Large Fibrous Connective Tissue Reduces Oxidative Stress to Form a Living Cell Scaffold in Adipose Grafts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graft Preparation
2.1.1. Particle Fat and Block Fat
2.1.2. LFC-Included Group
2.1.3. ECM Extraction
2.2. Mitochondrial Transplantation
2.2.1. Mitochondrial Extraction
2.2.2. Mitochondrial Concentration Detection
2.2.3. Mitochondrial Labeling
2.3. Animal Experiments
2.3.1. PF and BF Grafting
2.3.2. ALFC-ECM and DLFC-ECM Grafting
2.3.3. Mitochondrial Transferred Fat Grafting
2.3.4. LFC-Included Fat Grafting
2.4. Histology
2.5. Electron Microscopy
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Transmission Electron Microscopy (TEM)
2.6. Mitochondrial Membrane Potential (MMP) Detection
2.7. Measurement of Oxidative Stress and Antioxidants in the Graft
2.8. Measurement of Adenosine Triphosphate (ATP)
2.9. Oxygen Level Measurement in Grafts
2.10. qRT-PCR Analysis
2.11. In Vitro Experiments
2.11.1. Hypoxic Treatment of Adipose Tissue
2.11.2. Dedifferentiation of Adipocytes
2.11.3. Evaluation of ADSCs Activity with CCK-8
2.11.4. ADSCs Migration
2.11.5. Tri-Lineage Differentiation
Adipogenic Induction
Osteogenic Induction
Chondrogenic Induction
2.12. Transcriptome Sequencing
2.13. Proteomic Identification
2.14. Statistical Analysis
3. Results
3.1. Survival of Adipocytes Around the LFC in Block Fat Grafting
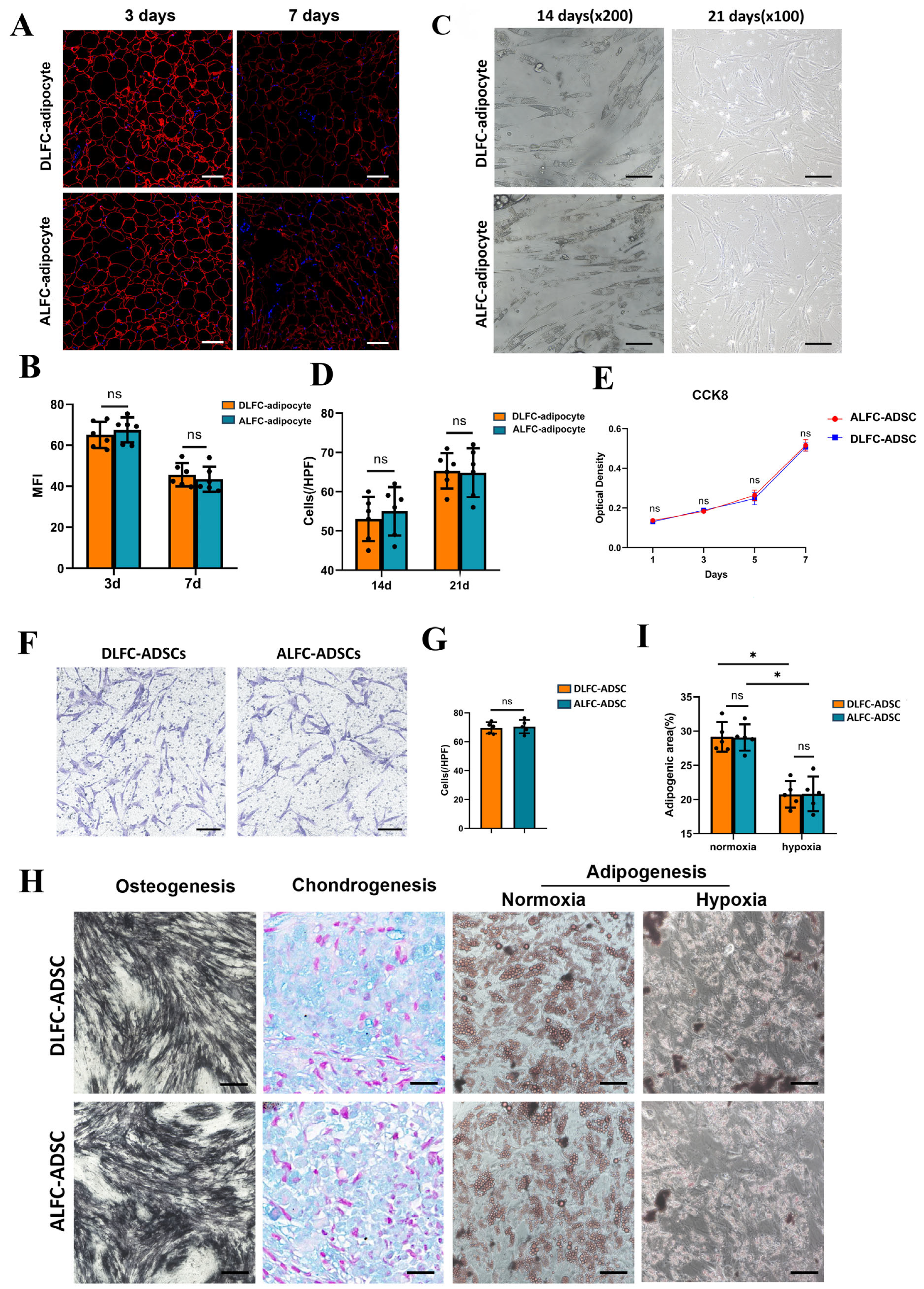
3.2. Characteristics of Adipocytes and ADSCs Show No Significant Differences
3.2.1. ALFC-Adipocyte and DLFC-Adipocyte
3.2.2. ALFC-ADSCs and DLFC-ADSCs
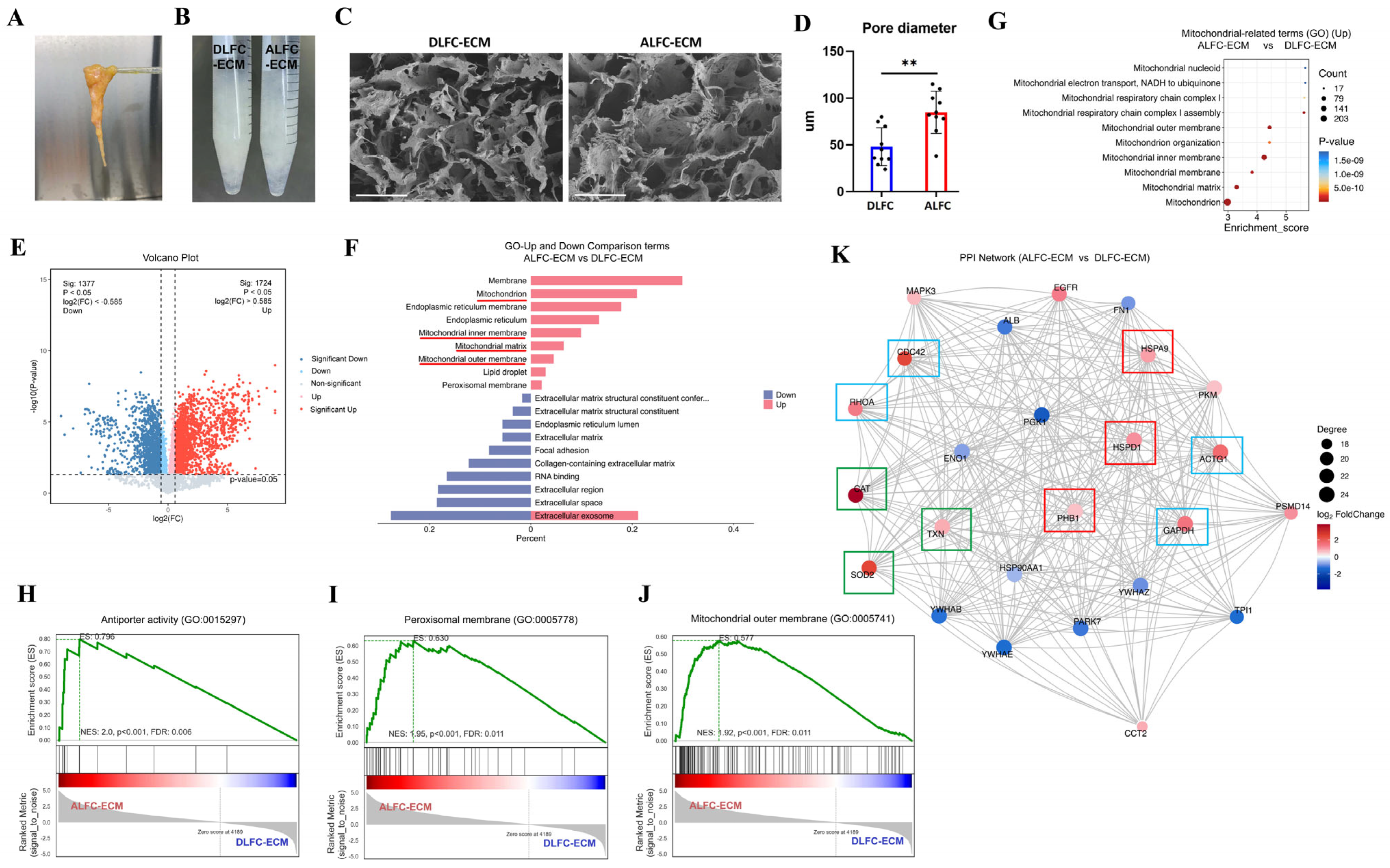
3.3. ALFC-ECM Contains More Mitochondria and Redox-Related Proteins than DLFC-ECM
3.4. Different Mitochondrial Distribution Between ALFC and DLFC Adipocytes After Grafting
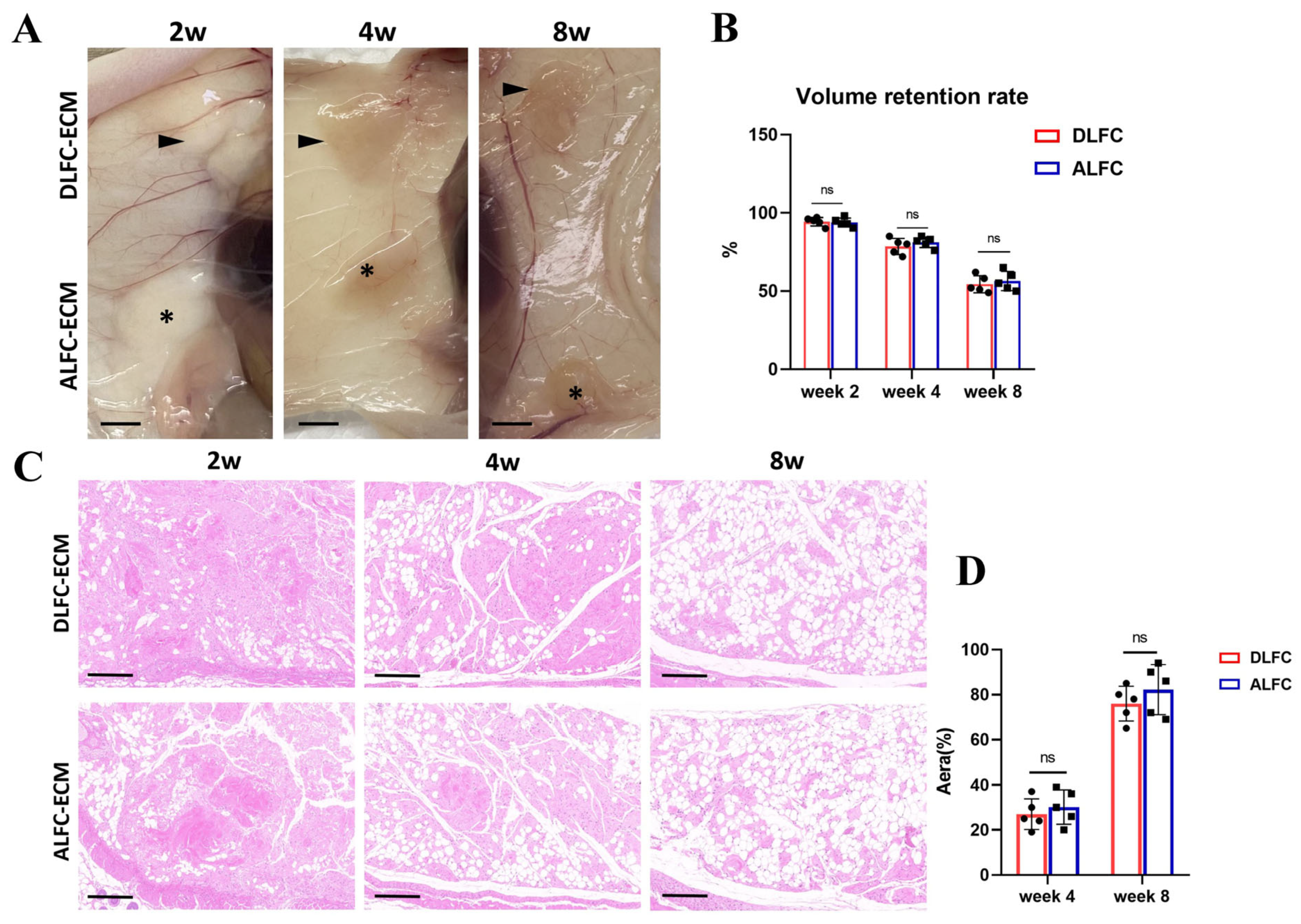
3.5. ALFC-ECM and DLFC-ECM Show No Significant Difference in Inducing Adipose Tissue Regeneration
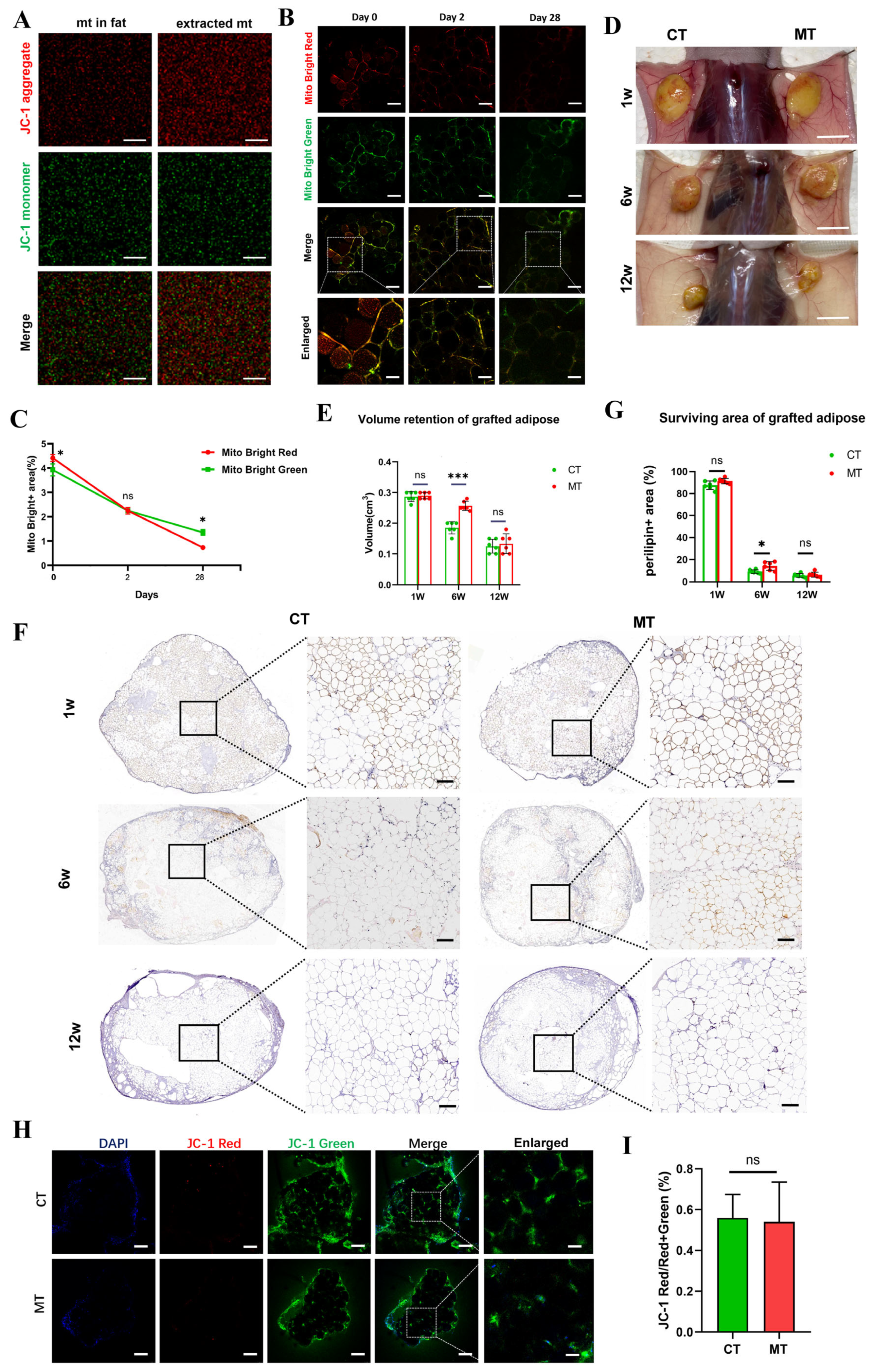
3.6. Mitochondrial Transplantation Can Promote Survival of Transplanted Fat by Reducing Oxidative Stress in the Early Stage
3.6.1. Free Mitochondria Survive Only Short-Term After Transplantation
3.6.2. Mitochondrial Transplantation Increases Early Volume Retention Rate
3.6.3. Transplanted Mitochondria Reduce Inflammation by Clearing ROS
