Mechanistic Insights and Analytical Advances in Food Antioxidants: A Comprehensive Review of Molecular Pathways, Detection Technologies, and Nutritional Applications
Abstract
:1. Introduction
2. Mechanisms of Action of Food Antioxidants
2.1. Basic Concept and Classification of Antioxidants
- (A)
- Classification by natureAntioxidants are divided into enzymatic and non-enzymatic categories (Figure 1A).
- Enzymatic antioxidants:
- Superoxide dismutase (SOD) catalyzes dismutation of superoxide radicals () with a catalytic efficiency Kcat/Km = 2 × 109 M−1S−1 [11].
- Catalase (CAT) and glutathione peroxidase (GPx) degrade hydrogen peroxide (H2O2) and lipid hydroperoxides, respectively.
- Glutathione reductase (GR) regenerates reduced glutathione (GSH) from oxidized glutathione (GSSG).
- Non-enzymatic antioxidants:
- Nutrient antioxidants include fat-soluble vitamin E (α-tocopherol, BDE = 78 kcal/mol [3]), water-soluble vitamin C (k = 1.1 × 1010 M−1S−1), carotenoids (e.g., β-carotene), and ω-3/6 fatty acids.
- Metabolic antioxidants comprise endogenous molecules like glutathione (GSH), lipoic acid, and L-arginine.
- (B)
- Classification by sourceAntioxidants originate from three primary sources (Figure 1B):
- Endogenous antioxidants:
- Synthesized intracellularly (e.g., GSH, uric acid) or through enzymatic pathways (SOD, CAT).
- Metal-binding proteins: ferritin (sequesters Fe3+), ceruloplasmin (binds Cu2+), and myoglobin (stores Fe2+).
- Dietary antioxidants:
- Derived from food sources: vitamin C (citrus fruits), vitamin E (nuts, seeds), polyphenols (green tea, berries), and carotenoids (tomatoes, carrots).
- Synthetic antioxidants:
- Industrially produced compounds like butylated hydroxytoluene (BHT) and tertiary butylhydroquinone (TBHQ), regulated under 21 CFR 172.115 (FDA) and EC/1333 (EFSA) [31].
- (C)
- Classification by mechanism of actionAntioxidants operate through four distinct mechanistic pathways (Figure 1C):
- Catalytic neutralization of ROS:
- Enzymes like SOD and CAT directly convert ROS into less harmful species.
- Example: SOD reduces the superoxide radical (O2−) to hydrogen peroxide (H2O2) [11].
- Metal ion chelation:
- Prevent Fenton reactions by binding transition metals (Fe2+, Cu+).
- Example: curcumin chelates Fe3+ with a stability constant log K = 8.2 [18], inhibiting hydroxyl radical (HO) formation: Fe2+ + H2O2 → Fe3+ + HO + OH− (inhibited).
- Chain-breaking radical scavenging:
- Donate hydrogen atoms to terminate radical chain reactions.
- Example: α-tocopherol neutralizes lipid peroxyl radicals (LOO∙) via: LOO + α-TOH → LOOH + α-TO. Bond dissociation energy (BDE) of the phenolic O–H bond is 78 kcal/mol [3].
- ROS quenching via energy absorption:
- Carotenoids (e.g., lycopene) and anthocyanins dissipate excess energy from singlet oxygen (1O2) through conjugated double-bond systems.

2.2. Effects of Antioxidants on Oxidative Stress
- Primary prevention: Antioxidants enhance endogenous defense systems by activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway, thereby upregulating phase II detoxifying enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [31]. This biological amplification effect significantly improves organisms’ capacity to neutralize reactive oxygen species (ROS) during stress conditions.
- Secondary interception: Through dual mechanisms of hydrogen atom transfer (HAT) and single electron transfer (SET), antioxidants directly scavenge free radicals (•OH, ROO•) and stabilize ROS (1O2, H2O2). Notably, lipophilic antioxidants (e.g., α-tocopherol) preferentially localize in cell membranes to prevent lipid peroxidation chain reactions, while hydrophilic species (e.g., ascorbic acid) function in cytoplasmic compartments [36,37].
- Tertiary repair: Certain antioxidants facilitate the repair of oxidized biomolecules through redox cycling mechanisms. For instance, glutathione reductase utilizes NADPH to regenerate reduced glutathione (GSH) from its oxidized form (GSSG), maintaining cellular redox homeostasis [38].
2.3. Mechanisms of Antioxidant Action
2.4. Comparative Efficacy and Limitations of Antioxidants
2.5. Dynamic Thresholds and Metabolic Networks
- Organelle-Specific Redox Thresholds
- 2.
- Antioxidant Metabolic Synergy
3. Analytical Strategies for Antioxidant Characterization
3.1. Antioxidant Activity Assays: Functional Screening
3.1.1. DPPH Radical Scavenging Assay
3.1.2. ORAC (Oxygen Radical Absorbance Capacity)
3.1.3. FRAP (Ferric Reducing Antioxidant Power)
3.1.4. Spectrophotometric Assays
3.2. Antioxidant Component Analysis (Targeted Identification)
3.2.1. HPLC with UV/FLD Detection
3.2.2. LC-MS/MS for Structural Elucidation
3.2.3. GC-MS for Volatile Antioxidants
- Derivatization: Silylation of non-volatile phenolics (e.g., gallic acid → TMS derivative).
- Separation: DB-5MS column, splitless injection.
- Quantification: Selected ion monitoring (SIM) mode.
3.3. Integrated Approaches and Emerging Technologies
3.3.1. Bioassay-Guided Fractionation
- Screen crude extract via DPPH/ORAC.
- Separate active fractions by preparative HPLC.
- Identify actives via NMR/MS.
