouse Brain
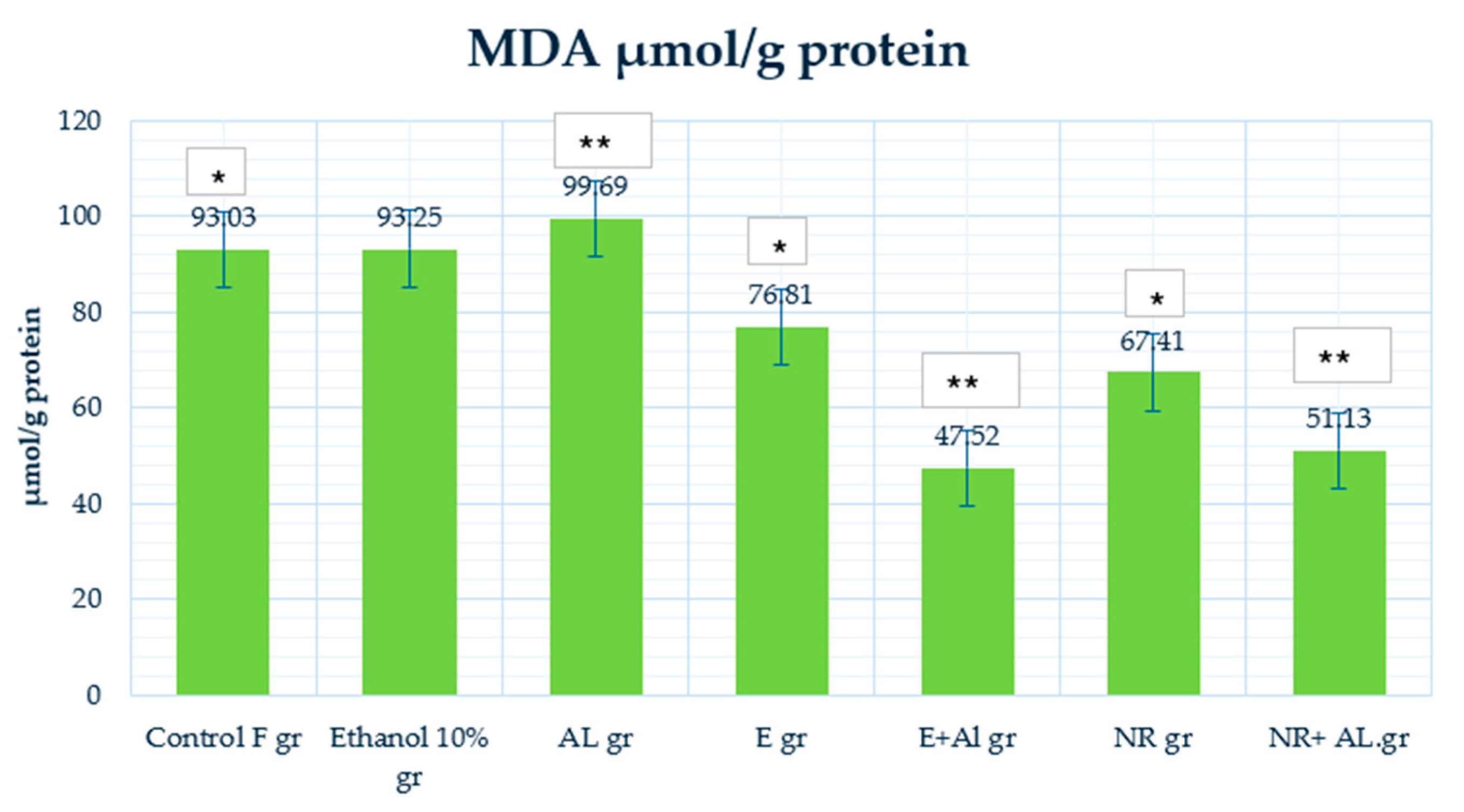
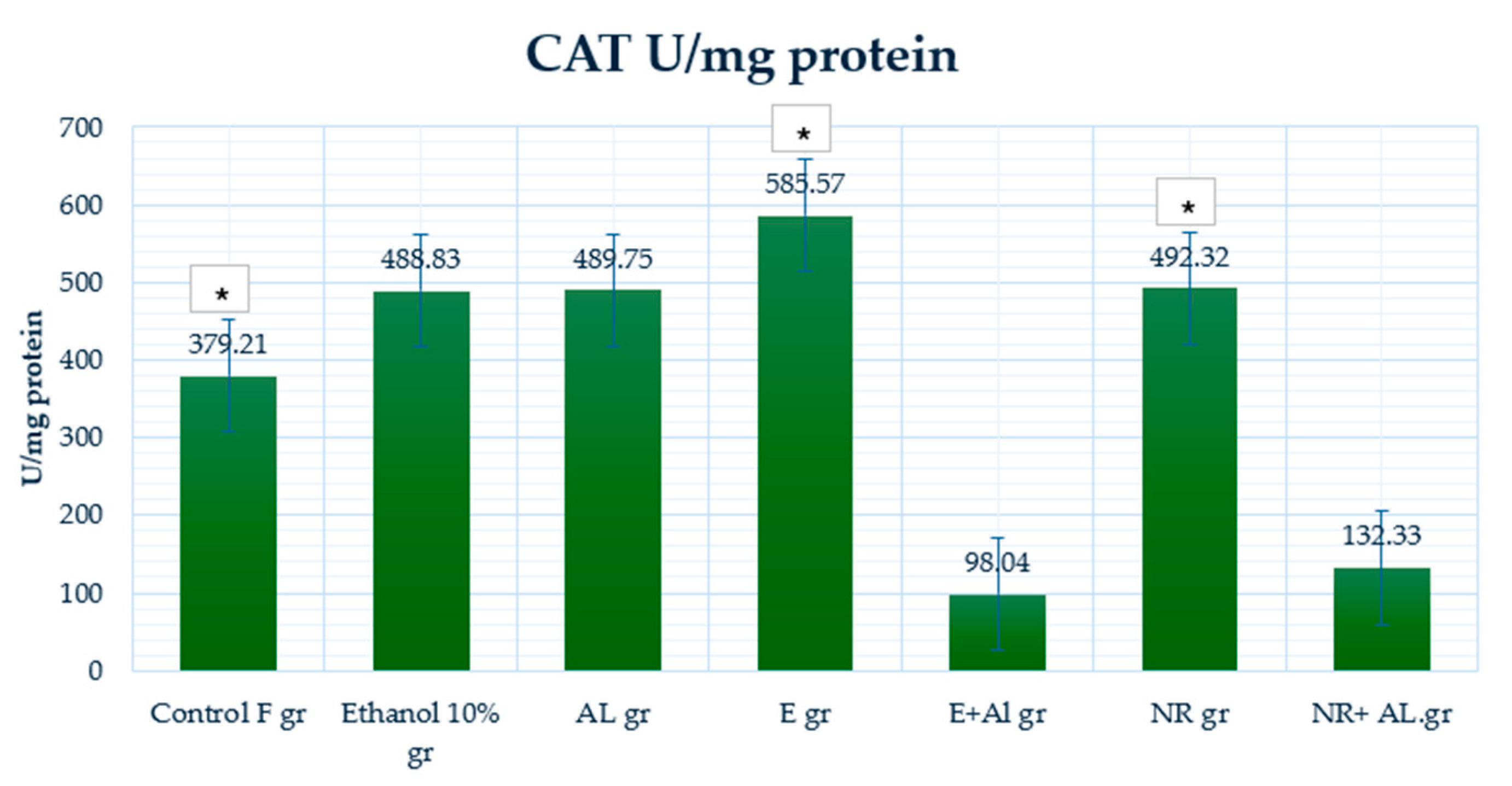
2.4. Result of GSH, MDA and CAT in Mouse Liver
3. Materials and Methods
3.1. Material
3.2. Preparation of Naringin Solution for In Vivo Studies
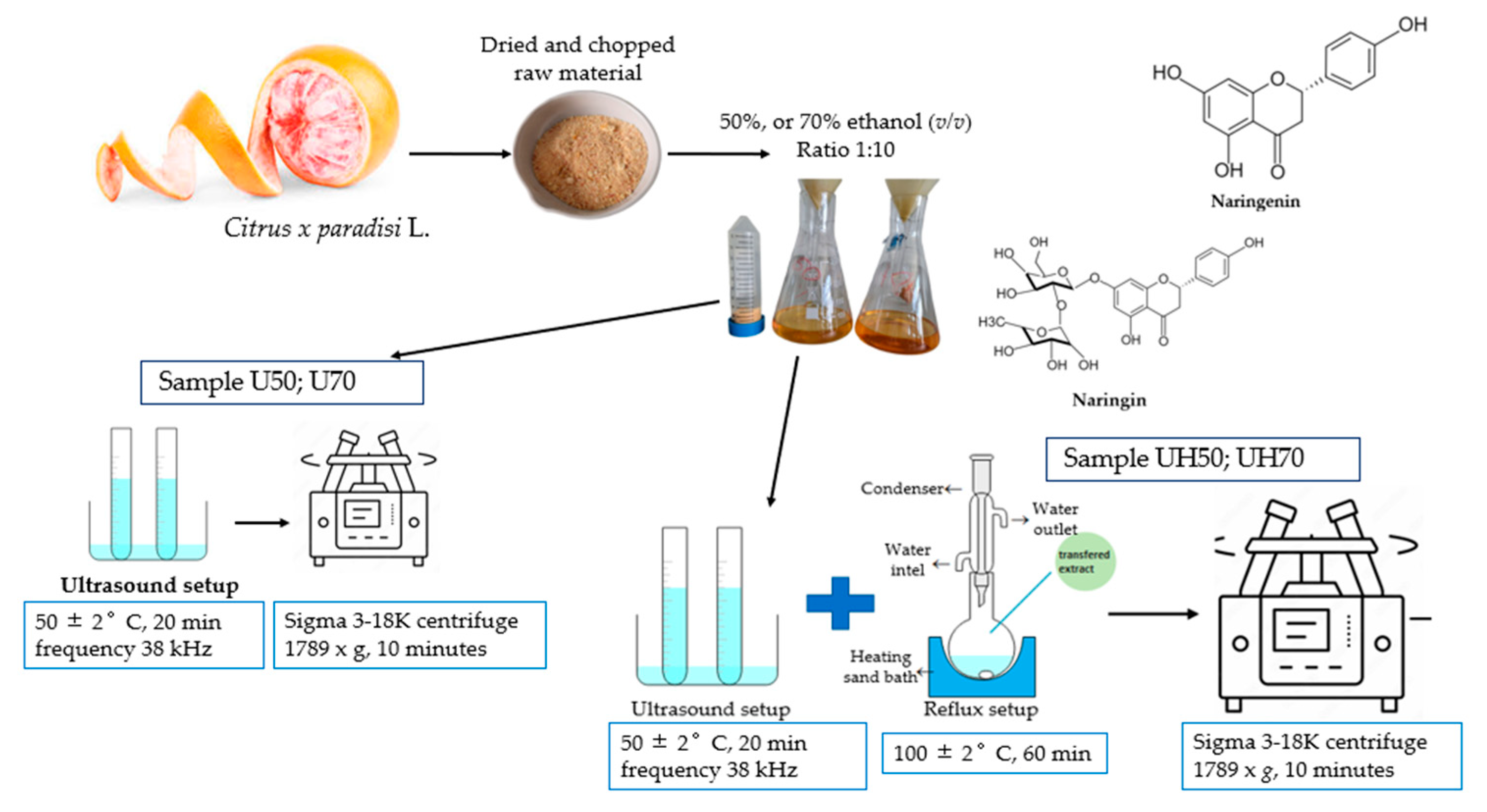
3.3. Preparation and Extraction of Flavonoids from Citrus x paradisi L. Peels
3.4. Chromatographic Analysis of Citrus x paradisi L. Peels Ethanolic Extract: HLPC Analysis for Phenolic Compounds
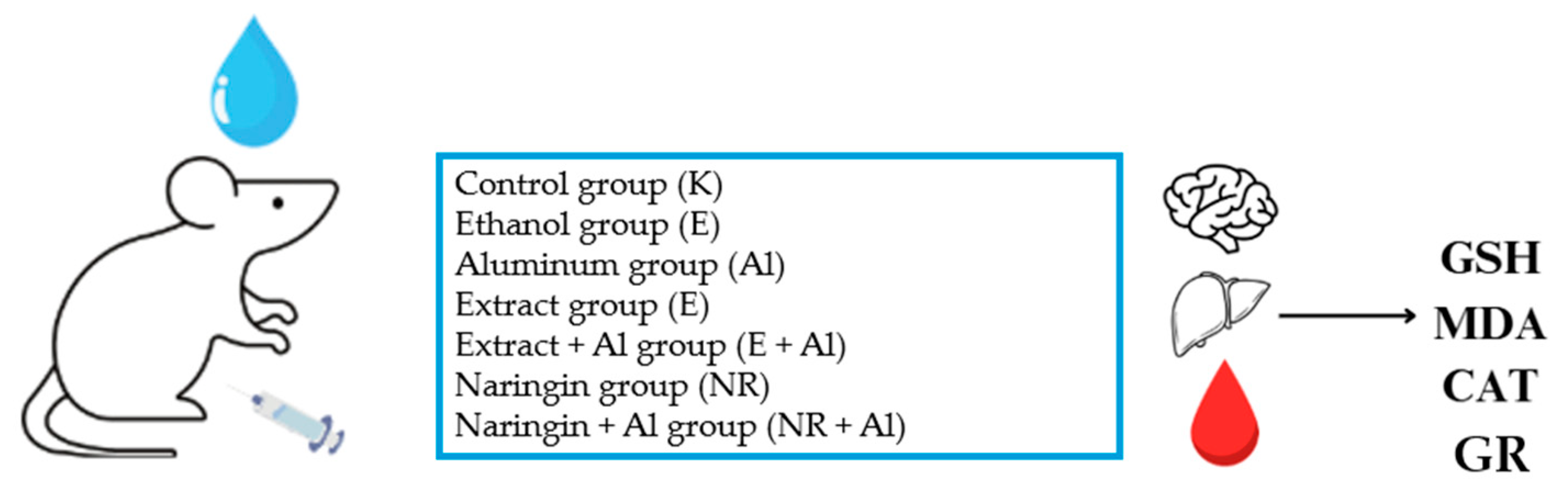
3.5. Animal Model
3.6. Determination of GSH Concentration in Laboratory Mice Blood
3.7. Determination of MDA Concentration in Laboratory Mice Blood
3.8. Determination of GSH Concentration in Laboratory Mouse Organs
3.9. GR Activity Assay
3.10. Determination of MDA Concentration in Laboratory Mouse Organs
3.11. (CAT) Activity Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Iova, G.M.; Calniceanu, H.; Popa, A.; Szuhanek, C.A.; Marcu, O.; Ciavoi, G.; Scrobota, I. The Antioxidant Effect of Curcumin and Rutin on Oxidative Stress Biomarkers in Experimentally Induced Periodontitis in Hyperglycemic Wistar Rats. Molecules 2021, 26, 1332. [Google Scholar] [CrossRef]
- Papaefthimiou, M.; Kontou, P.I.; Bagos, P.G.; Braliou, G.G. Antioxidant Activity of Leaf Extracts from Stevia rebaudiana Bertoni Exerts Attenuating Effect on Diseased Experimental Rats: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3325. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front. Pharmacol. 2020, 11, 364. [Google Scholar] [CrossRef]