3.7. Long-Term Survival of Mitochondria in the LFC Promotes Long-Term Fat Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debuc, B.; Gendron, N.; Cras, A.; Rancic, J.; Philippe, A.; Cetrulo, C.L., Jr.; Lellouch, A.G.; Smadja, D.M. Improving Autologous Fat Grafting in Regenerative Surgery through Stem Cell-Assisted Lipotransfer. Stem Cell Rev. Rep. 2023, 19, 1726–1754. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Jiang, Z.; Che, D.; Chen, G.; Shen, S.; Ding, W.; Zhang, Y.; Xiang, M.; Zhu, B.; Xie, J.; et al. Application of free serratus anterior muscle-fascial composite tissue flap and facial lipofilling in repairing progressive hemifacial atrophy. Asian J. Surg. 2024, 47, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Trotzier, C.; Sequeira, I.; Auxenfans, C.; Mojallal, A.A. Fat Graft Retention: Adipose Tissue, Adipose-Derived Stem Cells, and Aging. Plast. Reconstr. Surg. 2023, 151, 420e–431e. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Suga, H.; Eto, H. Adipose-derived stem/progenitor cells: Roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen. Med. 2009, 4, 265–273. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, T.; Liu, Y.; Liu, C.; Lin, Y.; Li, H.; Zhang, T.; Wang, Q.; Mu, D. Pyrroloquinoline Quinone (PQQ) Improves Long-term Survival of Fat Grafts by Alleviating Oxidative Stress and Promoting Angiogenesis During the Early Phase After Transplantation. Aesthetic Surg. J. 2023, 44, NP104–NP118. [Google Scholar] [CrossRef]
- Peer, L. Transplantation of Tissues: Skin, Cornea, Fat, Nerves, Teeth, Blood Vessels, Endocrine Glands, Organs, Peritoneum, Cancer Cells. Med. J. Aust. 1960, 1, 659–660. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Z.; Dai, Y.; Zhao, S.; Chen, H.; Wang, S.; Xie, H. Autologous fat grafting for postoperative breast reconstruction: A systemic review. Regen. Ther. 2024, 26, 1010–1017. [Google Scholar] [CrossRef]
- Puistola, P.; Kethiri, A.; Nurminen, A.; Turkki, J.; Hopia, K.; Miettinen, S.; Mörö, A.; Skottman, H. Cornea-Specific Human Adipose Stem Cell-Derived Extracellular Matrix for Corneal Stroma Tissue Engineering. ACS Appl. Mater. Interfaces 2024, 16, 15761–15772. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, Y.Q.; Cai, J.; Huang, S.S.; Wang, Y.F.; Yuan, S.S.; Wang, J.L.; Shi, K.Q.; Deng, J.J. Human Adipose Tissue Lysate-Based Hydrogel for Lasting Immunomodulation to Effectively Improve Spinal Cord Injury Repair. Small 2024, 20, e2304318. [Google Scholar] [CrossRef]
