Analysis of Singlet Oxygen Luminescence Generated By Protoporphyrin IX
Abstract
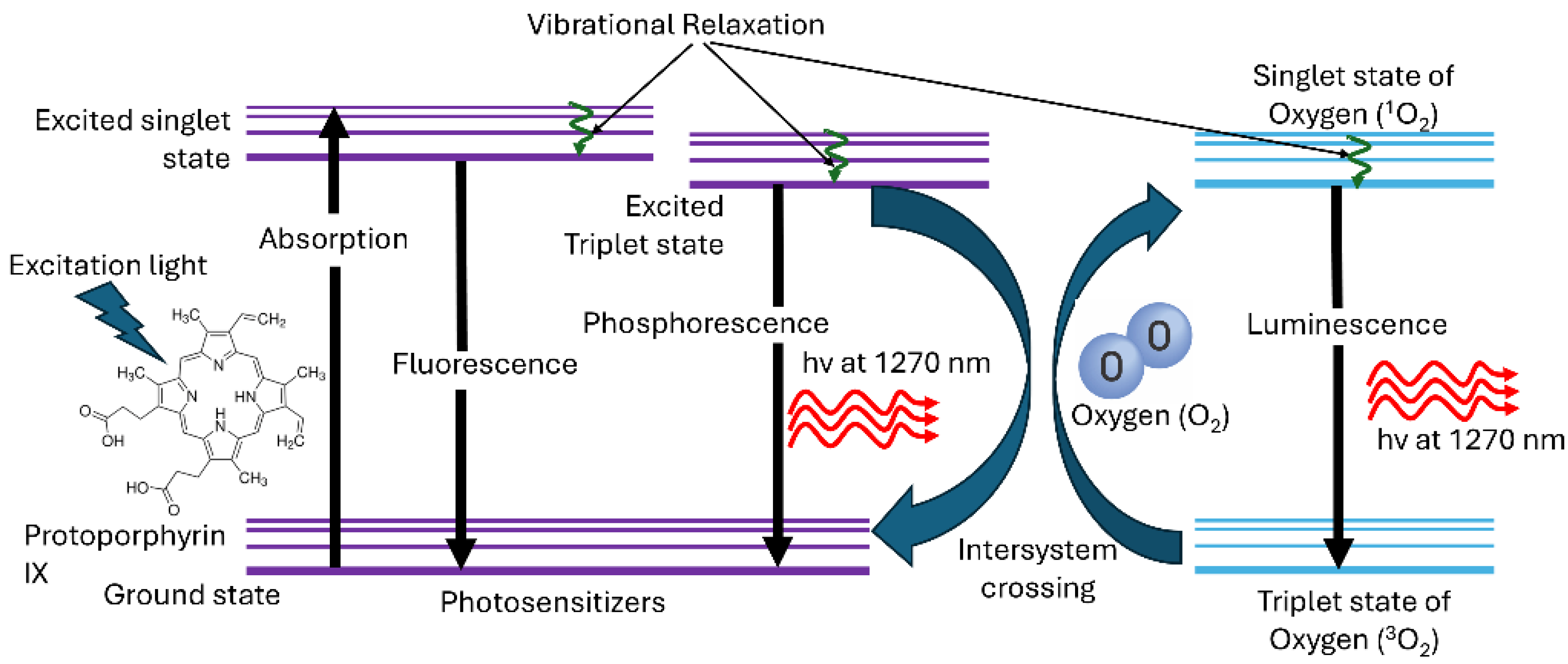
1. Introduction
2. Materials and Methods
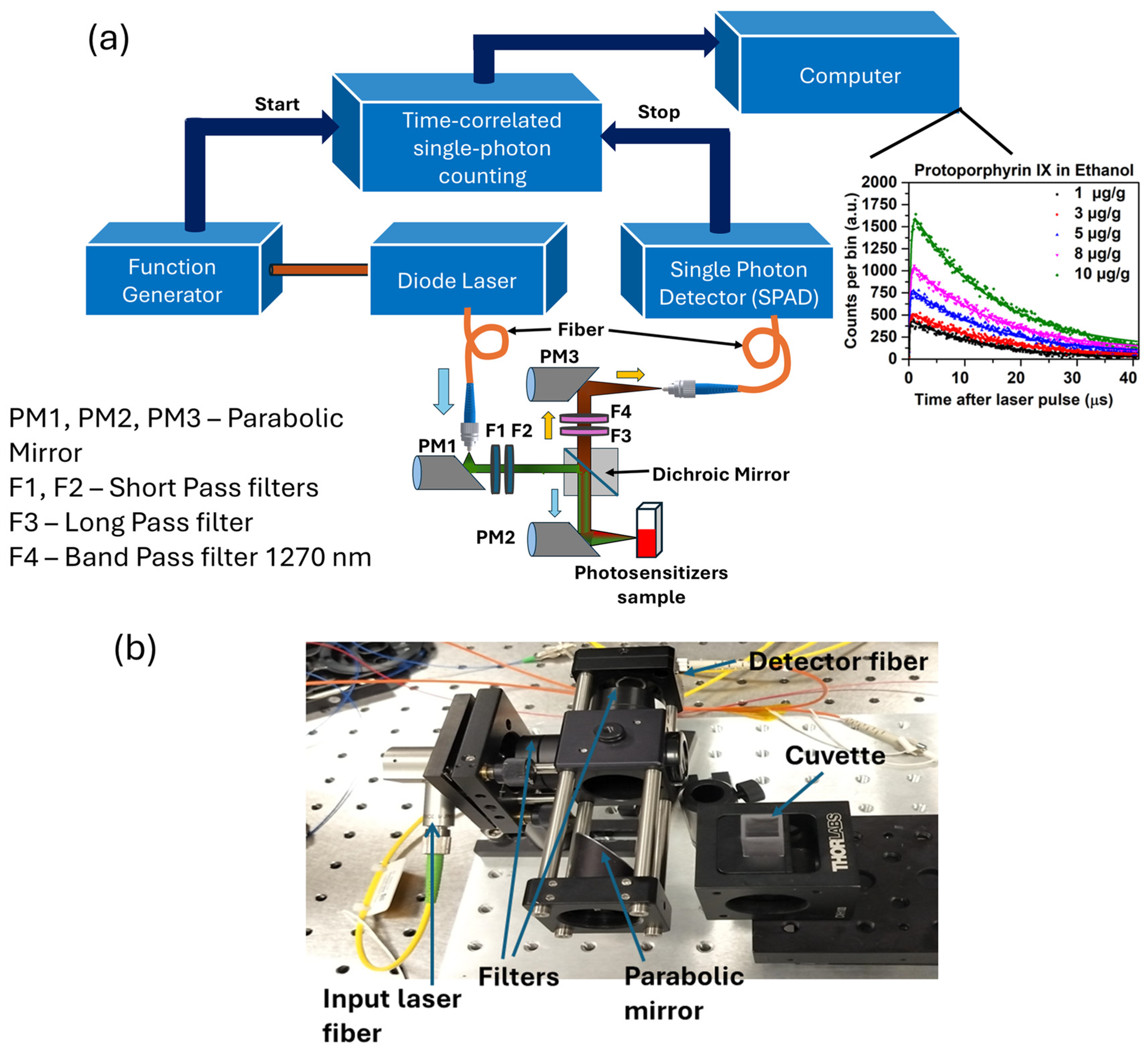
2.1. Time-Resolved 1O2 Luminescence System
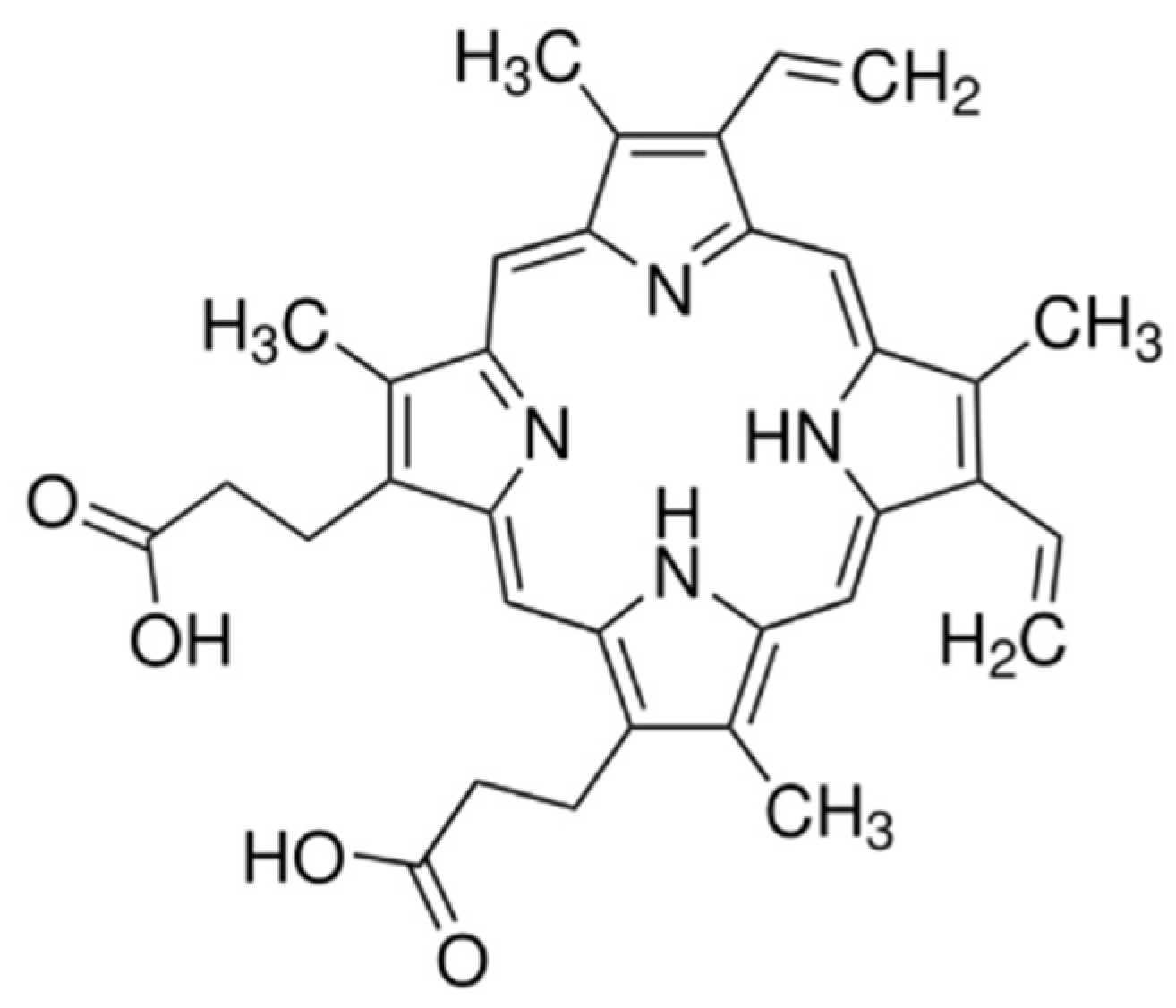
2.2. Photosensitizer in Various Media
2.3. Determination of Singlet Oxygen Lifetime
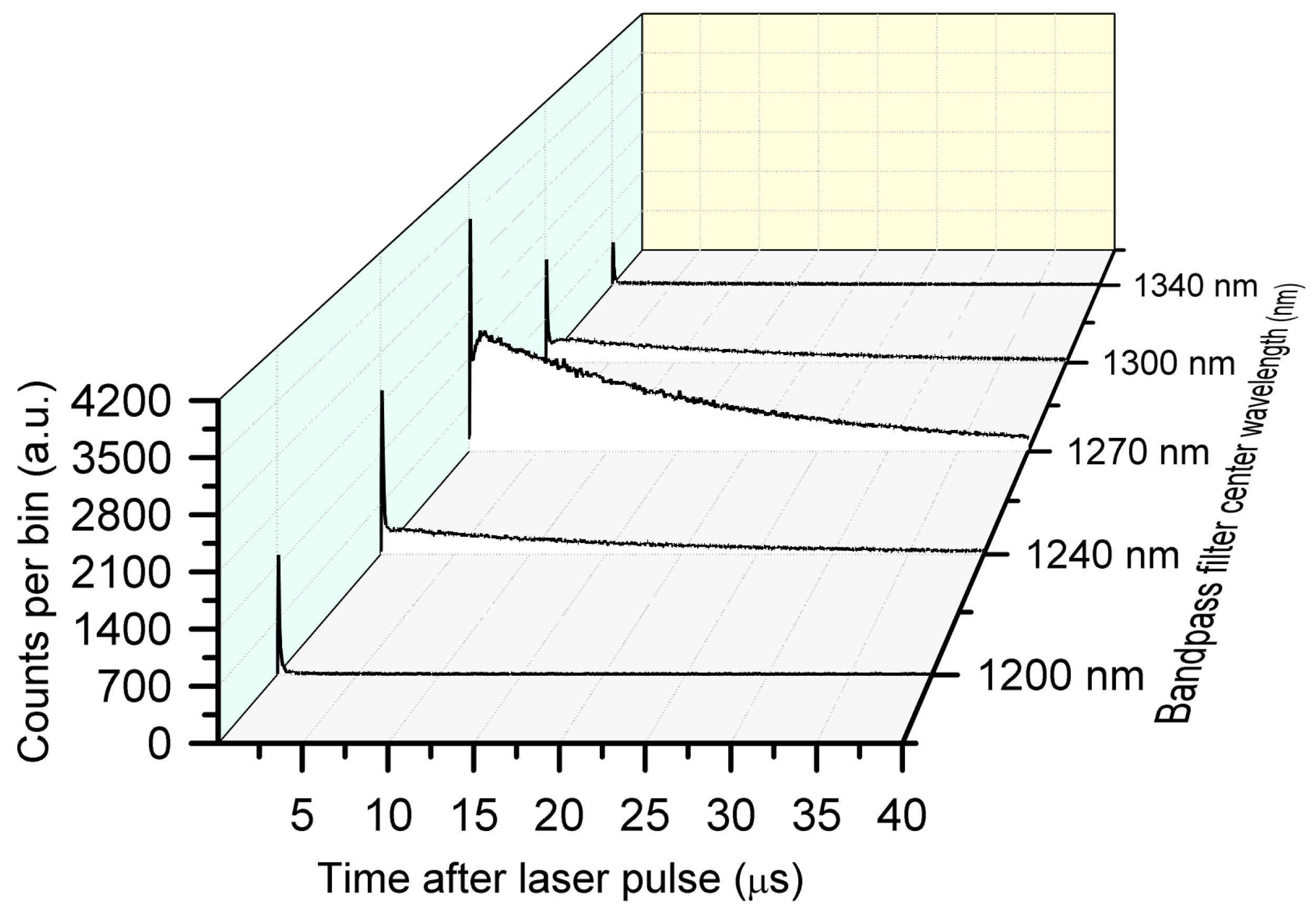
2.4. Validation of TSOLD System
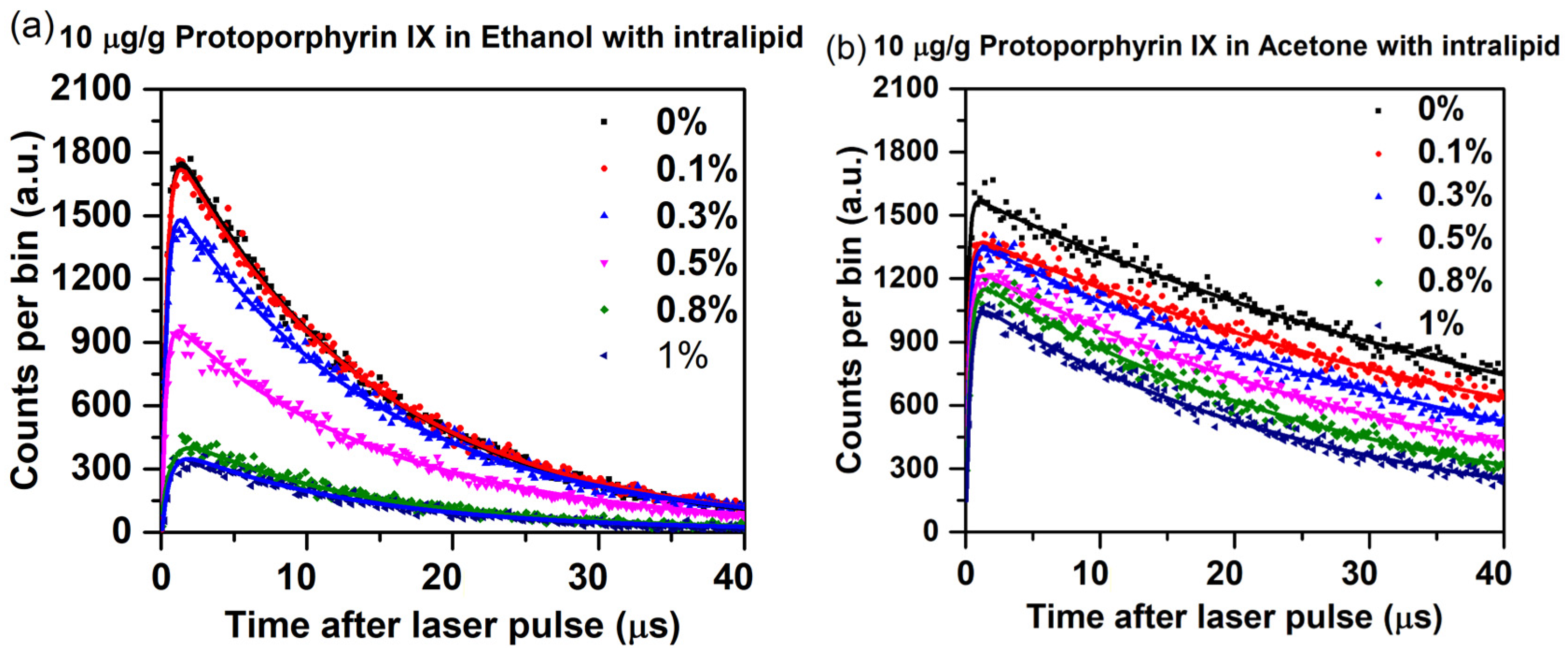
2.5. 1O2 Luminescence from PpIX in Ethanol and Acetone
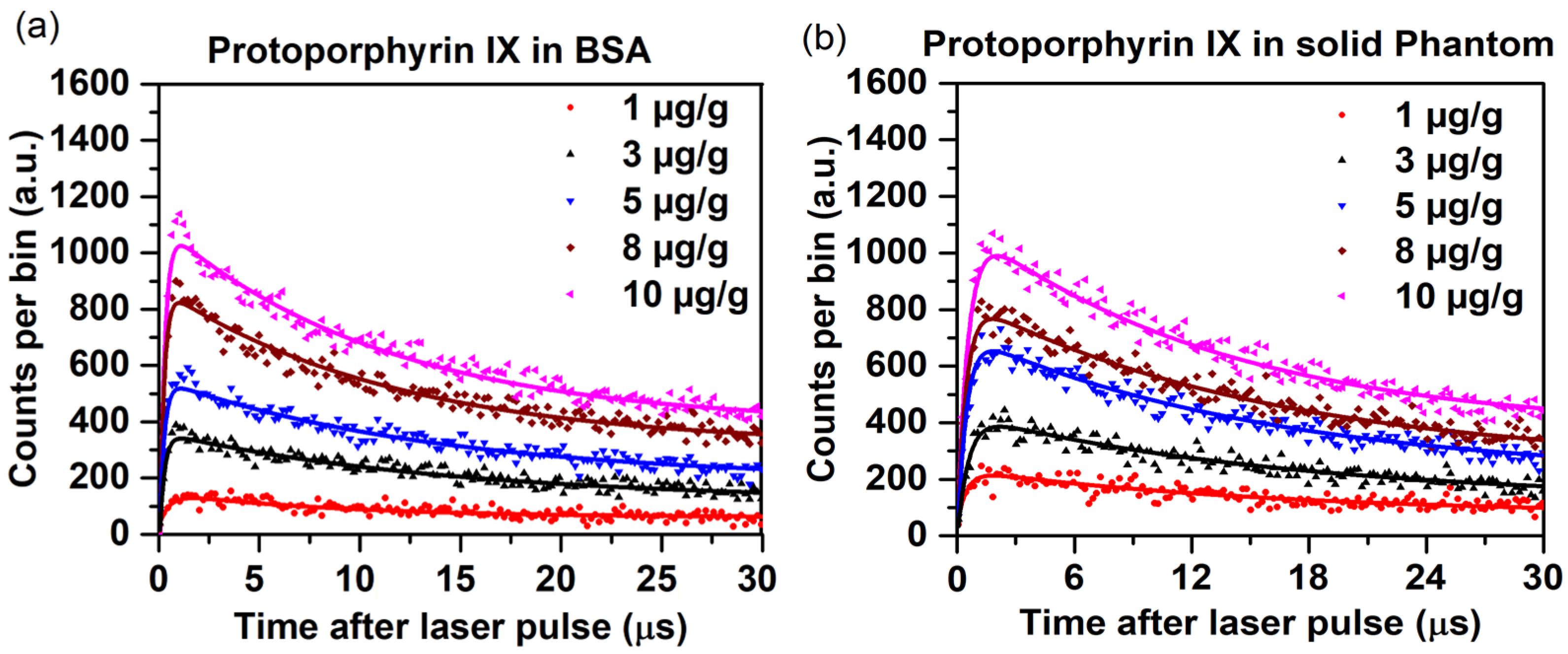
2.6. 1O2 Luminescence from PpIX in Biological Media
2.7. Impact of Scattering on Singlet Oxygen Generation and Luminescence Lifetime
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, S.B.; Brown, E.A.; Walker, I. The Present and Future Role of Photodynamic Therapy in Cancer Treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Wilson, B.C.; Patterson, M.S.; Lilge, L. Implicit