Abstract
Tyrosol (Tyr), hydroxytyrosol (TH), and 3,4-Dihydroxyacetophenone (3,4-DHAP) are three phenolic compounds naturally present in plants that have attracted considerable research attention due to their potent antioxidant, anti-inflammatory, anticancer, and cardiovascular protective properties. In recent years, mounting evidence has indicated that these phenolic compounds hold broad potential in both disease prevention and treatment. This paper reviews the chemical structures and synthetic methods of Tyr, HT, and 3,4-DHAP, as well as their multifaceted effects on human health, particularly their roles and mechanisms in antioxidation, anti-inflammation, cardiovascular protection, neuroprotection, and anticancer activity. In addition, this paper explores the future prospects of these compounds and the current challenges associated with their application—such as low bioavailability and long-term safety concerns—and proposes directions for further investigation.
1. Introduction
Phenolic compounds are a class of secondary metabolites widely found in plants, characterized by one or more benzene rings bearing one or more hydroxyl (-OH) groups [1]. Tyrosol (Tyr) is a representative phenolic compound; hydroxytyrosol (HT) is its hydroxylated product; while 3,4-Dihydroxyacetophenone (3,4-DHAP) results from the dehydration and hydroxylation of the Tyr isomer 1-(4-hydroxyphenyl)-ethanol [2]. In recent years, Tyr and its hydroxylated derivatives have garnered significant attention for their potential health benefits in preventing and treating chronic diseases, particularly due to crucial advances in antioxidant, anti-inflammatory, anticancer, and cardiovascular protective research [3,4,5,6]. The selection of Tyr, HT, and 3,4-DHAP in this context not only provides mutual validation of their mechanisms of action but also indicates possible directions for further mechanistic exploration.
In everyday diets, phenolic compounds are extensively present in fruits, vegetables, tea, red wine, and olive oil, with an especially pronounced role in the Mediterranean diet [7]. Owing to the abundance of phenolic compounds, notably Tyr and HT, this dietary pattern is believed to exert remarkable cardiovascular protective effects [8]. The most prominent mechanism attributed to phenolic compounds is their antioxidant capability [9], which safeguards cells by scavenging free radicals and reducing oxidative stress [10,11], thereby playing a vital role in chronic disease prevention. Moreover, phenolic compounds can mitigate the occurrence of cardiovascular disease [12], neurodegenerative conditions, and cancers [13] through mechanisms such as modulating inflammatory responses, promoting vasodilation, and inhibiting tumor cell proliferation.
Based on these findings, this paper aims to systematically review phenolic compounds, particularly Tyr, HT, and 3,4-DHAP, focusing on their structures, synthetic methods, and health impacts. Special attention is given to their modes of action in antioxidation, anti-inflammation, cardiovascular protection, neuroprotection, and anticancer effects [14], along with a discussion of their potential clinical applications and directions for future research.
2. Methods
A systematic literature search was conducted through the PubMed and Web of Science databases. Original articles on the synthesis methods and health effects of phenolic compounds (including Tyr, HT, and 3,4-DHAP) were identified using the following search terms: Tyr or HT or 3,4-DHAP and synthesis methods or bioactivity or antioxidant or anti-inflammatory or cardioprotective or neuroprotective or osteoprotective or anticancer or antidiabetic or antiobesity or antimicrobial or metabolism or bioavailability, covering the period from 2010 to 2025. These numerous articles were further manually screened for relevance to the research topic. The focus was on new insights related to the synthesis and bioactivity of phenolic compounds, as well as the confirmed effects on human health. Articles published prior to 2010 were carefully selected and incorporated when relevant to the topic.