- Saha, A.; Kolonin, M.G.; DiGiovanni, J. Obesity and prostate cancer-microenvironmental roles of adipose tissue. Nat. Rev. Urol. 2023, 20, 579–596. [Google Scholar] [CrossRef]
- Ramaut, L.; Moonen, L.; Laeremans, T.; Aerts, J.L.; Geeroms, M.; Hamdi, M. Push-Through Filtration of Emulsified Adipose Tissue Over a 500-µm Mesh Significantly Reduces the Amount of Stromal Vascular Fraction and Mesenchymal Stem Cells. Aesthetic Surg. J. 2023, 43, NP696–NP703. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, H.; Sun, Z.; Wang, S.; Zhao, X.; Zhao, B.; Zhang, A. Exploring Preclinical Experiments with Different Fat Types for Autologous Fat Grafting. Aesthetic Plast. Surg. 2024, 48, 2330–2342. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yue, Q.; Fu, S.; Liu, C.; Luan, J. Decellularized adipose-derived matrix from Superficial layers of abdominal adipose tissue exhibits superior capacity of adipogenesis compared to deep layers. Mater. Today Biol. 2024, 28, 101235. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Han, X.; Li, F. The Importance of Protecting the Structure and Viability of Adipose Tissue for Fat Grafting. Plast. Reconstr. Surg. 2022, 149, 1357–1368. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Qi, Y.; Gu, Y.; Liu, Z.; Ma, G.E. Volume Retention After Facial Fat Grafting and Relevant Factors: A Systematic Review and Meta-analysis. Aesthetic Plast. Surg. 2021, 45, 506–520. [Google Scholar] [CrossRef]
- Wen, X.; Tang, L.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Role of Mitophagy in Regulating Intestinal Oxidative Damage. Antioxidants 2023, 12, 480. [Google Scholar] [CrossRef]
- Fontdevila, J.; Guisantes, E.; Martínez, E.; Prades, E.; Berenguer, J. Double-Blind Clinical Trial to Compare Autologous Fat Grafts versus Autologous Fat Grafts with PDGF: No Effect of PDGF. Plast. Reconstr. Surg. 2014, 134, 219e–230e. [Google Scholar] [CrossRef]
