The Effects of Acute Temperature Changes on Transcriptomic Responses in the Liver of Leopard Coral Groupers (Plectropomus leopardus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
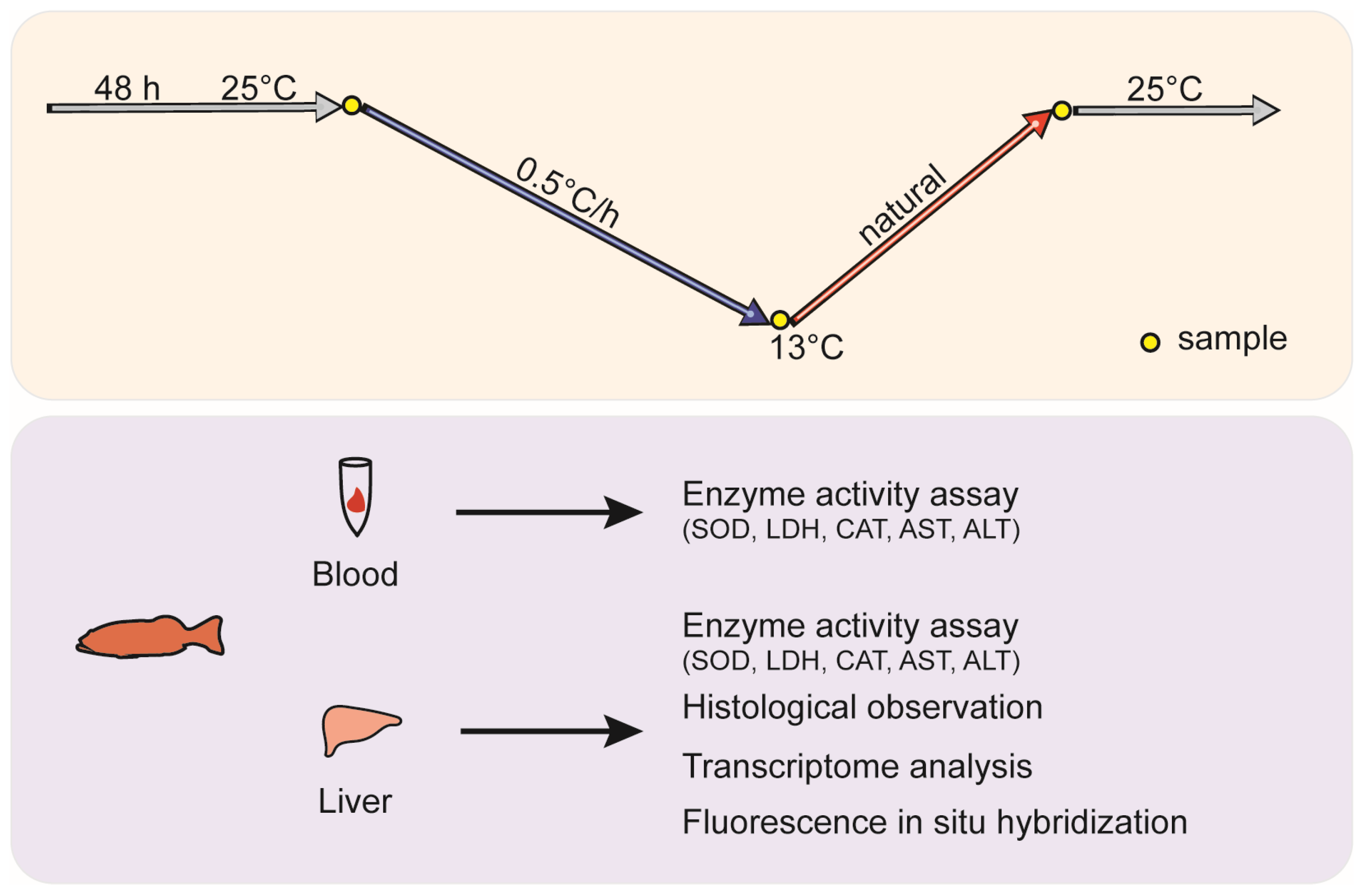
2.2. Experimental Design and Sampling
2.3. Enzyme Activity Assay
2.4. RNA Extraction, cDNA Library Construction, and Sequencing
2.5. Transcriptomic Data Processing and Analysis
2.6. cDNA Synthesis and qRT-PCR
2.7. HE Staining and Fluorescence in Situ Hybridization (FISH)
2.8. Statistical Analysis
3. Results
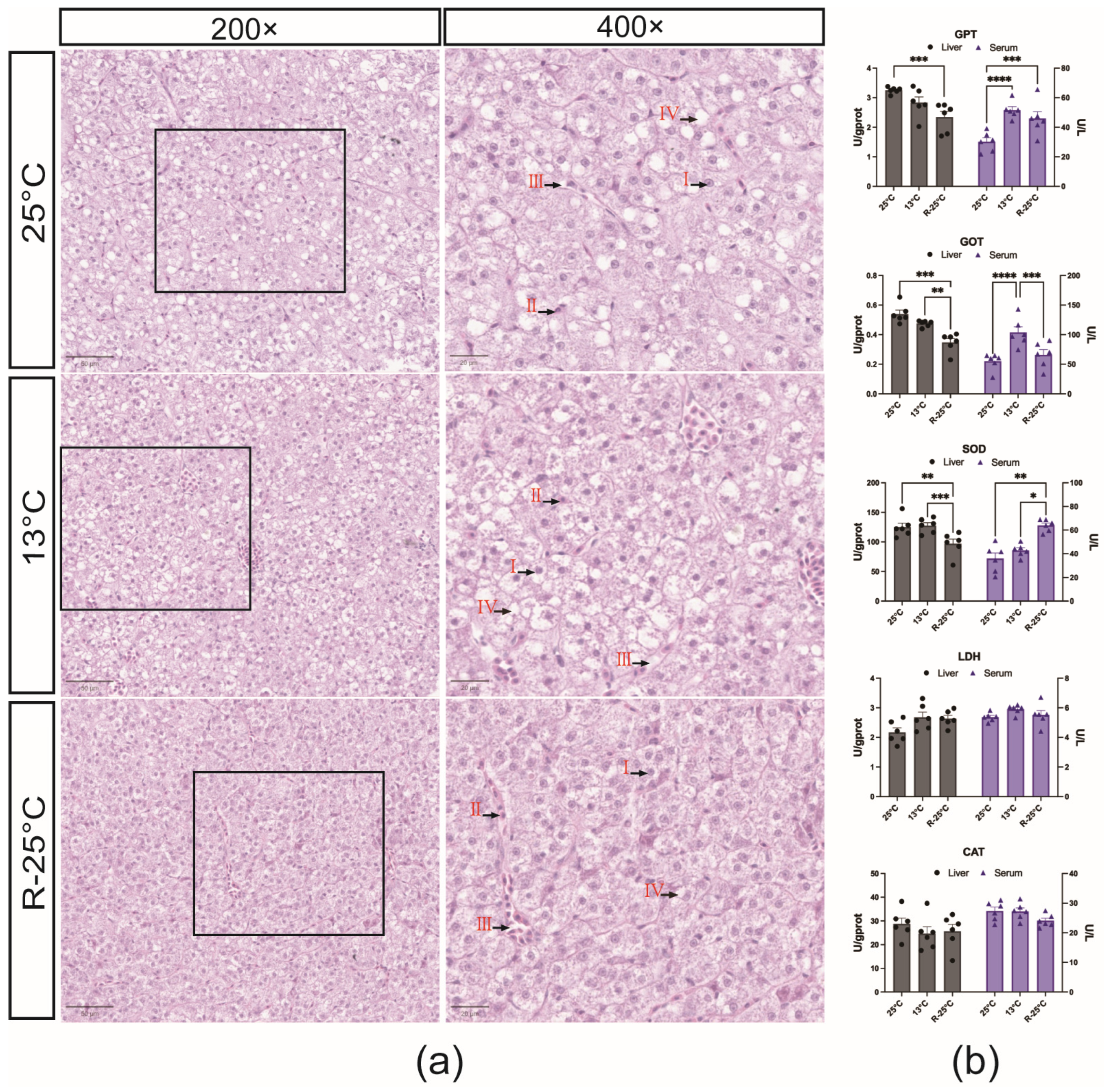
3.1. Effects of Temperature Stress on Hepatic Histology and Enzyme Activity in Serum and Liver
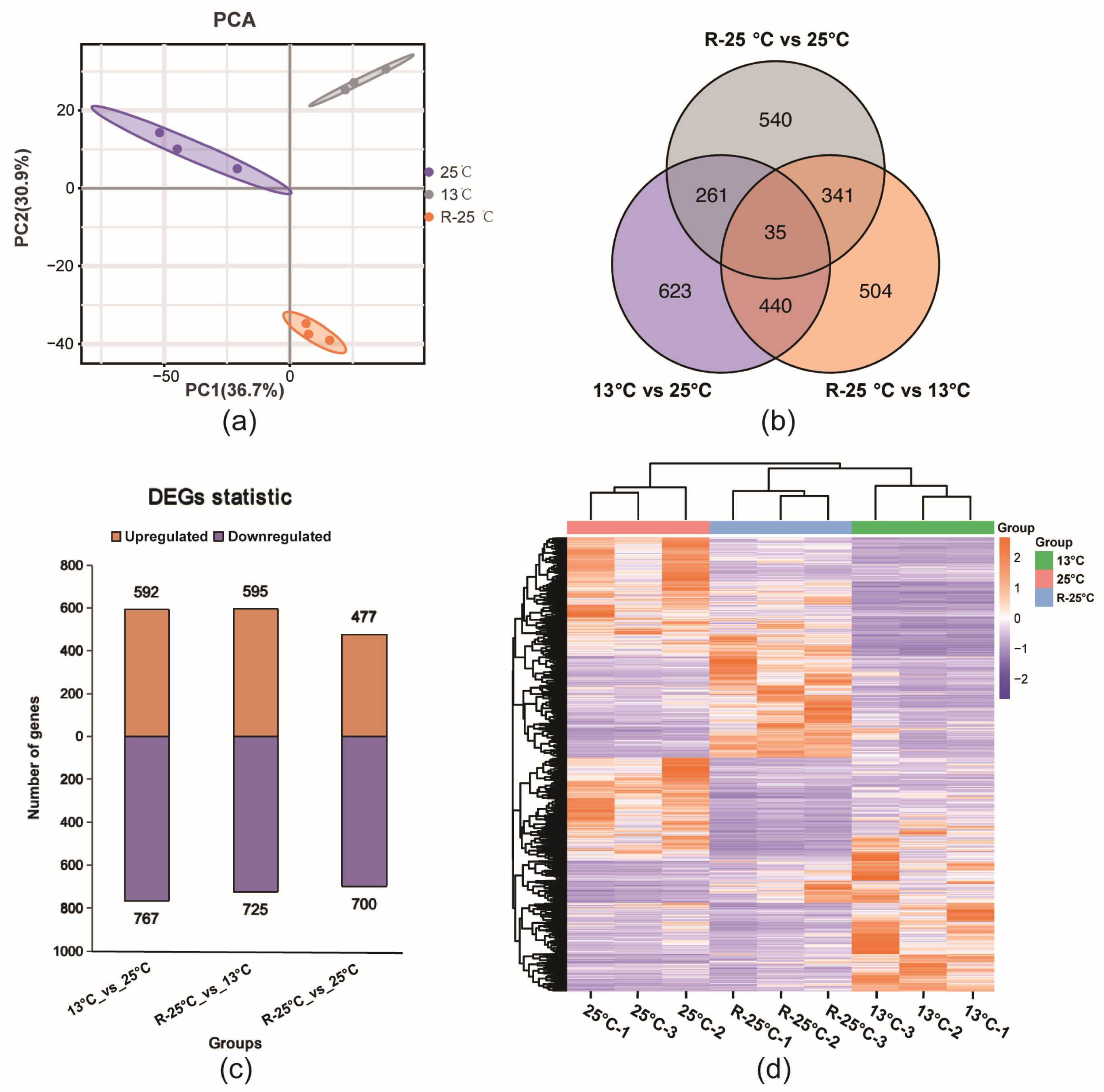
3.2. DEGs Identification Among Three Groups Based on Transcriptomic Data
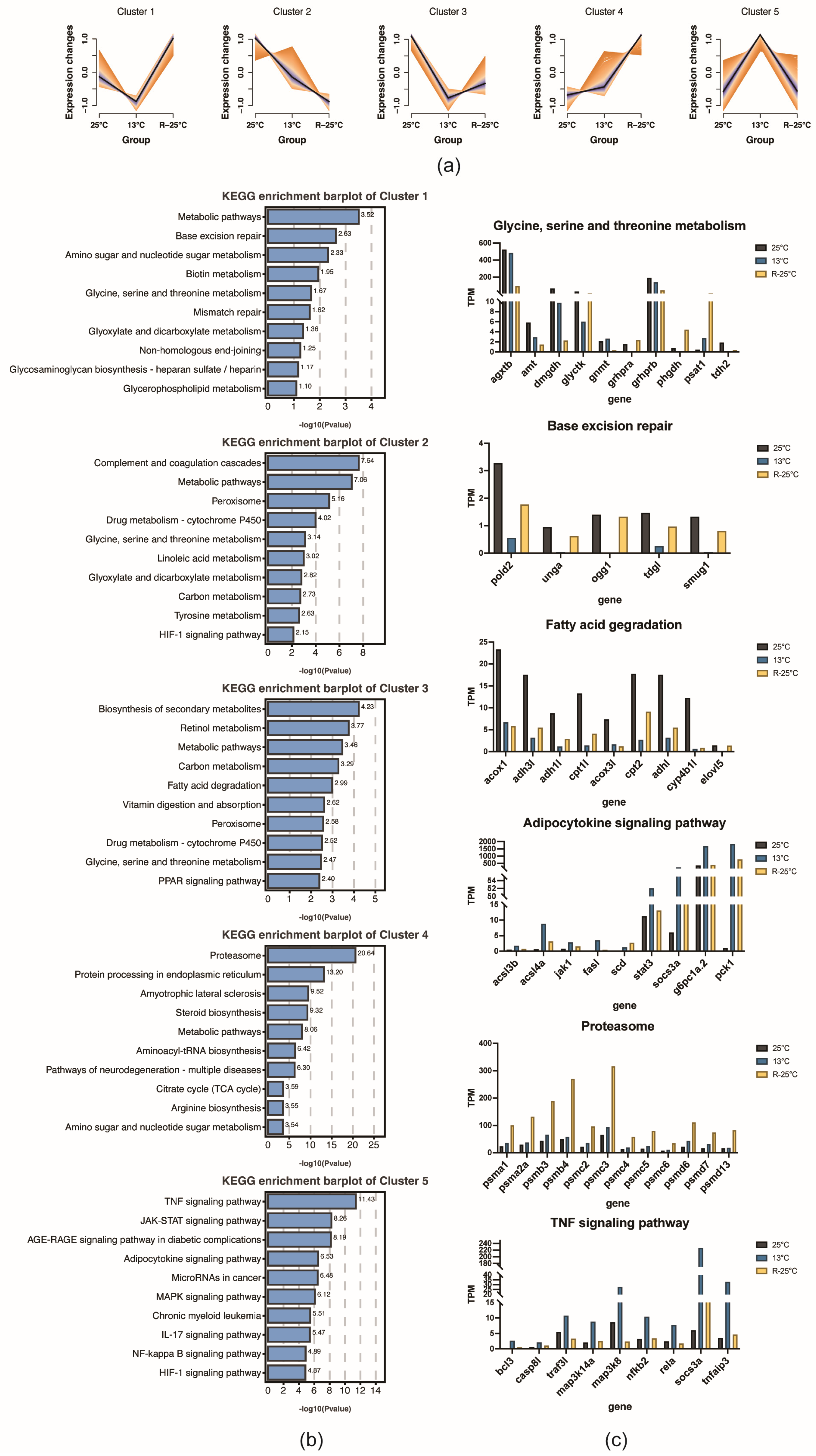
3.3. Functional Analysis of DEGs
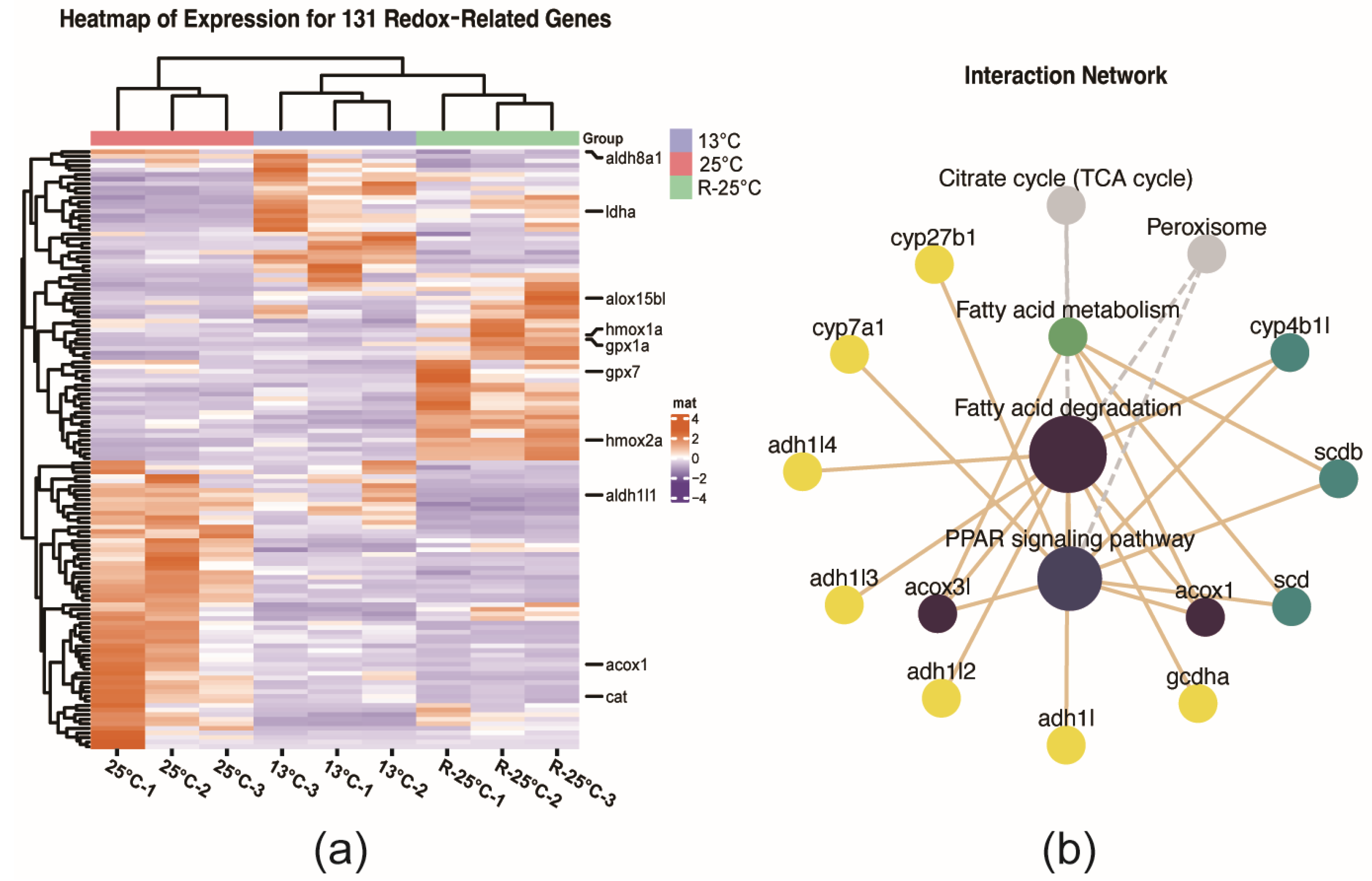
3.4. The Regulation of Redox Related DEGs Under the Temperature Fluctuations
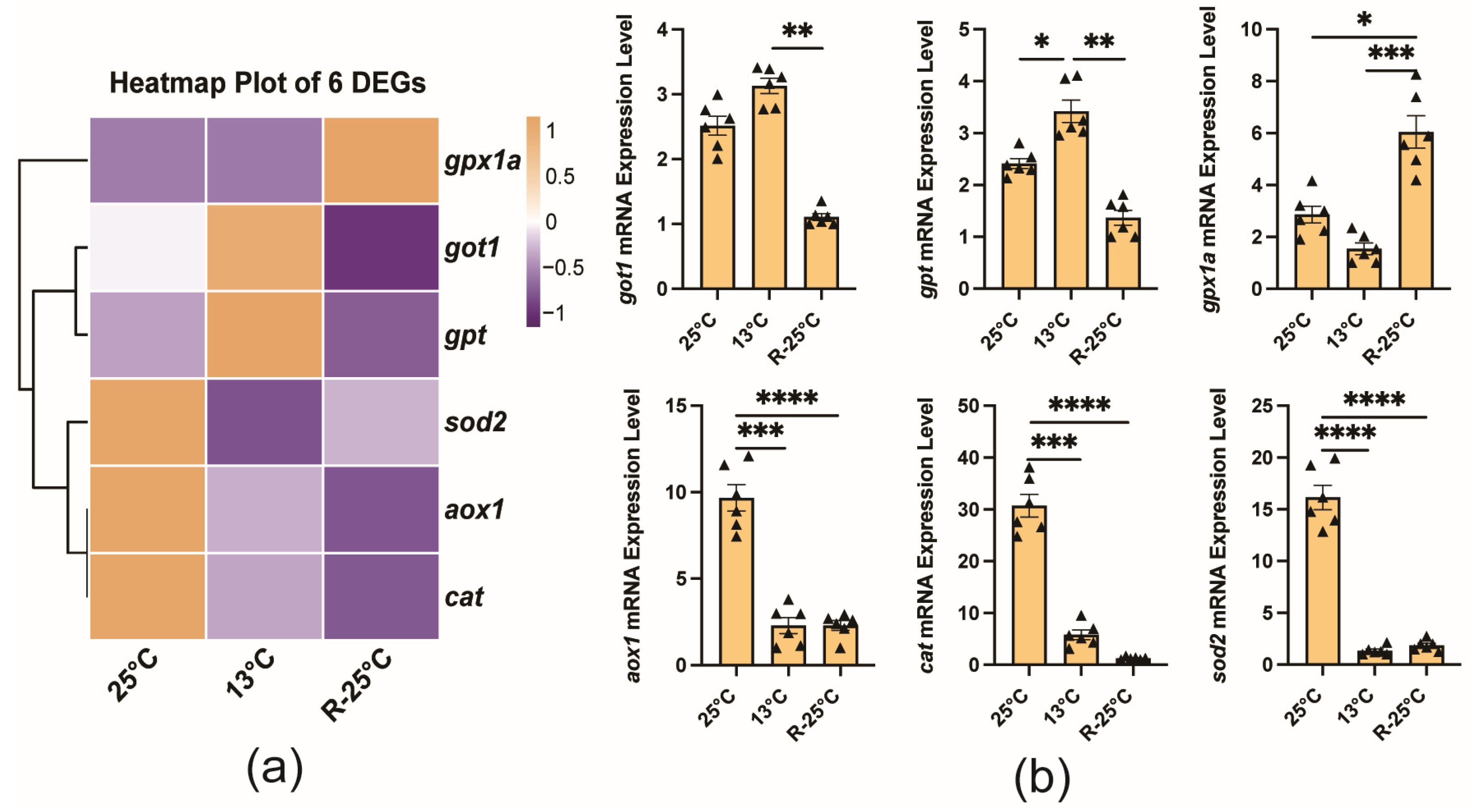
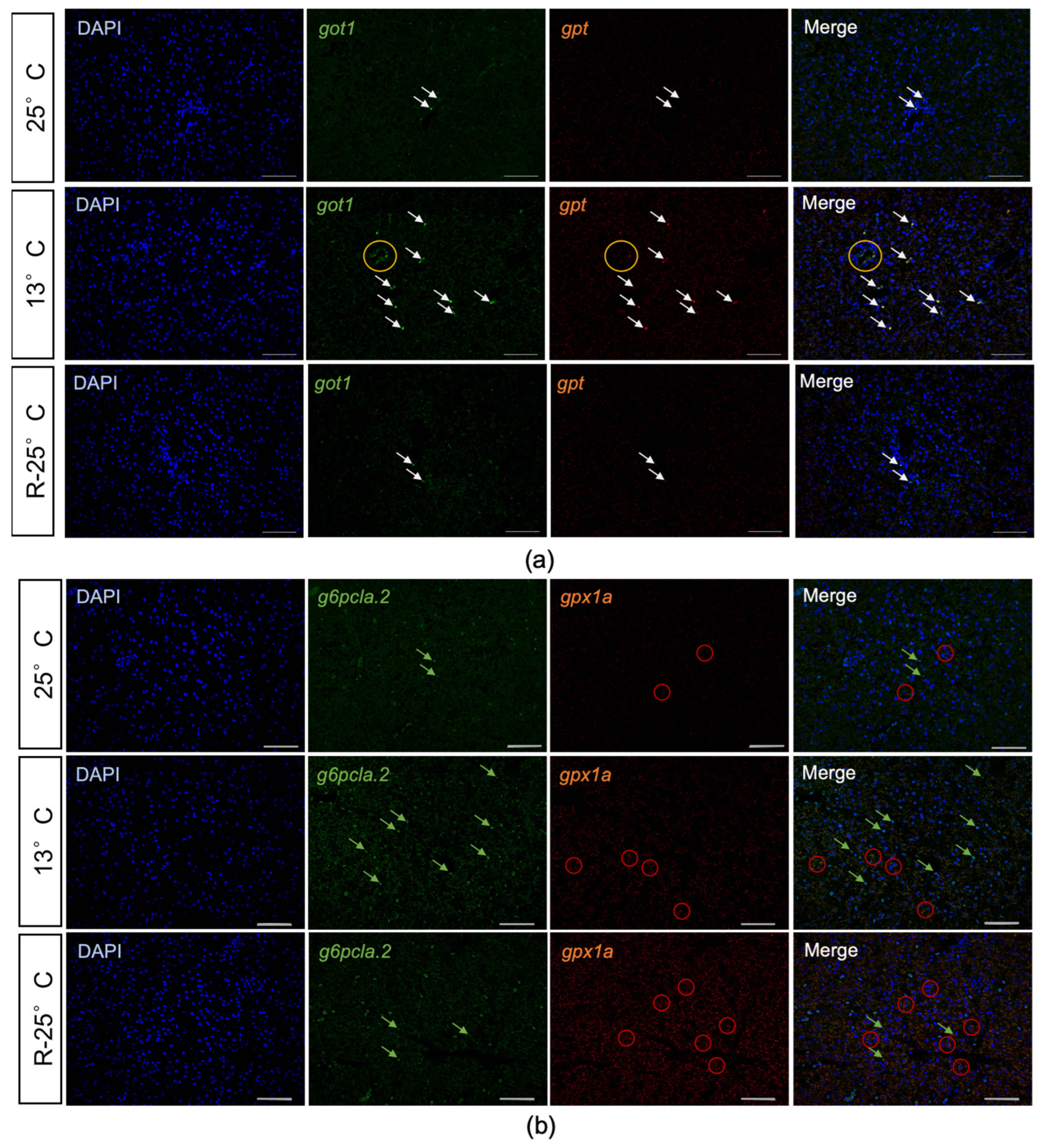
3.5. Validation of Gene Expression Profiles
4. Discussion
4.1. Low Temperature Leads to Expression Changes in Membrane Fluidity Maintenance-Related Genes
4.2. Low Temperature Promotes Upregulation of Hepatic Gluconeogenesis Related Genes
4.3. Hepatic Lipid Peroxidation Damage and Activation of Antioxidant Defense Under Cold Exposure
4.4. Upregulation of Genes Related Repair of Liver Damage upon Rewarming
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassahn, K.S.; Crozier, R.H.; Pörtner, H.O.; Caley, M.J. Animal Performance and Stress: Responses and Tolerance Limits at Different Levels of Biological Organisation. Biol. Rev. 2009, 84, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.A.; Fraser, K.P.P.; Bishop, C.M.; Peck, L.S.; Egginton, S. Hibernation in an Antarctic Fish: On Ice for Winter. PLoS ONE 2008, 3, e1743. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Long, Y.; Shi, L.; Ren, J.; Yan, J.; Li, C.; Li, Q.; Cui, Z. Transcriptomic Profiling Revealed Key Signaling Pathways for Cold Tolerance and Acclimation of Two Carp Species. BMC Genom. 2020, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Van der Walt, K.-A.; Porri, F.; Potts, W.M.; Duncan, M.I.; James, N.C. Thermal Tolerance, Safety Margins and Vulnerability of Coastal Species: Projected Impact of Climate Change Induced Cold Water Variability in a Temperate African Region. Mar. Environ. Res. 2021, 169, 105346. [Google Scholar] [CrossRef] [PubMed]