- Eom, S.; Lee, B.-B.; Lee, S.; Park, Y.; Yeom, H.D.; Kim, T.-H.; Nam, S.-H.; Lee, J.H. Antioxidative and Analgesic Effects of Naringin Through Selective Inhibition of Transient Receptor Potential Vanilloid Member 1. Antioxidants 2021, 11, 64. [Google Scholar] [CrossRef]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Turkekul, K. Naringin sensitizes human prostate cancer cells to paclitaxel therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-K.; Liu, X.-D.; Shen, L.; Zeng, W.-M.; Qiu, G.-Z. Natural plant polyphenols for alleviating oxidative damage in man: Current status and future perspectives. Trop. J. Pharm. Res. 2016, 15, 1089. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Abdelazim, A.M.; Abomughaid, M.M. Oxidative stress: An overview of past research and future insights. Life 2024, 17, 2316092. [Google Scholar] [CrossRef]
- Rogers, L.K.; Cismowski, M.J. Oxidative stress in the lung—The essential paradox. Curr. Opin. Toxicol. 2018, 7, 37–43. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Braga-Neto, M.B.; Costa, D.V.S.; Queiroz, D.M.M.; Maciel, F.S.; De Oliveira, M.S.; Viana-Junior, A.B.; Santos, F.A.; Leitao, R.F.C.; Brito, G.A.C.; Vasconcelos, P.R.L.; et al. Increased Oxidative Stress in Gastric Cancer Patients and Their First-Degree Relatives: A Prospective Study from Northeastern Brazil. Oxidative Med. Cell. Longev. 2021, 2021, 6657434. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Afshari-Kaveh, M.; Abbasalipourkabir, R.; Nourian, A.; Ziamajidi, N. The Protective Effects of Vitamins A and E on Titanium Dioxide Nanoparticles (nTiO2)-Induced Oxidative Stress in the Spleen Tissues of Male Wistar Rats. Biol. Trace Elem. Res. 2021, 199, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, E.; Rajasekaran, D.; Sivalingam, M.; Farooq, M.; Abdul, E.; Dhanapal, S. Naringenin Inhibits Oxidative Stress Induced Macromolecular Damage in N-methyl N-nitro N-nitrosoguanidine Induced Gastric Carcinogenesis in Wistar Rats. In Gastric Carcinoma—New Insights into Current Management; Lazar, D., Ed.; InTech: Houston, TX, USA, 2013; ISBN 978-953-51-0914-3. [Google Scholar]
- Ghezzi, P. Environmental risk factors and their footprints in vivo—A proposal for the classification of oxidative stress biomarkers. Redox Biol. 2020, 34, 101442. [Google Scholar] [CrossRef]
- Cammisotto, V.; Nocella, C.; Bartimoccia, S.; Sanguigni, V.; Francomano, D.; Sciarretta, S.; Pastori, D.; Peruzzi, M.; Cavarretta, E.; D’Amico, A.; et al. The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors. Antioxidants 2021, 10, 146. [Google Scholar] [CrossRef]
- Haro Girón, S.; Monserrat Sanz, J.; Ortega, M.A.; Garcia-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Boaru, D.L.; De Leon-Oliva, D.; Guijarro, L.G.; Atienza-Perez, M.; et al. Prognostic Value of Malondialdehyde (MDA) in the Temporal Progression of Chronic Spinal Cord Injury. J. Pers. Med. 2023, 13, 626. [Google Scholar] [CrossRef]
- Bergin, P.; Leggett, A.; Cardwell, C.R.; Woodside, J.V.; Thakkinstian, A.; Maxwell, A.P.; McKay, G.J. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in haemodialysis patients: A systematic review and meta-analysis. BMC Nephrol. 2021, 22, 126. [Google Scholar] [CrossRef]