- Willemsen, J.C.N.; Van Dongen, J.; Spiekman, M.; Vermeulen, K.M.; Harmsen, M.C.; van der Lei, B.; Stevens, H.P.J. The Addition of Platelet-Rich Plasma to Facial Lipofilling: A Double-Blind, Placebo-Controlled, Randomized Trial. Plast. Reconstr. Surg. 2018, 141, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, G.H. The Safety and Efficacy of Cell-Assisted Fat Grafting to Traditional Fat Grafting in the Anterior Mid-Face: An Indirect Assessment by 3D Imaging. Aesthetic Plast. Surg. 2015, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, X.; Li, Y.; Xu, M.; Yao, Y.; Liu, K.; Ma, C.; Zhang, Y.; Ru, J.; He, Y.; et al. Macrophage-mediated extracellular matrix remodeling after fat grafting in nude mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Li, F.W.; Dong, Y.X.; Xie, W.J.; Wang, H.B. PPAR-γ regulates the polarization of M2 macrophages to improve the microenvironment for autologous fat grafting. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23613. [Google Scholar] [CrossRef]
- Ali Pour, P.; Kenney, M.C.; Kheradvar, A. Bioenergetics Consequences of Mitochondrial Transplantation in Cardiomyocytes. J. Am. Heart Assoc. 2020, 9, e014501. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Xiang, Y.; Zhang, X. Therapeutic role of adipose-derived mesenchymal stem cells-derived extracellular vesicles in rats with obstructive sleep apnea hypopnea syndrome. Regen. Ther. 2023, 22, 210–223. [Google Scholar] [CrossRef]
- Shifa Ul Haq, H.M.; Ashfaq, R.; Mehmood, A.; Shahid, W.; Azam, G.; Azam, M.; Tasneem, S.; Akram, S.J.; Malik, K.; Riazuddin, S. Priming with caffeic acid enhances the potential and survival ability of human adipose-derived stem cells to counteract hypoxia. Regen. Ther. 2023, 22, 115–127. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [Google Scholar] [CrossRef]
- He, R.; Cui, M.; Lin, H.; Zhao, L.; Wang, J.; Chen, S.; Shao, Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018, 199, 122–130. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernández-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. Commun. Free. Radic. Res. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pietruski, P.; Paskal, W.; Paluch, Ł.; Paskal, A.M.; Nitek, Ż.; Włodarski, P.; Walecki, J.; Noszczyk, B. The Impact of N-Acetylcysteine on Autologous Fat Graft: First-in-Human Pilot Study. Aesthetic Plast. Surg. 2021, 45, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Kerfant, N.; Albacete, G.; Guernec, A.; Inizan, M.; Amerand, A.; Hu, W.; Theron, M.; Pichavant-Rafini, K. Fat grafting: Early hypoxia, oxidative stress, and inflammation developing prior to injection. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1775–1784. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell 2020, 183, 76–93.e22. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, Y.; Liu, B.; Wang, G.; Zhang, Y. TLR4 knockout upregulates the expression of Mfn2 and PGC-1α in a high-fat diet and ischemia-reperfusion mice model of liver injury. Life Sci. 2020, 254, 117762. [Google Scholar] [CrossRef]
- Han, X.; Li, B.; Wang, W.; Feng, B.; Tang, Q.; Qi, Y.; Zhao, R.; Qiu, W.; Zhao, S.; Pan, Z.; et al. Cerium Vanadate Nanozyme with pH-Dependent Dual Enzymatic Activity for Glioblastoma Targeted Therapy and Postradiotherapy Damage Protection. ACS Nano 2024, 18, 19836–19853. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, X.; Yang, Q.; Zhao, J.; Zhou, Q.; Zhou, Y. The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells. Front. Oncol. 2021, 11, 672781. [Google Scholar] [CrossRef]
- Nakamura, Y.; Park, J.H.; Hayakawa, K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol. 2020, 324, 113114. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Wang, L.L.; Chen, Y.Y. Mitochondrial Transfer in Cardiovascular Disease: From Mechanisms to Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 771298. [Google Scholar] [CrossRef]
- Hayakawa, K.; Chan, S.J.; Mandeville, E.T.; Park, J.H.; Bruzzese, M.; Montaner, J.; Arai, K.; Rosell, A.; Lo, E.H. Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 2018, 36, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Nakhle, J.; Griessinger, E.; Vignais, M.L. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle 2018, 17, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Shay, J.W. Mitochondrial transformation of mammalian cells. Nature 1982, 295, 605–607. [Google Scholar] [CrossRef] [PubMed]
- McCully, J.D.; Cowan, D.B.; Pacak, C.A.; Toumpoulis, I.K.; Dayalan, H.; Levitsky, S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H94–H105. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Kumar, V. A STING to inflammation and autoimmunity. J. Leukoc. Biol. 2019, 106, 171–185. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Yan, C.; Pu, K.; Wu, M.; Bai, J.; Li, Y.; Wang, Q. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav. Brain Res. 2019, 356, 322–331. [Google Scholar] [CrossRef]