and Explicit Dosimetry in Photodynamic Therapy: A New Paradigm. Laser Med. Sci. 1997, 12, 182–199. [Google Scholar] [CrossRef]
- Bankó, C.; Nagy, Z.L.; Nagy, M.; Szemán-Nagy, G.G.; Rebenku, I.; Imre, L.; Tiba, A.; Hajdu, A.; Szöllősi, J.; Kéki, S.; et al. Isocyanide Substitution in Acridine Orange Shifts DNA Damage-Mediated Phototoxicity to Permeabilization of the Lysosomal Membrane in Cancer Cells. Cancers 2021, 13, 5652. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One—Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Review: Organic Nanoparticle Based Active Targeting for Photodynamic Therapy Treatment of Breast Cancer Cells. Oncotarget 2020, 11, 2120–2136. [Google Scholar] [CrossRef]
- Kim, M.; Penjweini, R.; Gemmell, N.; Veilleux, I.; McCarthy, A.; Buller, G.; Hadfield, R.; Wilson, B.; Zhu, T. A Comparison of Singlet Oxygen Explicit Dosimetry (SOED) and Singlet Oxygen Luminescence Dosimetry (SOLD) for Photofrin-Mediated Photodynamic Therapy. Cancers 2016, 8, 109. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct Near-Infrared Luminescence Detection of Singlet Oxygen Generated by Photodynamic Therapy in Cells In Vitro and Tissues In Vivo. Photochem. Photobiol. 2002, 75, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, M.T.; Niedre, M.J.; Patterson, M.S.; Wilson, B.C. Singlet Oxygen Luminescence Dosimetry (SOLD) for Photodynamic Therapy: Current Status, Challenges and Future Prospects. Photochem. Photobiol. 2006, 82, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Hackbarth, S.; Islam, W.; Fang, J.; Subr, V.; Röder, B.; Etrych, T.; Maeda, H. Singlet Oxygen Phosphorescence Detection in Vivo Identifies PDT-Induced Anoxia in Solid Tumors. Photochem. Photobiol. Sci. 2019, 18, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-W.; Park, J.M.; Roh, Y.J.; Kim, J.H.; Choi, M.