- Messmer, V.; Pratchett, M.S.; Hoey, A.S.; Tobin, A.J.; Coker, D.J.; Cooke, S.J.; Clark, T.D. Global Warming May Disproportionately Affect Larger Adults in a Predatory Coral Reef Fish. Glob. Change Biol. 2017, 23, 2230–2240. [Google Scholar] [CrossRef]
- Soyano, K.; Mushirobira, Y. The Mechanism of Low-Temperature Tolerance in Fish. Adv. Exp. Med. Biol. 2018, 1081, 149–164. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, D.; Li, S.; Zhang, Z.; Mo, H.; Wang, L. Acute Temperature Stresses Trigger Liver Transcriptome and Microbial Community Remodeling in Largemouth Bass (Micropterus salmoides). Aquaculture 2023, 573, 739573. [Google Scholar] [CrossRef]
- Porter, E.S.; Clow, K.A.; Sandrelli, R.M.; Gamperl, A.K. Acute and Chronic Cold Exposure Differentially Affect Cardiac Control, but Not Cardiorespiratory Function, in Resting Atlantic Salmon (Salmo salar). Curr. Res. Physiol. 2022, 5, 158–170. [Google Scholar] [CrossRef]
- Mazumder, S.K.; Debi, S.; Das, S.K.; Salam, M.A.; Alam, M.S.; Rahman, M.L.; Mamun, M.A.A.; Ibrahim Khalil, S.M.; Pandit, D. Effects of Extreme-Ambient Temperatures in Silver Barb (Barbonymus gonionotus): Metabolic, Hemato-Biochemical Responses, Enzymatic Activity and Gill Histomorphology. Water 2024, 16, 292. [Google Scholar] [CrossRef]
- Nilsson, G.E. Gill Remodeling in Fish—A New Fashion or an Ancient Secret? J. Exp. Biol. 2007, 210, 2403–2409. [Google Scholar] [CrossRef]
- Liu, C.; Shen, W.; Hou, C.; Gao, X.; Wang, Q.; Wu, X.; Zhu, J. Low Temperature-Induced Variation in Plasma Biochemical Indices and Aquaglyceroporin Gene Expression in the Large Yellow Croaker Larimichthys crocea. Sci. Rep. 2019, 9, 2717. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Dong, X.; Liu, H.; Yan, X.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y.; et al. Effect of Dietary Oxidized Fish Oil on Liver Function in Hybrid Grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatus). Aquac. Rep. 2022, 22, 101000. [Google Scholar] [CrossRef]
- Jia, R.; Hou, Y.; Zhang, L.; Li, B.; Zhu, J. Effects of Berberine on Lipid Metabolism, Antioxidant Status, and Immune Response in Liver of Tilapia (Oreochromis niloticus) under a High-Fat Diet Feeding. Antioxidants 2024, 13, 548. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Li, B.; Ding, L.; Wei, X.; Wang, P.; Chen, Z.; Han, S.; Huang, T.; Wang, B.; et al. Physiological Responses to Heat Stress in the Liver of Rainbow Trout (Oncorhynchus mykiss) Revealed by UPLC-QTOF-MS Metabolomics and Biochemical Assays. Ecotoxicol. Environ. Saf. 2022, 242, 113949. [Google Scholar] [CrossRef]