3. Structure and Synthesis of Phenolic Compounds
3.1. Structure and Synthesis of Tyrosol
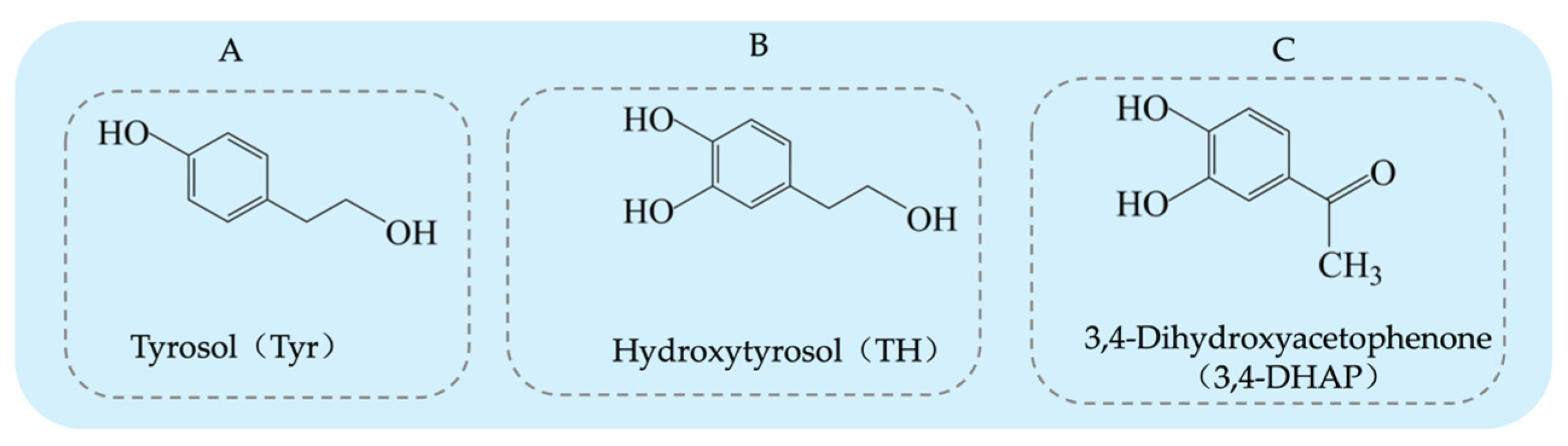
Tyrosol (Tyr) is a typical phenolic compound [15], with a chemical structure consisting of a benzene ring, a hydroxyl group (-OH), and a hydroxyethyl side chain (-CH2CH2OH) (see Figure 1A). Its chemical formula is C8H10O2 [16]. As an important chemical intermediate, Tyr has wide applications in various industries, including chemistry, pharmaceuticals, agriculture, and fragrances.
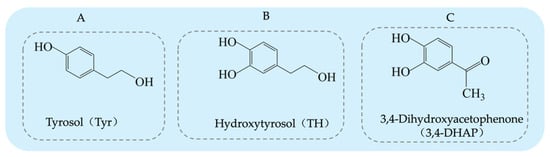
Figure 1.
Structural diagrams of tyrosol, hydroxytyrosol, and 3,4-Dihydroxyacetophenone: (A) tyrosol structure; (B) hydroxytyrosol structure; (C) 3,4-Dihydroxyacetophenone structure.