- Rahman, J.; Rahman, S. Mitochondrial medicine in the omics era. Lancet 2018, 391, 2560–2574. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Yao, Y.; Zhao, T.; Chen, Y.Y.; Shen, Y.L.; Wang, L.L.; Zhu, Y. Stem cell-derived mitochondria transplantation: A novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res. Ther. 2018, 9, 106. [Google Scholar] [CrossRef]
- Lin, R.Z.; Im, G.B.; Luo, A.C.; Zhu, Y.; Hong, X.; Neumeyer, J.; Tang, H.W.; Perrimon, N.; Melero-Martin, J.M. Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature 2024, 629, 660–668. [Google Scholar] [CrossRef]
- Schipper, J.A.M.; Vriend, L.; Tuin, A.J.; Dijkstra, P.U.; Schepers, R.H.; van der Lei, B.; Jansma, J.; Harmsen, M.C. Supplementation of Facial Fat Grafting to Increase Volume Retention: A Systematic Review. Aesthetic Surg. J. 2022, 42, NP711–NP727. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M.; Manes-Gravina, E.; Landi, F.; Bernabei, R.; Marzetti, E. Circulating Mitochondrial DNA at the Crossroads of Mitochondrial Dysfunction and Inflammation During Aging and Muscle Wasting Disorders. Rejuvenation Res. 2018, 21, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Merrick, D.; Sakers, A.; Irgebay, Z.; Okada, C.; Calvert, C.; Morley, M.P.; Percec, I.; Seale, P. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 2019, 364, 6438. [Google Scholar] [CrossRef] [PubMed]
- Egro, F.M.; Roy, E.; Rubin, J.P.; Coleman, S.R. Evolution of the Coleman Technique. Plast. Reconstr. Surg. 2022, 150, 329e–336e. [Google Scholar] [CrossRef]
- Ziegler, M.E.; Khabaz, K.; Khoshab, N.; Halaseh, F.F.; Chnari, E.; Chen, S.; Baldi, P.; Evans, G.R.D.; Widgerow, A.D. Combining Allograft Adipose and Fascia Matrix as an Off-the-Shelf Scaffold for Adipose Tissue Engineering Stimulates Angiogenic Responses and Activates a Proregenerative Macrophage Profile in a Rodent Model. Ann. Plast. Surg. 2023, 91, 294–300. [Google Scholar] [CrossRef]