- Duan, C.; Tian, C.; Guan, Y.; Xu, H.; Yang, L.; Chen, Y.; Liu, Y.; Shen, Y.; Zhang, Y.; Cao, S.; et al. Long-Term Thermal Stress Induces Hepatic Injury and Alters the Thermotolerance Response in Hong Kong Catfish (Clarias fuscus). Aquaculture 2024, 590, 741041. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Wang, X.; Zhu, H.; Tian, Z. Transcriptome Analysis Reveals Molecular Mechanisms Responsive to Acute Cold Stress in the Tropical Stenothermal Fish Tiger Barb (Puntius tetrazona). BMC Genom. 2020, 21, 737. [Google Scholar] [CrossRef]
- Xia, B.; Liu, Z.; Zhou, Y.; Wang, Y.; Huang, J.; Li, Y.; Kang, Y.; Jianfu, W.; Liu, X. Effects of Heat Stress on Biochemical Parameters and Heat Shock Protein Family A (Hsp70) Member 5 (HSPA5) mRNA Expression in Rainbow Trout (Oncorhynchus mykiss). Mar. Freshw. Res. 2018, 69, 1674–1680. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wei, M.; Lin, Z.; Shen, M.; Zhu, F.; Jia, C.; Meng, Q.; Xu, D.; Du, S. Liver Transcriptome Analysis of the Black Porgy (Acanthopagrus schlegelii) under Acute Low-Temperature Stress. Life 2023, 13, 721. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Su, J.; Zhou, Z.; Miao, Y.; Tian, X.; Tao, M.; Zhang, C.; Zhou, Y.; Qin, Q.; et al. Integration of Transcriptome and Metabolome Reveals Molecular Mechanisms Responsive to Cold Stress in Gynogenetic Mrigal Carp (Cirrhinus mrigala). Aquaculture 2024, 579, 740200. [Google Scholar] [CrossRef]
- Huang, D.; Liang, H.; Zhu, J.; Ren, M.; Ge, X. Transcriptome Reveals Insights into Hepatic Nutritional Metabolism and Gill Immune Responses Adapted to Cold Stress in Genetically Improved Farmed Tilapia (GIFT: Oreochromis niloticus). Aquac. Rep. 2022, 26, 101297. [Google Scholar] [CrossRef]
- Scott, M. Spatial and Temporal Variation in Movement Patterns and Behaviour of a Large, Coral Reef Mesopredator, Plectropomus leopardus, on the Great Barrier Reef, Australia. Ph.D. Thesis, James Cook University, Townsville, Australia, 2019. [Google Scholar]
- Jin, C.; Wang, M.; Gao, X.; Wu, S.; Ding, H.; Bao, Z.; Wang, B.; Hu, J. Deciphering Scavenger Receptors Reveals Key Regulators in the Intestine That Function in Carotenoid Coloration of Leopard Coral Groupers (Plectropomus leopardus). Int. J. Biol. Macromol. 2024, 260, 129387. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wang, Y.; Chen, F. Artificial Breeding and Aquaculture of Plectropomus leopardus; China Ocean Press: Beijing, China, 2018; ISBN 978-7-5210-0110-5. [Google Scholar]