3.3.2. AI-Driven Antioxidant Discovery
- Train QSAR models on ORAC data (n = 1200 compounds).
- Predict novel antioxidants (e.g., marine peptides).
3.3.3. Microscale Antioxidant Capacity Assays
3.4. Guidelines for Method Selection
4. Research Progress in the Applications of Antioxidants
4.1. The Use of Antioxidants in Different Animal Species
4.1.1. Poultry
4.1.2. Cud Chewers
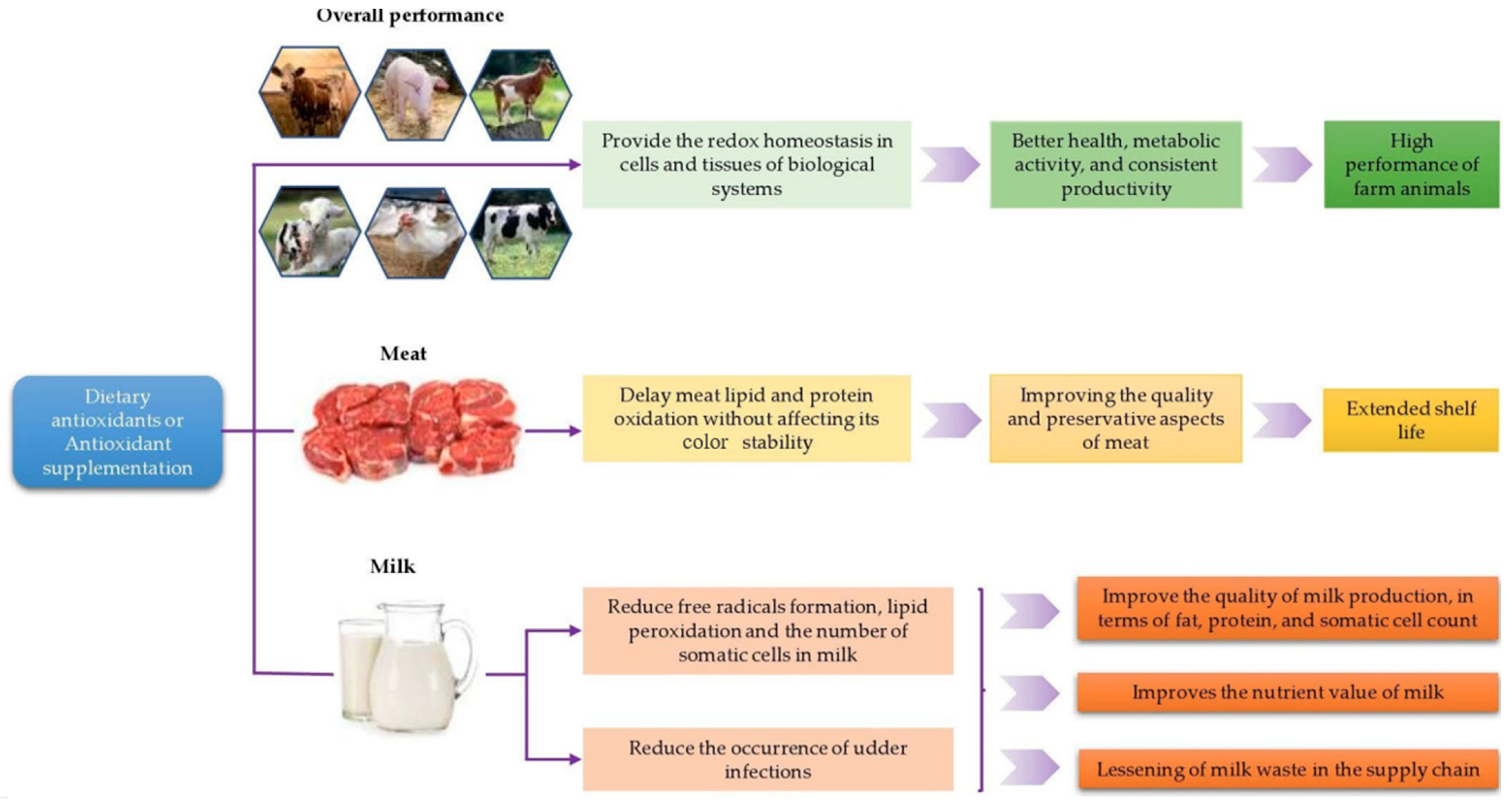
4.1.3. Farm Animals
4.1.4. In Vivo Metabolic Tracking
4.1.5. Global Antioxidant Regulatory Framework and Compliance Strategy
- European Union:
- United States:
- China:
4.2. Applications of Antioxidants in Human Nutrition
4.2.1. Bioavailability and Metabolic Dynamics
4.2.2. Dose–Response Relationships and Clinical Outcomes
- Resveratrol: A 6-month RCT (n = 75) demonstrated a U-shaped dose response: 100 mg/day improved endothelial function (flow-mediated dilation [FMD] +2.1%, p < 0.05), while 1000 mg/day elevated liver enzymes (ALT +18%) and attenuated benefits [25].
- Curcumin: In metabolic syndrome patients (n = 117), 1 g/day supplementation reduced CRP (−21%) and IL-6 (−18%) but failed to improve insulin sensitivity (HOMA-IR Δ = −0.3, p = 0.12) [16].
- Vitamin E: The SELECT trial (n = 35,533) revealed α-tocopherol supplementation (400 IU/day) increased prostate cancer risk (HR = 1.17, 95% CI: 1.004–1.36), whereas dietary γ-tocopherol from nuts showed protective effects (RR = 0.89) [61].
4.2.3. Population-Specific Responses
- Aging populations: A 2-year trial in elderly subjects (n = 289) showed combined vitamin C (500 mg) and EGCG (300 mg) supplementation reduced oxidative DNA damage (8-OHdG −28%, p < 0.001) but had no impact on cognitive decline (MMSE Δ = +0.2, p = 0.64) [25].