- Kong, A.S.-Y.; Lai, K.S.; Hee, C.-W.; Loh, J.Y.; Lim, S.H.E.; Sathiya, M. Oxidative Stress Parameters as Biomarkers of Cardiovascular Disease Towards the Development and Progression. Antioxidants 2022, 11, 1175. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Noori, S.; Rezaei Tavirani, M.; Deravi, N.; Mahboobi Rabbani, M.I.; Zarghi, A. Naringenin Enhances the Anti-Cancer Effect of Cyclophosphamide Against MDA-MB-231 Breast Cancer Cells via Targeting the STAT3 Signaling Pathway. Iran. J. Pharm. Res. 2020, 19, 122–133. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Baena, L.; Rios, L.A.; Calderón, J.A. The oxidative stability of biodiesel and its impact on the deterioration of metallic and polymeric materials: A review. J. Braz. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Takai, T.; Hisatomi, H. Cell Death via Lipid Peroxidation and Protein Aggregation Diseases. Biology 2021, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Lee, C.-W.; Huang, C.C.-Y.; Chi, M.-C.; Lee, K.-H.; Peng, K.-T.; Fang, M.-L.; Chiang, Y.-C.; Liu, J.-F. Naringenin Induces ROS-Mediated ER Stress, Autophagy, and Apoptosis in Human Osteosarcoma Cell Lines. Molecules 2022, 27, 373. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Ganjayi, M.S.; Meriga, B. Glutathione S-transferase is a good biomarker in acrylamide induced neurotoxicity and genotoxicity. Interdiscip. Toxicol. 2018, 11, 115–121. [Google Scholar] [CrossRef]
- Carmo De Carvalho E Martins, M.D.; Martins; Da Silva Santos Oliveira, A.S.; Da Silva, L.A.A.; Primo, M.G.S.; De Carvalho Lira, V.B. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. In Biomarkers in Nutrition; Patel, V.B., Preedy, V.R., Eds.; Biomarkers in Disease: Methods, Discoveries and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–25. ISBN 978-3-030-81304-8. [Google Scholar]