- Chaurasiya, V.; Nidhina Haridas, P.A.; Olkkonen, V.M. Adipocyte-endothelial cell interplay in adipose tissue physiology. Biochem. Pharmacol. 2024, 222, 116081. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Huang, J.; Qiu, G.; Zhang, T.; Wei, Q.; He, C.; Zhou, D.; Zhao, M.; Chen, C.; et al. HIF-2α inhibition disrupts leukemia stem cell metabolism and impairs vascular microenvironment to enhance chronic myeloid leukemia treatment. Cancer Lett. 2024, 597, 217060. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 65. [Google Scholar] [CrossRef]
- Nascimento-Dos-Santos, G.; de-Souza-Ferreira, E.; Linden, R.; Galina, A.; Petrs-Silva, H. Mitotherapy: Unraveling a Promising Treatment for Disorders of the Central Nervous System and Other Systemic Conditions. Cells 2021, 10, 1827. [Google Scholar] [CrossRef]
- Tang, B.L. MIRO GTPases in Mitochondrial Transport, Homeostasis and Pathology. Cells 2015, 5, 1. [Google Scholar] [CrossRef]
- Mao, J.; Li, C.; Wu, F.; She, Z.; Luo, S.; Chen, X.; Wen, C.; Tian, J. MSC-EVs transferring mitochondria and related components: A new hope for the treatment of kidney disease. Front. Immunol. 2022, 13, 978571. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, F.; Ortega, M.; Velarde, F.; Parra, E.; Gallardo, S.; Barba, D.; Soto, L.; Peña, G.; Pedroza, L.A.; Jorgensen, C.; et al. Primary allogeneic mitochondrial mix (PAMM) transfer/transplant by MitoCeption to address damage in PBMCs caused by ultraviolet radiation. BMC Biotechnol. 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Pinkert, C.A.; Irwin, M.H.; Johnson, L.W.; Moffatt, R.J. Mitochondria transfer into mouse ova by microinjection. Transgenic Res. 1997, 6, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Sagullo, E.; Case, D.; Zheng, X.; Li, Y.; Hong, J.S.; TeSlaa, T.; Patananan, A.N.; McCaffery, J.M.; Niazi, K.; et al. Mitochondrial Transfer by Photothermal Nanoblade Restores Metabolite Profile in Mammalian Cells. Cell Metab. 2016, 23, 921–929. [Google Scholar] [CrossRef]
- Lei, H.; Zheng, D.; Ma, G.E.; Li, Q. Assessment of effects of physical or chemical factors on fat particle viability by glucose transport test. Ann. Plast. Surg. 2014, 73, 225–230. [Google Scholar] [CrossRef]
- Zhang, T.G.; Miao, C.Y. Mitochondrial transplantation as a promising therapy for mitochondrial diseases. Acta Pharm. Sin. B 2023, 13, 1028–1035. [Google Scholar] [CrossRef]
- Rickenbach, C.; Gericke, C. Specificity of Adaptive Immune Responses in Central Nervous System Health, Aging and Diseases. Front. Neurosci. 2021, 15, 806260. [Google Scholar] [CrossRef]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef]