- Obesity: In obese individuals (BMI > 30), lycopene from processed tomatoes exhibited 32% higher bioavailability than raw sources, correlating with improved endothelial function (FMD +1.8%, p = 0.03) [21].
4.2.4. Safety and Long-Term Considerations
- Hepatotoxicity: High-dose EGCG (800 mg/day) induced hepatic CYP3A4 activity by 34%, potentially altering drug metabolism (e.g., simvastatin clearance +22%) [11].
- Pro-oxidant effects: In vitro studies reveal dose-dependent pro-oxidant activity for 22% of commercial polyphenol supplements at concentrations > 100 μM, as detected via microfluidic DPPH screening [45].
4.2.5. Food Matrix and Synergistic Effects
- Thermal processing: Lycopene bioavailability increases 1.5-fold in heat-treated tomato paste versus raw tomatoes due to cellular matrix disruption [21].
- Lipid synergy: Co-consumption of astragalus polysaccharides with dietary lipids enhances immunomodulatory effects 2.3-fold compared with isolated administration [10].
5. Conclusions and Outlook
5.1. Conclusions
- The mechanisms of antioxidants in nutrition can be explained from many aspects, including scavenging free radicals, preventing lipid peroxidation, inhibiting the activity of oxidase, chelating metal ions, etc. In addition, antioxidants can also protect the integrity of biological cell membranes and biomacromolecules by reducing oxidative stress responses so that biological body functions can be normal. Various studies have shown that different types of antioxidants have different mechanisms of action, but the ultimate goal of using antioxidants is maintaining the nutritional value of food and human health.
- The methods of detecting antioxidants include liquid chromatography, gas chromatography, liquid chromatography–mass spectrometry, and meteorological chromatography–mass spectrometry. Among them, gas chromatography and gas chromatography–mass spectrometry are the main detection methods used at present. The advantages of these two methods are fast analysis, high separation efficiency, sensitive detection, high precision, low detection limit, and accurate results. The results showed that these methods can be used for the quantitative analysis of antioxidants and can also evaluate the metabolism and residue of antioxidants in organisms. However, with the development of science and technology, it is necessary to continuously optimize the existing detection methods and study more simple, convenient, and economical new methods for use.
- Antioxidants are widely used to improve the nutritional value of food, prevent food oxidation and deterioration, and ensure healthy biological growth. Research and application of antioxidants in animal husbandry have made a lot of progress. Various studies have concluded that for maintaining the freshness of complementary foods, the rational use of antioxidants is necessary. Antioxidants can also reduce biological oxidative stress and prevent biological diseases caused by oxidative stress. In addition, with the improvement of people’s environmental awareness, the research and application of more natural plant sources and green, environmentally friendly antioxidants are undoubtedly the top priority of current antioxidant research.
5.2. Outlook
- Precision targeting via redox thresholds:Current dosing strategies ignore subcellular heterogeneity. Key steps include the following:
- (1)
- Mapping organelle-specific thresholds using genetically encoded ROS sensors (e.g., Mito-roGFP).
- (2)
- Developing delivery systems (e.g., mitochondrial-targeted nanoparticles) to confine antioxidants to high-ROS compartments.
- Engineering synergistic formulationsOptimizing antioxidant combinations requires
- (1)
- High-throughput screening of metabolite interactions (e.g., DHA-TrxR axis).
- (2)
- Kinetic modeling of Nrf2–GSH–NF-κB networks.
- (3)
- Validation in context-specific oxidative stress models (sepsis, metabolic syndrome).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Salami, S.A.; Guinguina, A.; Agboola, J.O.; Omede, A.A.; Agbonlahor, E.M.; Tayyab, U. Review: In Vivo and Postmortem Effects of Feed Antioxidants in Livestock: A Review of the Implications on Authorization of Antioxidant Feed Additives. Animal 2016, 10, 1375–1390. [Google Scholar]
- Shastak, Y.; Pelletier, W. Review of Liquid Vitamin A and E Formulations in Veterinary and Livestock Production: Applications and Perspectives. Vet. Sci. 2024, 11, 421. [Google Scholar] [CrossRef]
- Nakata, T.; Kyoui, D.; Takahashi, H.; Kimura, B.; Kuda, T. Inhibitory Effects of Soybean Oligosaccharides and Water-Soluble Soybean Fibre on Formation of Putrefactive Compounds from Soy Protein by Gut Microbiota. Int. J. Biol. Macromol. 2017, 97, 173–180. [Google Scholar]
- Chen, H.; Liu, L.; Zhu, J.; Xu, B.; Li, R. Effect of Soybean Oligosaccharides on Blood Lipid, Glucose Levels and Antioxidant Enzymes Activity in High Fat Rats. Food Chem. 2010, 119, 1633–1636. [Google Scholar]
- Xie, S.; Zhu, J.; Zhang, Y.; Shi, K.; Shi, Y.; Ma, X. Effects of Soya Oligosaccharides and Soya Oligopeptides on Lipid Metabolism in Hyperlipidaemic Rats. Br. J. Nutr. 2012, 108, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cai, S.; Ma, J. Evaluation of Cardio-Protective Effect of Soybean Oligosaccharides. Gene 2015, 555, 329–334. [Google Scholar]