-G.; Hasan, T. Direct Measurement of Singlet Oxygen by Using a Photomultiplier Tube-Based Detection System. J. Photochem. Photobiol. B Biol. 2016, 159, 14–23. [Google Scholar] [CrossRef]
- Boso, G.; Ke, D.; Korzh, B.; Bouilloux, J.; Lange, N.; Zbinden, H. Time-Resolved Singlet-Oxygen Luminescence Detection with an Efficient and Practical Semiconductor Single-Photon Detector. Biomed. Opt. Express 2016, 7, 211. [Google Scholar] [CrossRef]
- Gemmell, N.R.; McCarthy, A.; Liu, B.; Tanner, M.G.; Dorenbos, S.D.; Zwiller, V.; Patterson, M.S.; Buller, G.S.; Wilson, B.C.; Hadfield, R.H. Singlet Oxygen Luminescence Detection with a Fiber-Coupled Superconducting Nanowire Single-Photon Detector. Opt. Express 2013, 21, 5005. [Google Scholar] [CrossRef]
- Hadfield, R.H.; Leach, J.; Fleming, F.; Paul, D.J.; Tan, C.H.; Ng, J.S.; Henderson, R.K.; Buller, G.S. Single-Photon Detection for Long-Range Imaging and Sensing. Optica 2023, 10, 1124. [Google Scholar] [CrossRef]