- Van der Valk, J. Fetal bovine serum—A cell culture dilemma. Science 2022, 375, 143–144. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Xu, Y.; Yang, H.; Liao, Y.; Chang, Q.; Cai, J.; Lu, F.; Gao, J.; Yuan, Y. Lipophagy Enhances Fat Graft Retention by Stimulating Dedifferentiation of Mature Adipocytes and Modulating Inflammation. Plast. Reconstr. Surg. 2024; in press. [Google Scholar] [CrossRef]
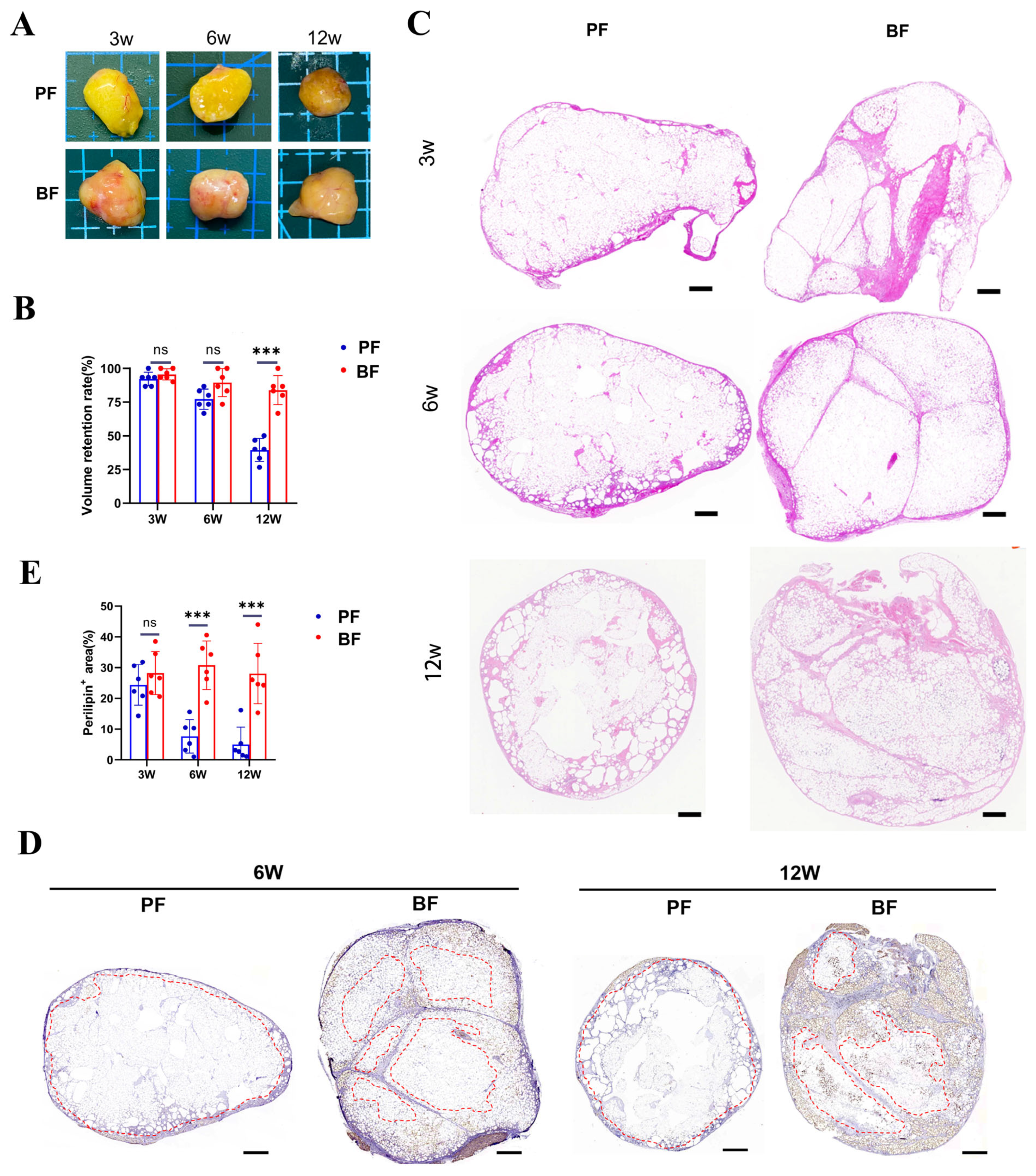



| Particle Fat | Block Fat | |
|---|---|---|
| Structural integrity | ||
| 3 weeks | 4.1 ± 0.61 | 4.5 ± 0.49 * |
| 6 weeks | 3.30 ± 0.45 | 4.65 ± 0.47 *** |
| 12 weeks | 1.25 ± 0.42 | 4.30 ± 0.45 *** |
| Necrotic area | ||
| 3 weeks | 1.1 ± 0.53 | 1.05 ± 0.49 |
| 6 weeks | 4.10 ± 0.53 | 1.35 ± 0.47 *** |
| 12 weeks | 4.95 ± 0.21 | 2.75 ± 0.87 *** |
| Vacuoles | ||
| 3 weeks | 0.6 ± 0.72 | 0.3 ± 0.45 |
| 6 weeks | 2.40 ± 0.48 | 0.40 ± 0.48 *** |
| 12 weeks | 3.9 ± 0.53 | 2.45 ± 0.84 *** |
| Fibrosis | ||
| 3 weeks | 0.9 ± 0.61 | 0.6 ± 0.48 |
| 6 weeks | 2.15 ± 0.35 | 0.70 ± 0.44 *** |
| 12 weeks | 3.85 ± 0.35 | 2.45 ± 0.84 *** |
| Inflammation | ||
| 3 weeks | 0.85 ± 0.64 | 0.4 ± 0.48 * |
| 6 weeks | 2.55 ± 0.49 | 0.5 ± 0.49 *** |
| 12 weeks | 2.6 ± 0.65 | 2.1 ± 0.61 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Q.; Cao, Z.; Zhang, T.; Yin, N.; Liu, L. Large Fibrous Connective Tissue Reduces Oxidative Stress to Form a Living Cell Scaffold in Adipose Grafts. Antioxidants 2025, 14, 270. https://doi.org/10.3390/antiox14030270
Yue Q, Cao Z, Zhang T, Yin N, Liu L. Large Fibrous Connective Tissue Reduces Oxidative Stress to Form a Living Cell Scaffold in Adipose Grafts. Antioxidants. 2025; 14(3):270. https://doi.org/10.3390/antiox14030270
Chicago/Turabian StyleYue, Qiang, Zilong Cao, Tiran Zhang, Ningbei Yin, and Liqiang Liu. 2025. "Large Fibrous Connective Tissue Reduces Oxidative Stress to Form a Living Cell Scaffold in Adipose Grafts" Antioxidants 14, no. 3: 270. https://doi.org/10.3390/antiox14030270
APA StyleYue, Q., Cao, Z., Zhang, T., Yin, N., & Liu, L. (2025). Large Fibrous Connective Tissue Reduces Oxidative Stress to Form a Living Cell Scaffold in Adipose Grafts. Antioxidants, 14(3), 270. https://doi.org/10.3390/antiox14030270