- Graziani, V.; Scognamiglio, M.; Esposito, A.; Fiorentino, A.; D’Abrosca, B. Chemical Diversity and Biological Activities of the Saponins Isolated from Astragalus Genus: Focus on Astragaloside IV. Phytochem. Rev. 2019, 18, 1133–1166. [Google Scholar]
- Park, H.S.; Lim, J.H.; Kim, M.Y.; Kim, Y.; Hong, Y.A.; Choi, S.R.; Chung, S.; Kim, H.W.; Choi, B.S.; Kim, Y.S.; et al. Resveratrol Increases AdipoR1 and AdipoR2 Expression in Type 2 Diabetic Nephropathy. J. Transl. Med. 2016, 14, 176. [Google Scholar]
- Guo, Z.; Xu, H.-Y.; Xu, L.; Wang, S.-S.; Zhang, X.-M. In vivo and in vitro immunomodulatory and anti-inflammatory effects of total flavonoids of astragalus. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 60–73. [Google Scholar]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant Capacity of Phytochemicals and Their Potential Effects on Oxidative Status in Animals—A Review. Asian-Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [PubMed]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Ali, S.; Sahin, N.; Hayirli, A. Epigallocatechin-3-Gallate Prevents Lipid Peroxidation and Enhances Antioxidant Defense System via Modulating Hepatic Nuclear Transcription Factors in Heat-Stressed Quails. Poult. Sci. 2010, 89, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Intrieri, M.; D’Agata, V.; Mangano, N.G.; Oriani, G.; Ontario, M.L.; Scapagnini, G. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Induces Heme Oxygenase in Rat Neurons and Acts as an Effective Neuroprotective Agent against Oxidative Stress. J. Am. Coll. Nutr. 2009, 28, 492S–499S. [Google Scholar]
- He, H.-J.; Wang, G.-Y.; Gao, Y.; Ling, W.-H.; Yu, Z.-W.; Jin, T.-R. Curcumin Attenuates Nrf2 Signaling Defect, Oxidative Stress in Muscle and Glucose Intolerance in High Fat Diet-Fed Mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar] [PubMed]
- Tapia, E.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; Pedraza-Chaverri, J. Curcumin Reverses Glomerular Hemodynamic Alterations and Oxidant Stress in 5/6 Nephrectomized Rats. Phytomedicine 2013, 20, 359–366. [Google Scholar]
- Garg, R.; Maru, G. Dietary Curcumin Enhances Benzo (a) Pyrene-Induced Apoptosis Resulting in a Decrease in BPDE-DNA Adducts in Mice. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 121–131. [Google Scholar]
- Ahmadi, F. Effect of Turmeric (Curcumin longa) Powder on Performance, Oxidative Stress State and Some of Blood Parameters in Broiler Fed on Diets Containing Aflatoxin B1. J. Anim. Feed. Sci. 2010, 5, 312–317. [Google Scholar]
- Sahin, K.; Orhan, C.; Tuzcu, Z.; Tuzcu, M.; Sahin, N. Curcumin Ameloriates Heat Stress via Inhibition of Oxidative Stress and Modulation of Nrf2/HO-1 Pathway in Quail. Food Chem. Toxicol. 2012, 50, 4035–4041. [Google Scholar]
- Ali, N.A.-L.; Mohammed, A.B.; Allow, A.A. Effect of Adding Different Levels of Lycopene to the Ration on Some Lipid Profile Traits of the Laying Hens ISA-Brown. J. Nat. Sci. Res. 2014, 4, 89–96. [Google Scholar]
- Palozza, P.; Catalano, A.; Simone, R.; Cittadini, A. Lycopene as a Guardian of Redox Signalling. Acta Biochim. Pol. 2012, 59, 21–25. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Ali, S.; Sahin, N. Tomato Powder Supplementation Activates Nrf-2 via ERK/Akt Signaling Pathway and Attenuates Heat Stress-Related Responses in Quails. Anim. Feed. Sci. Technol. 2011, 165, 230–237. [Google Scholar] [CrossRef]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure Activity Relationship of Carotenoid Derivatives in Activation of the Electrophile/Antioxidant Response Element Transcription System. Free. Radic. Biol. Med. 2009, 47, 659–667. [Google Scholar] [PubMed]
- Lian, F.; Wang, X. Enzymatic Metabolites of Lycopene Induce Nrf2-mediated Expression of Phase II Detoxifying/Antioxidant Enzymes in Human Bronchial Epithelial Cells. Int. J. Cancer 2008, 123, 1262–1268. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Iben, C.; Sahin, N. Resveratrol Protects Quail Hepatocytes against Heat Stress: Modulation of the Nrf2 Transcription Factor and Heat Shock Proteins. Anim. Physiol. Nutr. 2012, 96, 66–74. [Google Scholar] [CrossRef]
- Liu, Y.; Chan, F.; Sun, H.; Yan, J.; Fan, D.; Zhao, D.; An, J.; Zhou, D. Resveratrol Protects Human Keratinocytes HaCaT Cells from UVA-Induced Oxidative Stress Damage by Downregulating Keap1 Expression. Eur. J. Pharmacol. 2011, 650, 130–137. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, S.-C.; Ou, T.-T.; Chyau, C.-C.; Chang, Y.-C.; Wang, C.-J. Mulberry Leaf Polyphenol Extracts Reduced Hepatic Lipid Accumulation Involving Regulation of Adenosine Monophosphate Activated Protein Kinase and Lipogenic Enzymes. J. Funct. Foods 2013, 5, 1620–1632. [Google Scholar]
- Chan, K.-C.; Ho, H.-H.; Huang, C.-N.; Lin, M.-C.; Chen, H.-M.; Wang, C.-J. Mulberry Leaf Extract Inhibits Vascular Smooth Muscle Cell Migration Involving a Block of Small GTPase and Akt/NF-κB Signals. J. Agric. Food Chem. 2009, 57, 9147–9153. [Google Scholar] [CrossRef]
- Gundogdu, M.; Muradoglu, F.; Sensoy, R.I.G.; Yilmaz, H. Determination of Fruit Chemical Properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar]