- Gemmell, N.R.; McCarthy, A.; Kim, M.M.; Veilleux, I.; Zhu, T.C.; Buller, G.S.; Wilson, B.C.; Hadfield, R.H. A Compact Fiber-optic Probe-based Singlet Oxygen Luminescence Detection System. J. Biophotonics 2017, 10, 320–326. [Google Scholar] [CrossRef]
- Morozov, P.; Lukina, M.; Shirmanova, M.; Divochiy, A.; Dudenkova, V.; Gol’tsman, G.N.; Becker, W.; Shcheslavskiy, V.I. Singlet Oxygen Phosphorescence Imaging by Superconducting Single-Photon Detector and Time-Correlated Single-Photon Counting. Opt. Lett. 2021, 46, 1217. [Google Scholar] [CrossRef]
- Hackbarth, S.; Pfitzner, M.; Pohl, J.; Röder, B. Time-Resolved Singlet Oxygen Luminescence Ex Vivo. In Singlet Oxygen Detection and Imaging; Synthesis Lectures on Materials and Optics; Springer International Publishing: Cham, Switzerland, 2021; pp. 61–80. ISBN 978-3-031-01263-1. [Google Scholar]
- Niedre, M.J.; Patterson, M.S.; Giles, A.; Wilson, B.C. Imaging of Photodynamically Generated Singlet Oxygen Luminescence In Vivo. Photochem. Photobiol. 2005, 81, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, R.; Guo, Y.; Song, J.; Li, Z.; Song, F. Boosting Type-I and Type-II ROS Production of Water-Soluble Porphyrin for Efficient Hypoxic Tumor Therapy. Mol. Pharm. 2023, 20, 606–615. [Google Scholar] [CrossRef]
- Yan, T.; Alimu, G.; Zhu, L.; Fan, H.; Zhang, L.; Du, Z.; Ma, R.; Chen, S.; Alifu, N.; Zhang, X. PpIX/IR-820 Dual-Modal Therapeutic Agents for Enhanced PDT/PTT Synergistic Therapy in Cervical Cancer. ACS Omega 2022, 7, 44643–44656. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef]
- Vikas, V.; Kumar, R.; Soni, S. Dynamic Change in Optical Properties of a Nanoparticle Embedded Tumor Phantom for Plasmonic Photothermal Cancer Therapeutics. J. Biophotonics 2023, 16, e202200179. [Google Scholar] [CrossRef]
- Protoporphyrin IX = 95 553-12-8. Available online: http://www.sigmaaldrich.com/ (accessed on 6 May 2024).