- Wang, J.; Yu, X.; Wu, S.; Jin, C.; Wang, M.; Ding, H.; Song, S.; Bao, Z.; Wang, B.; Hu, J. Identification of Candidate SNPs and Genes Associated with Resistance to Nervous Necrosis Virus in Leopard Coral Grouper (Plectropomus leopardus) Using GWAS. Fish. Shellfish. Immunol. 2024, 144, 109295. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, S.; Ding, H.; Wang, M.; Ma, J.; Xiao, J.; Wang, B.; Bao, Z.; Hu, J. Dietary Antarctic Krill Improves Antioxidant Capacity, Immunity and Reduces Lipid Accumulation, Insights from Physiological and Transcriptomic Analysis of Plectropomus leopardus. BMC Genom. 2024, 25, 210. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, M.; Wang, M.; Wu, S.; Guo, Y.; Gao, Y.; Li, L.; Bao, Z.; Wang, B.; Hu, J. Synchronously Sexual Maturity in Hermaphrodite Fish as Revealed by Transcriptome Analysis in Plectropomus leopardus. Gene 2024, 901, 148166. [Google Scholar] [CrossRef]
- Farkas, T.; Fodor, E.; Kitajka, K.; Halver, J. Response of Fish Membranes to Environmental Temperature. Aquac. Res. 2001, 32, 645–655. [Google Scholar] [CrossRef]
- Kempf, S.; Windisch, H.; Kunz, K.; Pörtner, H.-O.; Mark, F.; Lucassen, M. Hepatic Transcriptomic Responsiveness of Polar Cod, Boreogadus Saida, to Ocean Acidification and Warming. Fishes 2024, 9, 271. [Google Scholar] [CrossRef]
- Baccouch, R.; Shi, Y.; Vernay, E.; Mathelié-Guinlet, M.; Taib-Maamar, N.; Villette, S.; Feuillie, C.; Rascol, E.; Nuss, P.; Lecomte, S.; et al. The Impact of Lipid Polyunsaturation on the Physical and Mechanical Properties of Lipid Membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2023, 1865, 184084. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Dong, M.; Han, X. Effect of Cholesterol on the Fluidity of Supported Lipid Bilayers. Colloids Surf. B Biointerfaces 2020, 196, 111353. [Google Scholar] [CrossRef]
- Bu, S.Y.; Mashek, M.T.; Mashek, D.G. Suppression of Long Chain Acyl-CoA Synthetase 3 Decreases Hepatic de Novo Fatty Acid Synthesis through Decreased Transcriptional Activity. J. Biol. Chem. 2009, 284, 30474–30483. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Zhu, Y.; Jiang, Y.; Feng, J.; Zhao, Z.; Xu, J. Contribution of Elovl5a to Docosahexaenoic Acid (DHA) Synthesis at the Transcriptional Regulation Level in Common Carp, Cyprinus carpio. Animals 2024, 14, 544. [Google Scholar] [CrossRef]