- Da Silva, J.K.; Cazarin, C.B.B.; Colomeu, T.C.; Batista, Â.G.; Meletti, L.M.M.; Paschoal, J.A.R.; Bogusz Júnior, S.; Furlan, M.F.; Reyes, F.G.R.; Augusto, F.; et al. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: In vitro and in vivo study. Food Res. Int. 2013, 53, 882–890. [Google Scholar] [CrossRef]
- Gajewska, J.; Chełchowska, M.; Rychłowska-Pruszyńska, M.; Klepacka, T.; Ambroszkiewicz, J. Oxidative and Antioxidative Status Expressed as OSI Index and GSH/GSSG Ratio in Children with Bone Tumors After Anticancer Therapy Completion. J. Clin. Med. 2022, 11, 1663. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Pardo-Botello, R.; Chamizo-Calero, F.; Monago-Maraña, O.; Rodríguez-Corchado, R.; De La Torre-Carreras, R.; Galeano-Díaz, T. Evaluation of Hydrophilic and Lipophilic Antioxidant Capacity in Spanish Tomato Paste: Usefulness of Front-Face Total Fluorescence Signal Combined with Parafac. Food Anal. Methods 2022, 15, 981–992. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Viskelis, P.; Viskelis, J.; Bernatoniene, J. Citrus × paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential. Nutrients 2023, 15, 1276. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Pisati, M.; Cestone, E.; Insolia, V.; Zaccaria, V.; Malfa, G.A. Antioxidant Efficacy of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Elderly Subjects: A Randomized, Double Blind, Controlled Study. Nutrients 2022, 14, 4235. [Google Scholar] [CrossRef]