- Tsimvrakidis, K.; Gemmell, N.R.; Erotokritou, K.; Miki, S.; Yabuno, M.; Yamashita, T.; Terai, H.; Hadfield, R.H. Enhanced Optics for Time-Resolved Singlet Oxygen Luminescence Detection. IEEE J. Select. Top. Quantum Electron. 2019, 25, 7000107. [Google Scholar] [CrossRef]
- Jones, L.R.; Grossweiner, L.I. Singlet Oxygen Generation by Photofrin® in Homogeneous and Light-Scattering Media. J. Photochem. Photobiol. B Biol. 1994, 26, 249–256. [Google Scholar] [CrossRef]
- Flock, S.T.; Jacques, S.L.; Wilson, B.C.; Star, W.M.; van Gemert, M.J.C. Optical Properties of Intralipid: A Phantom Medium for Light Propagation Studies. Lasers Surg. Med. 1992, 12, 510–519. [Google Scholar] [CrossRef]
- Krasnovsky, A.A. Singlet Molecular Oxygen in Photobiochemical Systems: IR Phosphorescence Studies. Membr. Cell Biol. 1998, 12, 665–690. [Google Scholar]
- Vinklárek, I.S.; Scholz, M.; Dědic, R.; Hála, J. Singlet Oxygen Feedback Delayed Fluorescence of Protoporphyrin IX in Organic Solutions. Photochem. Photobiol. Sci. 2017, 16, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Serafin, I.; Batóg-Szczęch, K.; Dynarowicz, K.; Chodurek, E.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic Therapy in the Treatment of Cancer—The Selection of Synthetic Photosensitizers. Pharmaceuticals 2024, 17, 932. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Ricchelli, F. Photophysical Properties of Porphyrins in Biological Membranes. J. Photochem. Photobiol. B Biol. 1995, 29, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ogilby, P.R. Singlet Oxygen: There Is Indeed Something New under the Sun. Chem. Soc. Rev. 2010, 39, 3181. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, L.; Liu, L.; Yu, Y.; Liao, X.; Gu, Y.; Li, B. Detection of Singlet Oxygen Luminescence in Skin Phantom Based on Optical Fiber Detection. In Proceedings of the Optics in Health Care and Biomedical Optics IX; Luo, Q., Li, X., Tang, Y., Gu, Y., Zhu, D., Eds.; SPIE: Hangzhou, China, 2019; p. 106. [Google Scholar]
- Sitte, E.; Senge, M.O. The Red Color of Life Transformed—Synthetic Advances and Emerging Applications of Protoporphyrin IX in Chemical Biology. Eur. J. Org. Chem. 2020, 2020, 3171–3191. [Google Scholar] [CrossRef]
- Yeh, P.-S.; Li, C.-C.; Lu, Y.-S.; Chiang, Y.-W. Structural Insights into the Binding and Degradation Mechanisms of Protoporphyrin IX by the Translocator Protein TSPO. JACS Au 2023, 3, 2918–2929. [Google Scholar] [CrossRef]
- Hussain, M.; Razi, S.S.; Tao, T.; Hartl, F. Triplet-Triplet Annihilation Photon up-Conversion: Accessing Triplet Excited States with Minimum Energy Loss. J. Photochem. Photobiol. C Photochem. Rev. 2023, 56, 100618. [Google Scholar] [CrossRef]
- Solubility of Softisan 100 in Ethanol + Water and Acetone + Water Solutions. J. Chem. Eng. Data 2010, 55, 2333–2337. [CrossRef]
- Sakamoto, K.; Ohno-Okumura, E. Syntheses and Functional Properties of Phthalocyanines. Materials 2009, 2, 1127–1179. [Google Scholar] [CrossRef]
- Tournaire, C.; Croux, S.; Maurette, M.-T.; Beck, I.; Hocquaux, M.; Braun, A.M.; Oliveros, E. Antioxidant Activity of Flavonoids: Efficiency of Singlet Oxygen (1Δg) Quenching. J. Photochem. Photobiol. B Biol. 1993, 19, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Soares, H.T.; Campos, J.R.S.; Gomes-da-Silva, L.C.; Schaberle, F.A.; Dabrowski, J.M.; Arnaut, L.G. Pro-Oxidant and Antioxidant Effects in Photodynamic Therapy: Cells Recognise That Not All Exogenous ROS Are Alike. Chembiochem 2016, 17, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.L.C.; Johnstone, R.A.W.; Sørensen, A.M.P.S.; Burget, D.; Jacques, P. Determination of Fluorescence Yields, Singlet Lifetimes and Singlet Oxygen Yields of Water-Insoluble Porphyrins and Metalloporphyrins in Organic Solvents and in Aqueous Media. Photochem. Photobiol. 1999, 69, 517–528. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Mano, C.M.; Prado, F.M.; Massari, J.; Ronsein, G.E.; Martinez, G.R.; Miyamoto, S.; Cadet, J.; Sies, H.; Medeiros, M.H.G.; Bechara, E.J.H.; et al. Excited Singlet Molecular O2 (1Δg) Is Generated Enzymatically from Excited Carbonyls in the Dark. Sci. Rep. 2014, 4, 5938. [Google Scholar] [CrossRef]