- Liang, X.-F.; Ogata, H.Y.; Oku, H. Effect of Dietary Fatty Acids on Lipoprotein Lipase Gene Expression in the Liver and Visceral Adipose Tissue of Fed and Starved Red Sea Bream Pagrus Major. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, Y.; Miao, X.-M.; Tao, Y.-X.; Xie, L.; Li, Y. A Model Construction of Starvation Induces Hepatic Steatosis and Transcriptome Analysis in Zebrafish Larvae. Biology 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Qiang, J.; He, J.; Gabriel, N.N.; Xu, P. Changes in the Physiological Parameters, Fatty Acid Metabolism, and SCD Activity and Expression in Juvenile GIFT Tilapia (Oreochromis niloticus) Reared at Three Different Temperatures. Fish Physiol. Biochem. 2015, 41, 937–950. [Google Scholar] [CrossRef]
- Arts, M.T.; Kohler, C.C. Health and Condition in Fish: The Influence of Lipids on Membrane Competency and Immune Response. In Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009. [Google Scholar]
- Hsieh, S.L.; Kuo, C.-M. Stearoyl–CoA Desaturase Expression and Fatty Acid Composition in Milkfish (Chanos chanos) and Grass Carp (Ctenopharyngodon idella) during Cold Acclimation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 141, 95–101. [Google Scholar] [CrossRef]
- Hafner, M.; Rezen, T.; Rozman, D. Regulation of Hepatic Cytochromes P450 by Lipids and Cholesterol. Curr. Drug Metab. 2011, 12, 173–185. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, W.; Wang, L.; Zhou, Y.; Nong, Q.; Valencak, T.G.; Wang, Y.; Xie, J.; Shan, T. Cold Exposure Affects Lipid Metabolism, Fatty Acids Composition and Transcription in Pig Skeletal Muscle. Front. Physiol. 2021, 12, 748801. [Google Scholar] [CrossRef]
- Sun, S.; Cao, X.; Gao, J. C24:0 Avoids Cold Exposure-Induced Oxidative Stress and Fatty Acid β-Oxidation Damage. iScience 2021, 24, 103409. [Google Scholar] [CrossRef]
- Huang, S.; Yan, C.; Xu, Y.; Jiao, H.; Zhang, M.; Cheng, J.; Wang, W.; Cui, Z.; Chen, L.; Hu, P.; et al. Integrated Transcriptomic and Epigenomic Analyses to Disclose the Transcriptional Regulatory Mechanisms of Lipid and Energy Metabolism under Cold Stress in Grass Carp. Aquaculture 2025, 595, 741526. [Google Scholar] [CrossRef]
- Chen, T.; Wong, M.K.H.; Chan, B.C.B.; Wong, A.O.L. Mechanisms for Temperature Modulation of Feeding in Goldfish and Implications on Seasonal Changes in Feeding Behavior and Food Intake. Front. Endocrinol. 2019, 10, 133. [Google Scholar] [CrossRef]