- Andallu, B.; Shankaran, M.; Ullagaddi, R.; Iyer, S. In Vitro Free Radical Scavenging and In Vivo Antioxidant Potential of Mulberry (Morus indica L.) Leaves. J. Herb. Med. 2014, 4, 10–17. [Google Scholar]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Parajó, J.C. Natural Antioxidants from Residual Sources. Food Chem. 2001, 72, 145–171. [Google Scholar]
- Yang, Y.; Yang, W.; Hu, T.; Sun, M.; Wang, J.; Shen, J.; Ding, E. Protective Effect of Biochanin A on Gamma Radiation-Induced Oxidative Stress, Antioxidant Status, Apoptotic, and DNA Repairing Molecules in Swiss Albino Mice. Cell Biochem. Funct. 2024, 42, e70005. [Google Scholar]
- Qian, Z.; Huang, Q.; Wu, M.; Yang, W.; Wei, Z.; Cheng, X.; Li, D. Analysis of Antioxidants from Three Parts of Polygonum chinense Based on Online Extraction High Performance Liquid Chromatography Antioxidant Analysis System. Chem. Biodivers. 2024, 22, e202401771. [Google Scholar]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A.M. Antioxidants an overview on the natural and synthetic types. Eur. Chem. Bull 2017, 6, 365. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Jamshidi-kia, F.; Wibowo, J.P.; Elachouri, M.; Masumi, R.; Salehifard-Jouneghani, A.; Abolhasanzadeh, Z.; Lorigooini, Z. Battle between Plants as Antioxidants with Free Radicals in Human Body. J. Herbmed Pharmacol. 2020, 9, 191–199. [Google Scholar]
- Adrar, N.; Gulsunoglu-Konuskan, Z.; Ceylan, F.D.; Capanoglu, E. Overview and Trends in Electrochemical Sensors, Biosensors and Cellular Antioxidant Assays for Oxidant and Antioxidant Determination in Food. Talanta 2025, 283, 127058. [Google Scholar]
- Karpinska, B.; Foyer, C.H. Superoxide Signalling and Antioxidant Processing in the Plant Nucleus. J. Exp. Bot. 2024, 75, 4599–4610. [Google Scholar] [CrossRef]
- Burri, S.C.M.; Granheimer, K.; Rémy, M.; Ekholm, A.; Håkansson, Å.; Rumpunen, K.; Tornberg, E. Lipid Oxidation Inhibition Capacity of 11 Plant Materials and Extracts Evaluated in Highly Oxidised Cooked Meatballs. Foods 2019, 8, 406. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, Z.; Su, Y.; Wang, T. Full-length transcriptome analysis of multiple organs and identification of adaptive genes and pathways in Mikania micrantha. Sci. Rep. 2022, 12, 3272. [Google Scholar]
- Villar, A.; Vázquez-González, I.; Vicente, F.; Salcedo, G.; González, L.; Botana, A.; Royo, L.J.; Eguinoa, P.; Busqué, J. Study of the Variability in Fatty Acids and Carotenoid Profiles: Laying the Ground for Tank Milk Authentication. Sustainability 2021, 13, 4506. [Google Scholar] [CrossRef]
- Prince, R.C.; Dutton, P.L.; Gunner, M.R. The Aprotic Electrochemistry of Quinones. Biochim. Biophys. Acta (BBA)-Bioenerg. 2022, 1863, 148558. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pu, H.; Tang, P.; Tang, B.; Sun, Q.; Li, H. Propyl Gallate/Cyclodextrin Supramolecular Complexes with Enhanced Solubility and Radical Scavenging Capacity. Food Chem. 2018, 245, 1062–1069. [Google Scholar] [CrossRef]
- Qian, J.; Liang, T.; Xu, Y.; Liu, Z.-P.; Jing, L.-L.; Luo, H.-B. Effect of the Novel Free Radical Scavenger 4′-Hydroxyl-2-Substituted Phenylnitronyl Nitroxide on Oxidative Stress, Mitochondrial Dysfunction and Apoptosis Induced by Cerebral Ischemia–Reperfusion in Rats. Neuroscience 2024, 540, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Widyarti, S.; Sabarudin, A.; Soeatmadji, D.W.; Sumitro, S.B. DFT and Molecular Dynamics Studies of Astaxanthin-Metal Ions (Cu2+ and Zn2+) Complex to Prevent Glycated Human Serum Albumin from Possible Unfolding. Heliyon 2021, 7, e06548. [Google Scholar] [CrossRef] [PubMed]
- Casoni, D.; Simion, I.M.; Cobzac, S.C.A.; Kiraly, A.-G. Development of a new micro-hptlc protocol for total antioxidant potential determination of redox-active drugs. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 235–247. [Google Scholar] [CrossRef]
- Fernández-Dueñas, D.M.; Mariscal, G.; Ramírez, E.; Cuarón, J.A. Vitamin C and β-Carotene in Diets for Pigs at Weaning. Anim. Feed. Sci. Technol. 2008, 146, 313–326. [Google Scholar]
- Pashtetsky, V.; Ostapchuk, P.; Il’yazov, R.; Zubochenko, D.; Kuevda, T. Use of Antioxidants in Poultry Farming (Review). IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012042. [Google Scholar] [CrossRef]
- Deng, Q.; Xu, J.; Yu, B.; He, J.; Zhang, K.; Ding, X.; Chen, D. Effect of Dietary Tea Polyphenols on Growth Performance and Cell-Mediated Immune Response of Post-Weaning Piglets under Oxidative Stress. Arch. Anim. Nutr. 2010, 64, 12–21. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Rao, D.; Zhao, R.; Hu, Y.; Li, H.; Chun, Z.; Zheng, S. Revealing of Intracellular Antioxidants in Dendrobium Nobile by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Metabolites 2023, 13, 702. [Google Scholar] [CrossRef]
- Liu, M.; Xie, H.; Ma, Y.; Li, H.; Li, C.; Chen, L.; Jiang, B.; Nian, B.; Guo, T.; Zhang, Z.; et al. High performance liquid chromatography and metabolomics analysis of tannase metabolism of gallic acid and gallates in tea leaves. J. Agric. Food Chem. 2020, 68, 4946–4954. [Google Scholar]
- High-Performance Liquid Chromatography—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Courses/Providence_College/CHM_331_Advanced_Analytical_Chemistry_1/14:_Liquid_Chromatography/14.02:_High-Performance_Liquid_Chromatography (accessed on 15 May 2024). (Hosted by University of California, Davis, CA, USA).