- Kubiliene, A.; Munius, E.; Songailaite, G.; Kokyte, I.; Baranauskaite, J.; Liekis, A.; Sadauskiene, I. A Comparative Evaluation of Antioxidant Activity of Extract and Essential Oil of Origanum onites L. In Vivo. Molecules 2023, 28, 5302. [Google Scholar] [CrossRef]
- Sadauskiene, I.; Liekis, A.; Bernotiene, R.; Sulinskiene, J.; Kasauskas, A.; Zekonis, G. The Effects of Buckwheat Leaf and Flower Extracts on Antioxidant Status in Mouse Organs. Oxidative Med. Cell. Longev. 2018, 2018, 6712407. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin Against Human Malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Li, N.; Zhang, J.; Wooley, P.; Xu, Z. Therapeutic potentials of naringin on polymethylmethacrylate induced osteoclastogenesis and osteolysis, in vitro and in vivo assessments. Drug Des. Devel. Ther. 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D. Preclinical Evidence for the Pharmacological Actions of Naringin: A Review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.-L. HPLC in Natural Product Analysis: The Detection Issue. Planta Medica 2009, 75, 719–734. [Google Scholar] [CrossRef]
- Bakare, O.; Adedugbe, O.; Owoloye, A. Aluminum Chloride-Induced Oxidative Damage to Serum and Combined Intervention of Ascorbic Acids and Massularia Acuminata On Selected Markers Of In Vivo Antioxidant Enzymes in Wistar Rats. J. Chem. Nutr. Biochem. 2021, 2, 13–28. [Google Scholar] [CrossRef]
- Staneviciene, I.; Ivanov, L.; Kursvietiene, L.; Viezeliene, D. Short-term effects of aluminum and selenium on redox status in mice brain and blood. Trace Elem. Electrolytes 2017, 34, 74–80. [Google Scholar] [CrossRef]
- Official Journal of the European Union Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 23 January 2025).