- Kehoe, A.S.; Volkoff, H. The Effects of Temperature on Feeding and Expression of Two Appetite-Related Factors, Neuropeptide Y and Cocaine- and Amphetamine-Regulated Transcript, in Atlantic Cod, Gadus Morhua. J. World Aquac. Soc. 2008, 39, 790–796. [Google Scholar] [CrossRef]
- Blanco, A.M.; Soengas, J.L. Leptin Signalling in Teleost Fish with Emphasis in Food Intake Regulation. Mol. Cell. Endocrinol. 2021, 526, 111209. [Google Scholar] [CrossRef] [PubMed]
- Claxton, D.P.; Overway, E.M.; Oeser, J.K.; O’Brien, R.M.; Mchaourab, H.S. Biophysical and Functional Properties of Purified Glucose-6-Phosphatase Catalytic Subunit 1. J. Biol. Chem. 2022, 298, 101520. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Valadés, A.G.; Méndez-Lucas, A.; Vidal-Alabró, A.; Blasco, F.X.; Chillon, M.; Bartrons, R.; Bermúdez, J.; Perales, J.C. Pck1 Gene Silencing in the Liver Improves Glycemia Control, Insulin Sensitivity, and Dyslipidemia in Db/Db Mice. Diabetes 2008, 57, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; He, J.; Yang, H.; Wang, H.; Kpundeh, M.D.; Xu, P.; Zhu, Z.X. Temperature Modulates Hepatic Carbohydrate Metabolic Enzyme Activity and Gene Expression in Juvenile GIFT Tilapia (Oreochromis niloticus) Fed a Carbohydrate-Enriched Diet. J. Therm. Biol. 2014, 40, 25–31. [Google Scholar] [CrossRef]
- Parihar, M.S.; Dubey, A.K.; Javeri, T.; Prakash, P. Changes in Lipid Peroxidation, Superoxide Dismutase Activity, Ascorbic Acid and Phospholipid Content in Liver of Freshwater Catfish Heteropneustes Fossilis Exposed to Elevated Temperature. J. Therm. Biol. 1996, 21, 323–330. [Google Scholar] [CrossRef]
- Ibarz, A.; Martín-Pérez, M.; Blasco, J.; Bellido, D.; de Oliveira, E.; Fernández-Borràs, J. Gilthead Sea Bream Liver Proteome Altered at Low Temperatures by Oxidative Stress. Proteomics 2010, 10, 963–975. [Google Scholar] [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. CB 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a Biomarker and Contributor of Ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-mediated Generation of Lipid Peroxides Enhances Ferroptosis Induced by Erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic Transferrin Plays a Role in Systemic Iron Homeostasis and Liver Ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Wiczew, D.; Szulc, N.; Tarek, M. Molecular Dynamics Simulations of the Effects of Lipid Oxidation on the Permeability of Cell Membranes. Bioelectrochemistry 2021, 141, 107869. [Google Scholar] [CrossRef] [PubMed]