- Giménez, R.E.; Vargová, V.; Rey, V.; Turbay, M.B.E.; Abatedaga, I.; Morán Vieyra, F.E.; Paz Zanini, V.I.; Mecchia Ortiz, J.H.; Katz, N.E.; Ostatná, V.; et al. Interaction of Singlet Oxygen with Bovine Serum Albumin and the Role of the Protein Nano-Compartmentalization. Free Radic. Biol. Med. 2016, 94, 99–109. [Google Scholar] [CrossRef]
- Miranda-Apodaca, J.; Hananya, N.; Velázquez-Campoy, A.; Shabat, D.; Arellano, J.B. Emissive Enhancement of the Singlet Oxygen Chemiluminescence Probe after Binding to Bovine Serum Albumin. Molecules 2019, 24, 2422. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Yahioglu, G.; Ogilby, P.R. Singlet Oxygen in a Cell: Spatially Dependent Lifetimes and Quenching Rate Constants. J. Am. Chem. Soc. 2009, 131, 332–340. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Takemura, T.; Ohta, N.; Nakajima, S.; Sakata, I. Critical Importance of the Triplet Lifetime of Photosensitizer in Photodynamic Therapy of Tumor. Photochem. Photobiol. 1989, 50, 339–344. [Google Scholar] [CrossRef]
- Codognato, D.C.K.; Pena, F.S.; dos Reis, E.R.; Ramos, A.P.; Borissevitch, I.E. Effects of Serum Albumin on the Photophysical Characteristics of Synthetic and Endogenous Protoporphyrin IX. Braz. J. Med. Biol. Res. 2022, 55, e12272. [Google Scholar] [CrossRef] [PubMed]
- Reinert, M.; Piffaretti, D.; Wilzbach, M.; Hauger, C.; Guckler, R.; Marchi, F.; D’Angelo, M.L. Quantitative Modulation of PpIX Fluorescence and Improved Glioma Visualization. Front. Surg. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.A.; Patterson, M.S. Chapter 33. Singlet Oxygen Dosimetry in Biological Media. In Comprehensive Series in Photochemical & Photobiological Sciences; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; Volume 2, pp. 151–168. ISBN 978-1-78262-697-8. [Google Scholar]

| PpIX Concentration (µg/g) | PpIX in Ethanol | PpIX in Acetone | ||
|---|---|---|---|---|
| Singlet Oxygen Lifetime (µs) | PpIX Triplet-State Lifetime (µs) | Singlet Oxygen Lifetime (µs) | PpIX Triplet-State Lifetime (µs) | |
| 1 | 13.8 | 0.10 | 47.9 | 0.16 |
| 3 | 14.4 | 0.13 | 48.2 | 0.21 |
| 5 | 14.6 | 0.14 | 48.3 | 0.27 |
| 8 | 14.4 | 0.18 | 48.4 | 0.17 |
| 10 | 14.6 | 0.22 | 48.6 | 0.25 |
| PpIX Concentration (µg/g) | PpIX in BSA | PpIX in Agarose-Based Solid Phantoms | ||
|---|---|---|---|---|
| Singlet Oxygen Lifetime (µs) | PpIX Triplet-State Lifetime (µs) | Singlet Oxygen Lifetime (µs) | PpIX Triplet-State Lifetime (µs) | |
| 1 | 18.9 | 0.51 | 21.1 | 0.54 |
| 3 | 23.0 | 0.20 | 25.4 | 0.44 |
| 5 | 26.9 | 0.19 | 27.5 | 0.38 |
| 8 | 28.4 | 0.17 | 28.8 | 0.36 |
| 10 | 28.5 | 0.16 | 28.9 | 0.34 |
| Intralipid Concentration (%) | PpIX in Ethanol | PpIX in Acetone | ||
|---|---|---|---|---|
| Singlet Oxygen Lifetime (µs) | PpIX Triplet-State Lifetime (µs) | Singlet Oxygen Lifetime (µs) | PpIX Triplet-State Lifetime (µs) | |
| 0 | 14.6 | 0.30 | 48.6 | 0.18 |
| 0.1 | 14.4 | 0.32 | 48.0 | 0.24 |
| 0.3 | 14.2 | 0.34 | 45.9 | 0.25 |
| 0.5 | 13.8 | 0.36 | 42.1 | 0.28 |
| 0.8 | 13.3 | 0.51 | 40.4 | 0.31 |
| 1.0 | 12.7 | 0.55 | 38.7 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikas, V.; Yang, W.; Wilson, B.C.; Zhu, T.C.; Hadfield, R.H. Analysis of Singlet Oxygen Luminescence Generated By Protoporphyrin IX. Antioxidants 2025, 14, 176. https://doi.org/10.3390/antiox14020176
Vikas V, Yang W, Wilson BC, Zhu TC, Hadfield RH. Analysis of Singlet Oxygen Luminescence Generated By Protoporphyrin IX. Antioxidants. 2025; 14(2):176. https://doi.org/10.3390/antiox14020176
Chicago/Turabian StyleVikas, Vikas, Weibing Yang, Brian C. Wilson, Timothy C. Zhu, and Robert H. Hadfield. 2025. "Analysis of Singlet Oxygen Luminescence Generated By Protoporphyrin IX" Antioxidants 14, no. 2: 176. https://doi.org/10.3390/antiox14020176
APA StyleVikas, V., Yang, W., Wilson, B. C., Zhu, T. C., & Hadfield, R. H. (2025). Analysis of Singlet Oxygen Luminescence Generated By Protoporphyrin IX. Antioxidants, 14(2), 176. https://doi.org/10.3390/antiox14020176