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Non Protein Sulfhydryl Groups in Tissue by Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–208. [Google Scholar] [CrossRef]
- Rajurkar, N.; Hande, S. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian. J. Pharm. Sci. 2011, 73, 146. [Google Scholar] [CrossRef]
- Seliutina, C.N.; AIu, S.; Pal, A.I. Modifikatsiia opredeleniia kontsentratsii TBK-aktivnykh produktov syvorotki krovi [Modification of estimation the concentrations of serum TBA-active product]. Klin. Lab. Diagn. 2000, 2, 8–10. [Google Scholar]
- Bernotiene, R.; Ivanoviene, L.; Sadauskiene, I.; Liekis, A.; Ivanov, L. Effects of cadmium and zinc ions on mice brain lipid peroxidation and amounts of thiol-rich compounds. Trace Elem. Electrolytes 2016, 33, 83–88. [Google Scholar] [CrossRef]
- Sadauskiene, I.; Liekis, A.; Staneviciene, I.; Naginiene, R.; Ivanov, L. Effects of Long-Term Supplementation with Aluminum or Selenium on the Activities of Antioxidant Enzymes in Mouse Brain and Liver. Catalysts 2020, 10, 585. [Google Scholar] [CrossRef]





| Sample ID | Naringin (mg/g) | Naringenin (µg/g) |
|---|---|---|
| U50 | 42.04 ± 2.1 | 56.81 ± 2.84 |
| U70 | 40.36 ± 2.02 | 49.76 ± 2.49 |
| UH50 | 49.13 ± 2.46 | 63.99 ± 3.17 |
| UH70 | 51.94 ± 2.6 | 64.22 ± 3.21 |
| Component | Calibration Equation | Coefficient of Determination ‘R2’ | Coefficient of Correlation R | LOD * µg/mL | LOQ ** µg/mL |
|---|---|---|---|---|---|
| Naringin | Y = 25.500x + 6720 | 0.999923 | 0.99996 | 0.146 | 0.583 |
| Naringenin | ±Y = 33.300x + 3570 | 0.999924 | 0.99996 | 0.118 | 0.430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabrauskiene, J.; Sadauskiene, I.; Liekis, A.; Mikniene, Z.; Bernatoniene, J. Naringin vs. Citrus x paradisi L. Peel Extract: An In Vivo Journey into Oxidative Stress Modulation. Antioxidants 2025, 14, 157. https://doi.org/10.3390/antiox14020157
Stabrauskiene J, Sadauskiene I, Liekis A, Mikniene Z, Bernatoniene J. Naringin vs. Citrus x paradisi L. Peel Extract: An In Vivo Journey into Oxidative Stress Modulation. Antioxidants. 2025; 14(2):157. https://doi.org/10.3390/antiox14020157
Chicago/Turabian StyleStabrauskiene, Jolita, Ilona Sadauskiene, Arunas Liekis, Zoja Mikniene, and Jurga Bernatoniene. 2025. "Naringin vs. Citrus x paradisi L. Peel Extract: An In Vivo Journey into Oxidative Stress Modulation" Antioxidants 14, no. 2: 157. https://doi.org/10.3390/antiox14020157
APA StyleStabrauskiene, J., Sadauskiene, I., Liekis, A., Mikniene, Z., & Bernatoniene, J. (2025). Naringin vs. Citrus x paradisi L. Peel Extract: An In Vivo Journey into Oxidative Stress Modulation. Antioxidants, 14(2), 157. https://doi.org/10.3390/antiox14020157