- Morel, I.; Lescoat, G.; Cillard, J.; Pasdeloup, N.; Brissot, P.; Cillard, P. Kinetic Evaluation of Free Malondialdehyde and Enzyme Leakage as Indices of Iron Damage in Rat Hepatocyte Cultures: Involvement of Free Radicals. Biochem. Pharmacol. 1990, 39, 1647–1655. [Google Scholar] [CrossRef]
- Ramaiah, S.K. A Toxicologist Guide to the Diagnostic Interpretation of Hepatic Biochemical Parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef]
- Santos, C.R.; Bastos, F.F.; Dantas, R.F.; Hauser-Davis, R.A.; Rodrigues, L.C.; Cunha Bastos, V.L.F.; Bastos, J.C. Glutathione Peroxidase and Glutathione S-Transferase in Blood and Liver from a Hypoxia-Tolerant Fish under Oxygen Deprivation. Ecotoxicol. Environ. Saf. 2018, 163, 604–611. [Google Scholar] [CrossRef]
- Grim, J.M.; Hyndman, K.A.; Kriska, T.; Girotti, A.W.; Crockett, E.L. Relationship between Oxidizable Fatty Acid Content and Level of Antioxidant Glutathione Peroxidases in Marine Fish. J. Exp. Biol. 2011, 214, 3751–3759. [Google Scholar] [CrossRef]
- Hegazi, M.M.; Attia, Z.I.; Ashour, O.A. Oxidative Stress and Antioxidant Enzymes in Liver and White Muscle of Nile Tilapia Juveniles in Chronic Ammonia Exposure. Aquat. Toxicol. 2010, 99, 118–125. [Google Scholar] [CrossRef]
- Folch-Puy, E.; Panisello, A.; Oliva, J.; Lopez, A.; Castro Benítez, C.; Adam, R.; Roselló-Catafau, J. Relevance of Endoplasmic Reticulum Stress Cell Signaling in Liver Cold Ischemia Reperfusion Injury. Int. J. Mol. Sci. 2016, 17, 807. [Google Scholar] [CrossRef]
- Mininni, A.N.; Milan, M.; Ferraresso, S.; Petochi, T.; Di Marco, P.; Marino, G.; Livi, S.; Romualdi, C.; Bargelloni, L.; Patarnello, T. Liver Transcriptome Analysis in Gilthead Sea Bream upon Exposure to Low Temperature. BMC Genom. 2014, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, X.; Song, G.; Zhou, T.; Long, Y.; Li, Q.; Zhong, S.; Cui, Z. Deficiency in the Membrane Protein Tmbim3a/Grinaa Initiates Cold-Induced ER Stress and Cell Death by Activating an Intrinsic Apoptotic Pathway in Zebrafish. J. Biol. Chem. 2019, 294, 11445–11457. [Google Scholar] [CrossRef] [PubMed]
- Topal, A.; Özdemir, S.; Arslan, H.; Çomaklı, S. How Does Elevated Water Temperature Affect Fish Brain? (A Neurophysiological and Experimental Study: Assessment of Brain Derived Neurotrophic Factor, cFOS, Apoptotic Genes, Heat Shock Genes, ER-Stress Genes and Oxidative Stress Genes). Fish Shellfish. Immunol. 2021, 115, 198–204. [Google Scholar] [CrossRef]