- Thinh, B.B.; Thin, D.B.; Ogunwande, I.A. Chemical Composition, Antimicrobial and Antioxidant Properties of Essential Oil From the Leaves of Vernonia volkameriifolia DC. Nat. Prod. Commun. 2024, 19, 1934578X241239477. [Google Scholar]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas Chromatography–Mass Spectrometry Data Processing Made Easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [PubMed]
- Pobłocka-Olech, L.; Isidorov, V.A.; Krauze-Baranowska, M. Characterization of Secondary Metabolites of Leaf Buds from Some Species and Hybrids of Populus by Gas Chromatography Coupled with Mass Detection and Two-Dimensional High-Performance Thin-Layer Chromatography Methods with Assessment of Their Antioxidant Activity. Int. J. Mol. 2024, 25, 3971. [Google Scholar]
- Aksenov, A.A.; Laponogov, I.; Zhang, Z.; Doran, S.L.; Belluomo, I.; Veselkov, D.; Bittremieux, W.; Nothias, L.F.; Nothias-Esposito, M.; Maloney, K.N.; et al. Auto-Deconvolution and Molecular Networking of Gas Chromatography-Mass Spectrometry Data. Nat. Biotechnol. 2021, 39, 169–173. [Google Scholar] [PubMed]
- Głód, B.K.; Wantusiak, P.M.; Piszcz, P.; Elwira Lewczuk, E.; Zarzycki, P.K. Application of micro-TLC to the total antioxidant potential (TAP) measurement. Food Chem. 2015, 173, 749–754. [Google Scholar]
- Hawrył, M.A.; Waksmundzka-Hajnos, M. Micro 2D-TLC of selected plant extracts in screening of their composition and antioxidative properties. Chromatographia 2013, 76, 1347–1352. [Google Scholar]
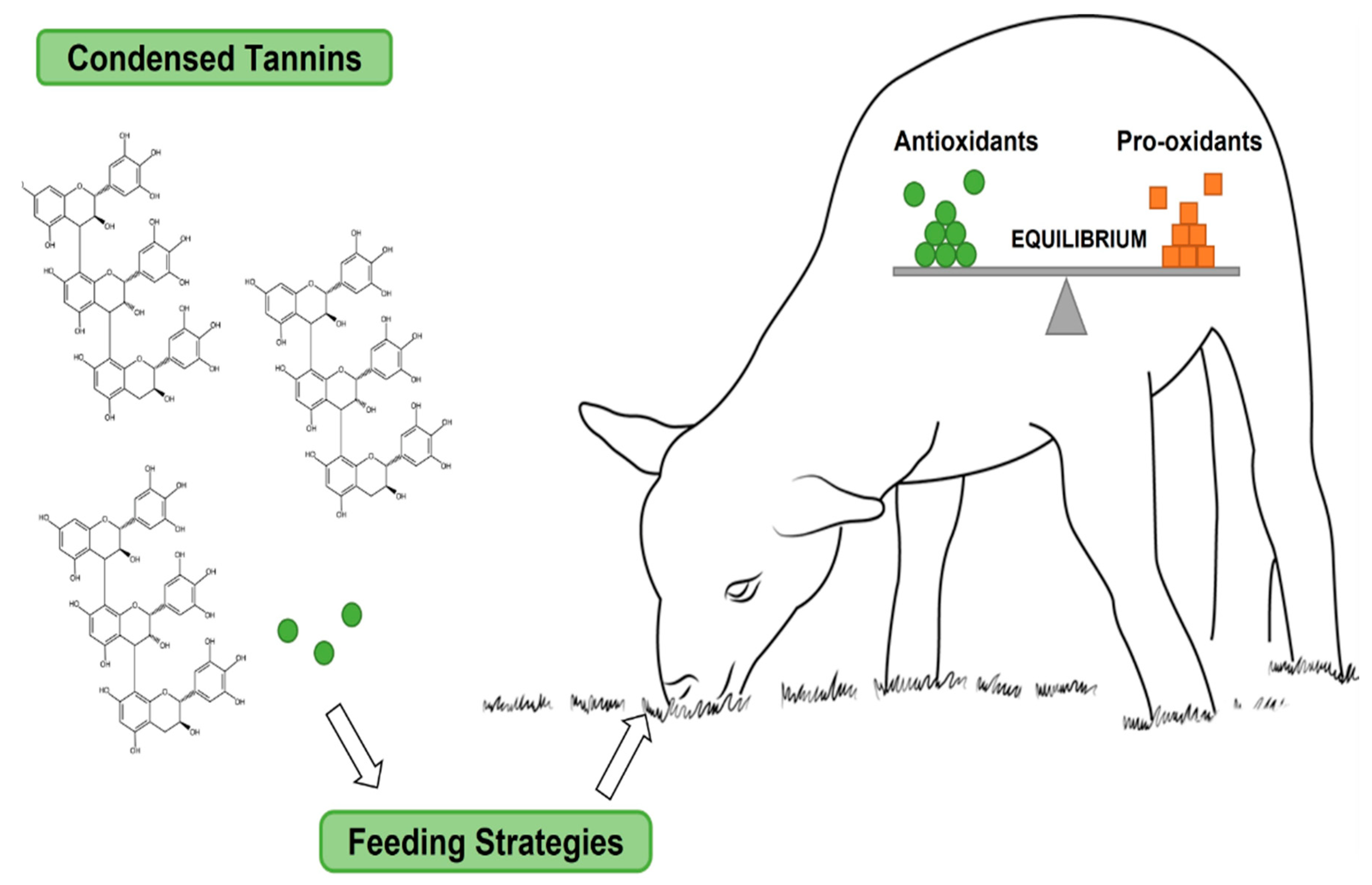
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Politis, I. Reevaluation of Vitamin E Supplementation of Dairy Cows: Bioavailability, Animal Health and Milk Quality. Animal 2012, 6, 1427–1434. [Google Scholar]
- GB 2760-2014; National Food Safety Standard for Uses of Food Additives. National Health and Family Planning Commission: Beijing, China, 2014.
- ISO 22000:2018; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- Bialasiewicz, P.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Markowski, J.; Rutkowski, K.P.; Nowak, D. Sour Cherries but Not Apples Added to the Regular Diet Decrease Resting and fMLP-Stimulated Chemiluminescence of Fasting Whole Blood in Healthy Subjects. J. Am. Coll. Nutr. 2018, 37, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries Improve Biomarkers of Cardiometabolic Function in Participants with Metabolic Syndrome—Results from a 6-Month, Double-Blind, Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [PubMed]

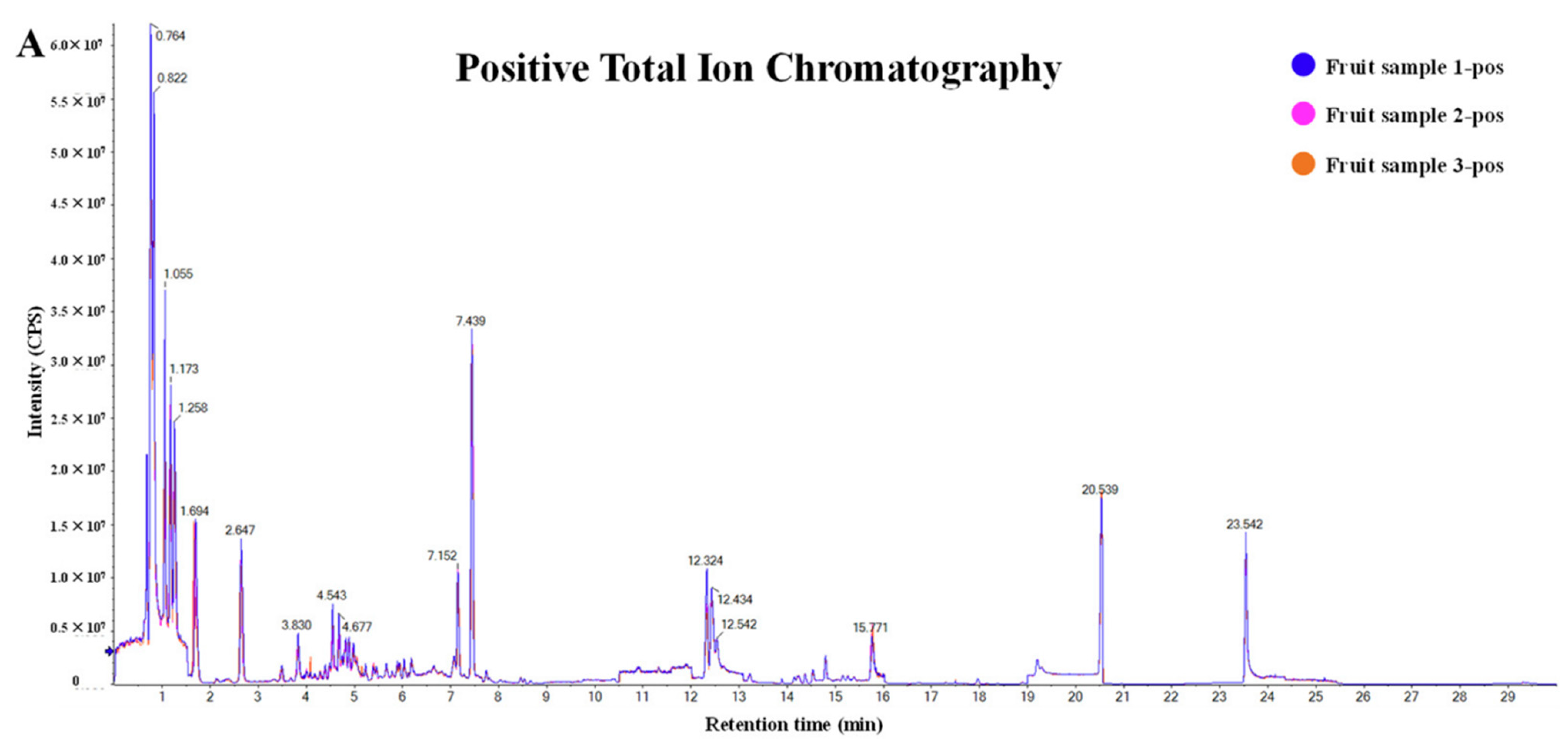
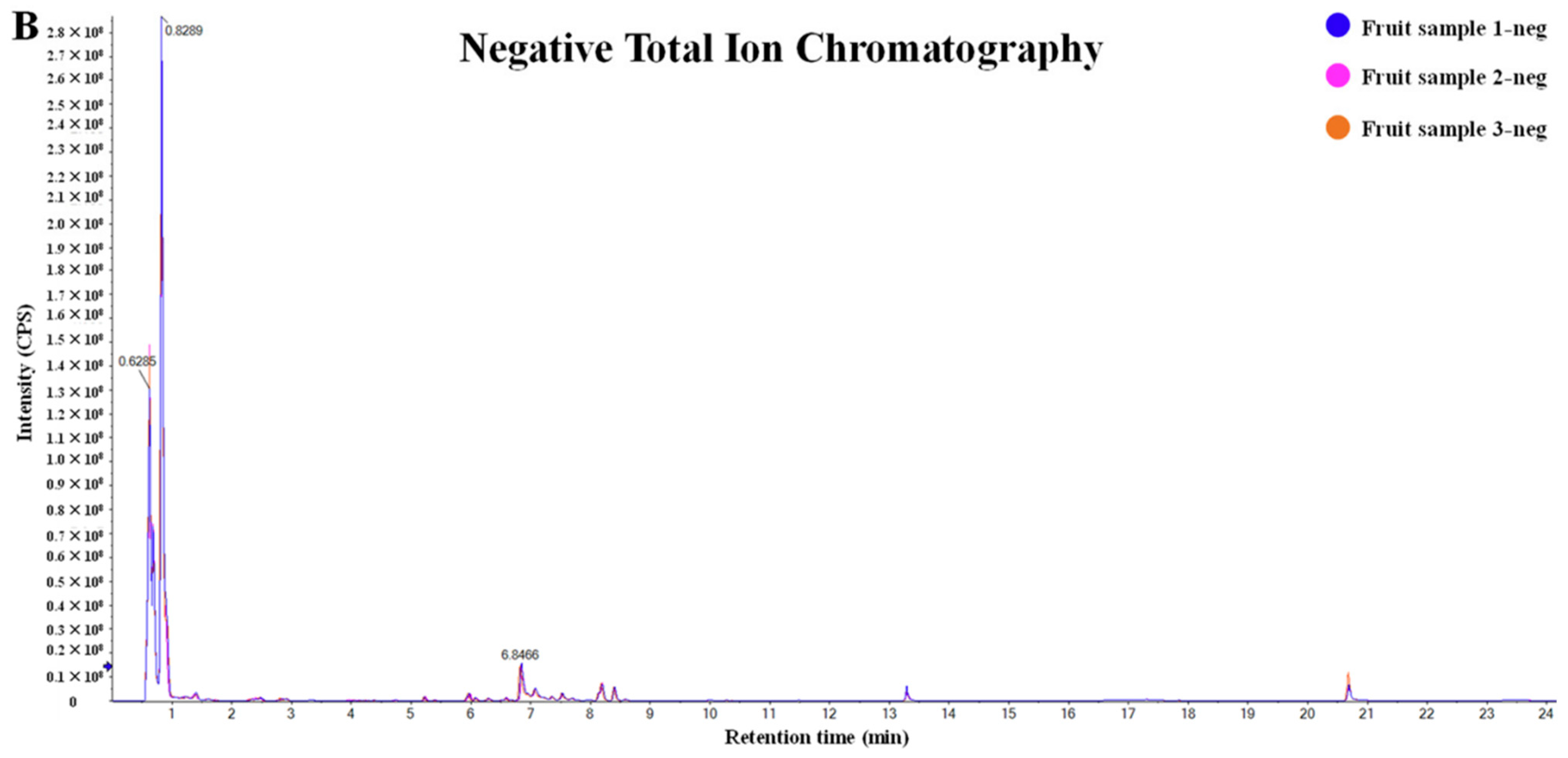

| Classification Basis | Example Study | Key Limitation | Enhanced Approach |
|---|---|---|---|
| Chemical Structure | [33] | Inability to distinguish synergistic effects of natural/synthetic antioxidants (e.g., reference [33] points to the mixing of semi-synthetic derivatives) | Introduction of metabolic network model (animal model method in reference [2]) |
| Source (Natural/Synthetic) | [11] | Ignoring dose dependence (e.g., reference [14] shows curcumin promotes oxidation at high doses) | Integration dynamic parameters (Nrf2 activation threshold in reference [22]) |