- Venkatesan, A.; Satin, L.S.; Raghavan, M. Roles of Calreticulin in Protein Folding, Immunity, Calcium Signaling and Cell Transformation. Prog. Mol. Subcell. Biol. 2021, 59, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.C.; Zhang, B. Receptor-Mediated Protein Transport in the Early Secretory Pathway. Trends Biochem. Sci. 2007, 32, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Yamashita, S.; Uchino, K.; Suga, T.; Ikeda, T.; Takamatsu, K.; Ishizaki, M.; Koide, T.; Kimura, E.; Mita, S.; et al. Derlin-1 Overexpression Ameliorates Mutant SOD1-Induced Endoplasmic Reticulum Stress by Reducing Mutant SOD1 Accumulation. Neurochem. Int. 2011, 58, 344–353. [Google Scholar] [CrossRef]
- Yagishita, N.; Yamasaki, S.; Nishioka, K.; Nakajima, T. Synoviolin, Protein Folding and the Maintenance of Joint Homeostasis. Nat. Rev. Rheumatol. 2008, 4, 91–97. [Google Scholar] [CrossRef]
- Ding, W.-X.; Yin, X.-M. Sorting, Recognition and Activation of the Misfolded Protein Degradation Pathways through Macroautophagy and the Proteasome. Autophagy 2008, 4, 141–150. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Jin, C.; Wei, C.; Zhong, K.; Gao, Y.; Li, P.; Qu, Z.; Bao, Z.; Wang, B.; Hu, J. The Effects of Acute Temperature Changes on Transcriptomic Responses in the Liver of Leopard Coral Groupers (Plectropomus leopardus). Antioxidants 2025, 14, 223. https://doi.org/10.3390/antiox14020223
Guo Y, Jin C, Wei C, Zhong K, Gao Y, Li P, Qu Z, Bao Z, Wang B, Hu J. The Effects of Acute Temperature Changes on Transcriptomic Responses in the Liver of Leopard Coral Groupers (Plectropomus leopardus). Antioxidants. 2025; 14(2):223. https://doi.org/10.3390/antiox14020223
Chicago/Turabian StyleGuo, Yilan, Chaofan Jin, Cun Wei, Kangning Zhong, Yurui Gao, Peiyu Li, Zhe Qu, Zhenmin Bao, Bo Wang, and Jingjie Hu. 2025. "The Effects of Acute Temperature Changes on Transcriptomic Responses in the Liver of Leopard Coral Groupers (Plectropomus leopardus)" Antioxidants 14, no. 2: 223. https://doi.org/10.3390/antiox14020223
APA StyleGuo, Y., Jin, C., Wei, C., Zhong, K., Gao, Y., Li, P., Qu, Z., Bao, Z., Wang, B., & Hu, J. (2025). The Effects of Acute Temperature Changes on Transcriptomic Responses in the Liver of Leopard Coral Groupers (Plectropomus leopardus). Antioxidants, 14(2), 223. https://doi.org/10.3390/antiox14020223