| Mechanism (Enzymatic/Non-enzymatic) | [34] | Cross-species bioavailability differences in anthocyanins were not considered (reference [34] figure in below) | Classification by tissue-specific distribution (skin model in reference [25]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Zhu, Z.; Pi, H.; Chen, J.; Cai, J.; Wu, Y. Mechanistic Insights and Analytical Advances in Food Antioxidants: A Comprehensive Review of Molecular Pathways, Detection Technologies, and Nutritional Applications. Antioxidants 2025, 14, 438. https://doi.org/10.3390/antiox14040438
Duan M, Zhu Z, Pi H, Chen J, Cai J, Wu Y. Mechanistic Insights and Analytical Advances in Food Antioxidants: A Comprehensive Review of Molecular Pathways, Detection Technologies, and Nutritional Applications. Antioxidants. 2025; 14(4):438. https://doi.org/10.3390/antiox14040438
Chicago/Turabian StyleDuan, Mingyu, Zhiting Zhu, Hao Pi, Jibing Chen, Jie Cai, and Yiping Wu. 2025. "Mechanistic Insights and Analytical Advances in Food Antioxidants: A Comprehensive Review of Molecular Pathways, Detection Technologies, and Nutritional Applications" Antioxidants 14, no. 4: 438. https://doi.org/10.3390/antiox14040438
APA StyleDuan, M., Zhu, Z., Pi, H., Chen, J., Cai, J., & Wu, Y. (2025). Mechanistic Insights and Analytical Advances in Food Antioxidants: A Comprehensive Review of Molecular Pathways, Detection Technologies, and Nutritional Applications. Antioxidants, 14(4), 438. https://doi.org/10.3390/antiox14040438