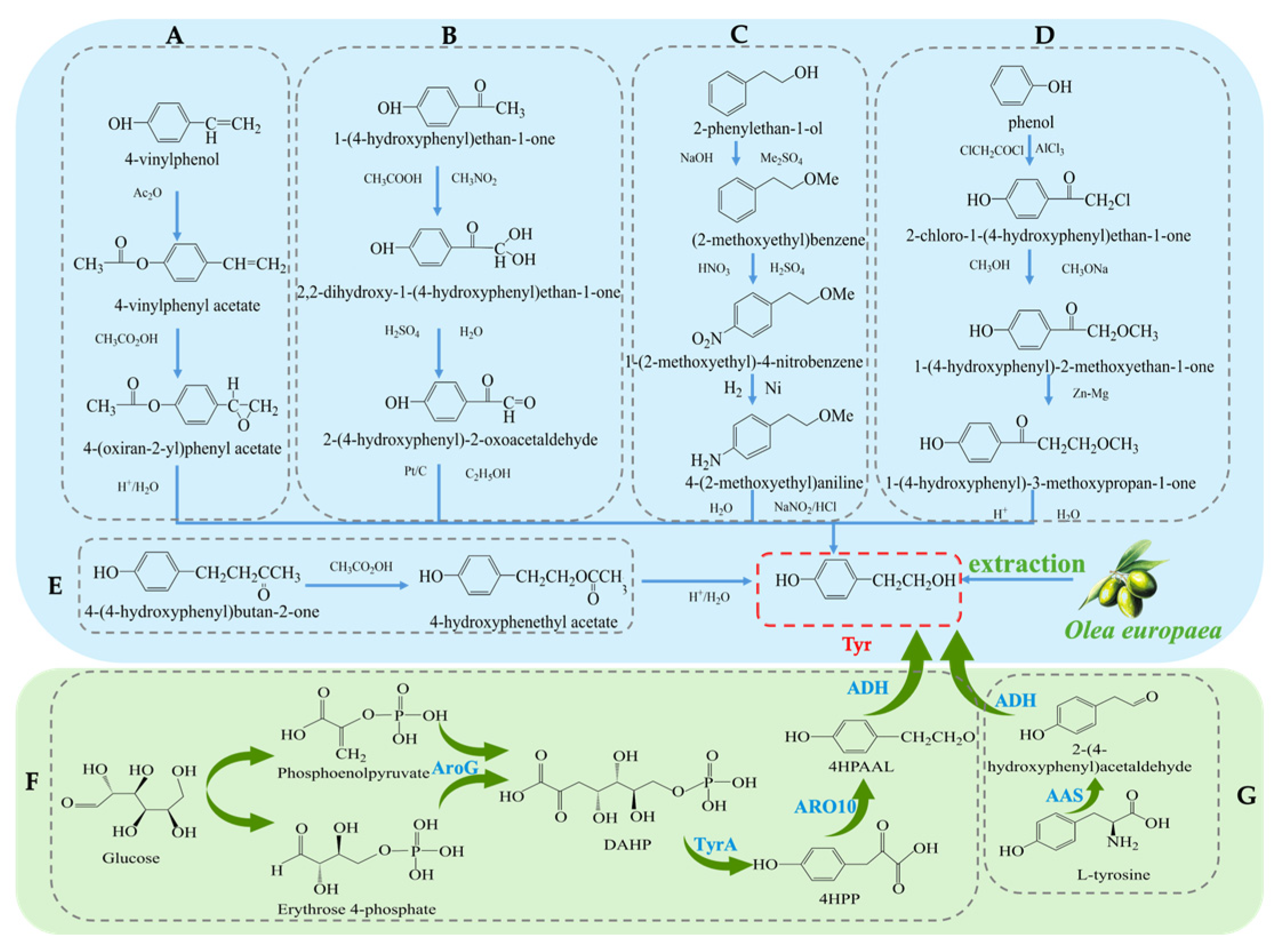
The synthesis methods of Tyr have undergone more than a century of development, and currently, there are several synthetic routes available. Traditional methods for obtaining Tyr mainly include plant extraction and chemical synthesis. The plant extraction method involves extraction from the olive plant (Olea europaea) [17]. However, plant extraction faces challenges such as long plant growth cycles, low yields, and high raw material costs, which limit its widespread application in industrial production. To overcome the limitations of plant extraction, chemical synthesis methods have been widely applied in the production of Tyr. Several chemical synthesis strategies have been developed to achieve efficient Tyr synthesis [18], each with its own characteristics. Firstly, the synthesis method using para-hydroxyphenylethylene (see Figure 2A) has gained attention due to its simplicity in operation, achieving a high yield of 96% and purity of 99% during the synthesis process [19]. From a technical perspective, this method shows considerable potential for application; however, the high cost of raw materials remains a major bottleneck to its large-scale industrial adoption. Secondly, the para-hydroxyacetophenone method [20] (see Figure 2B) is praised for its simple steps, but the first reaction step requires a temperature of −30 °C, which not only increases equipment and energy costs but also affects the universality of the process to some extent. Considering economic benefits and environmental friendliness, this method still demonstrates competitiveness in certain special applications. Another method uses phenylethanol [21] as the starting material (see Figure 2C), with the basic process including hydroxyl protection, followed by nitration, reduction, diazotization, and hydrolysis, ultimately yielding Tyr with an overall yield of approximately 70%. Despite the numerous steps involved, this route has advantages in terms of raw material availability and operational processes. Additionally, there is a biosynthetic route using phenol [22] as the starting material (see Figure 2D), which involves steps such as chloroacetylation, etherification, Clemmensen reduction, and hydrolysis, efficiently producing the target product. Lastly, the hydrolysis of carboxylate esters [23] (see Figure 2E) is also a method of interest due to its simplicity, but like other methods, environmental concerns still need attention. Overall, although chemical synthesis methods have distinct advantages in improving the yield and purity of Tyr, some reagents and process conditions used in these reactions may still have negative environmental impacts. Future research should focus on optimizing the greening of the process and reducing costs.
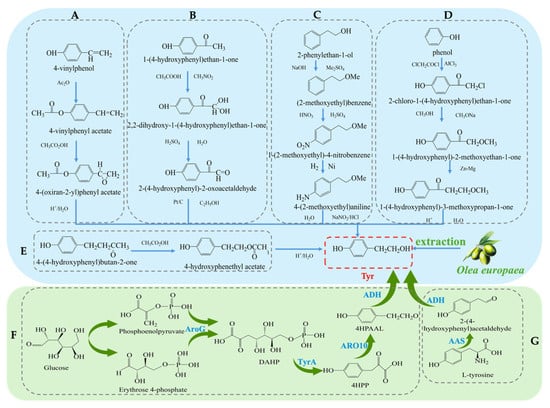
Figure 2.
Overall synthesis scheme of tyrosol: (A) para-hydroxyphenylethylene synthesis method; (B) para-hydroxyacetophenone synthesis method; (C) phenylethanol synthesis method; (D) phenol synthesis method; (E) carboxylate ester synthesis method; (F) Glucose-based synthesis method; (G) Tyrosine-based synthesis method; AroG: 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase; TyrA: chorismate mutase or prephenate, dehydrogenase; 4HPP: 4-hydroxyphenylpyruvate; ARO10: phenylpyruvate decarboxylase; 4HPAAL: 4- hydroxyphenyacetaldehyde; ADH: alcohol dehydrogenase; AAS: Aromatic Aldehyde Synthase.
In recent years, with the development of biotechnology, biosynthesis has emerged as a new direction for obtaining Tyr. These methods not only offer high efficiency and environmental benefits but also effectively reduce production costs [24]. Common biosynthetic pathways include biosynthesis using Escherichia coli, where a dual-plasmid system carrying the tyrosine hydroxylase and decarboxylase genes is constructed. The process (see Figure 2G) involves converting 10 mM tyrosine to 9.56 ± 0.64 mM Tyr at 45 °C with a conversion efficiency exceeding 95% [25]. Another study utilized the overexpression of phenylpyruvate decarboxylase (ARO10) in Saccharomyces cerevisiae to construct a recombinant E. coli strain. The process (see Figure 2F) synthesizes Tyr from glucose and optimizes the induction conditions, resulting in an increase in Tyr yield to 9.53 mM [26]. In summary, although traditional plant extraction and chemical synthesis methods remain important routes for Tyr production, biosynthesis technologies are gradually becoming the mainstream method for Tyr production due to their high efficiency, environmental friendliness, low cost, and adjustability in the production process.
3.2. Structure and Synthesis of Hydroxytyrosol
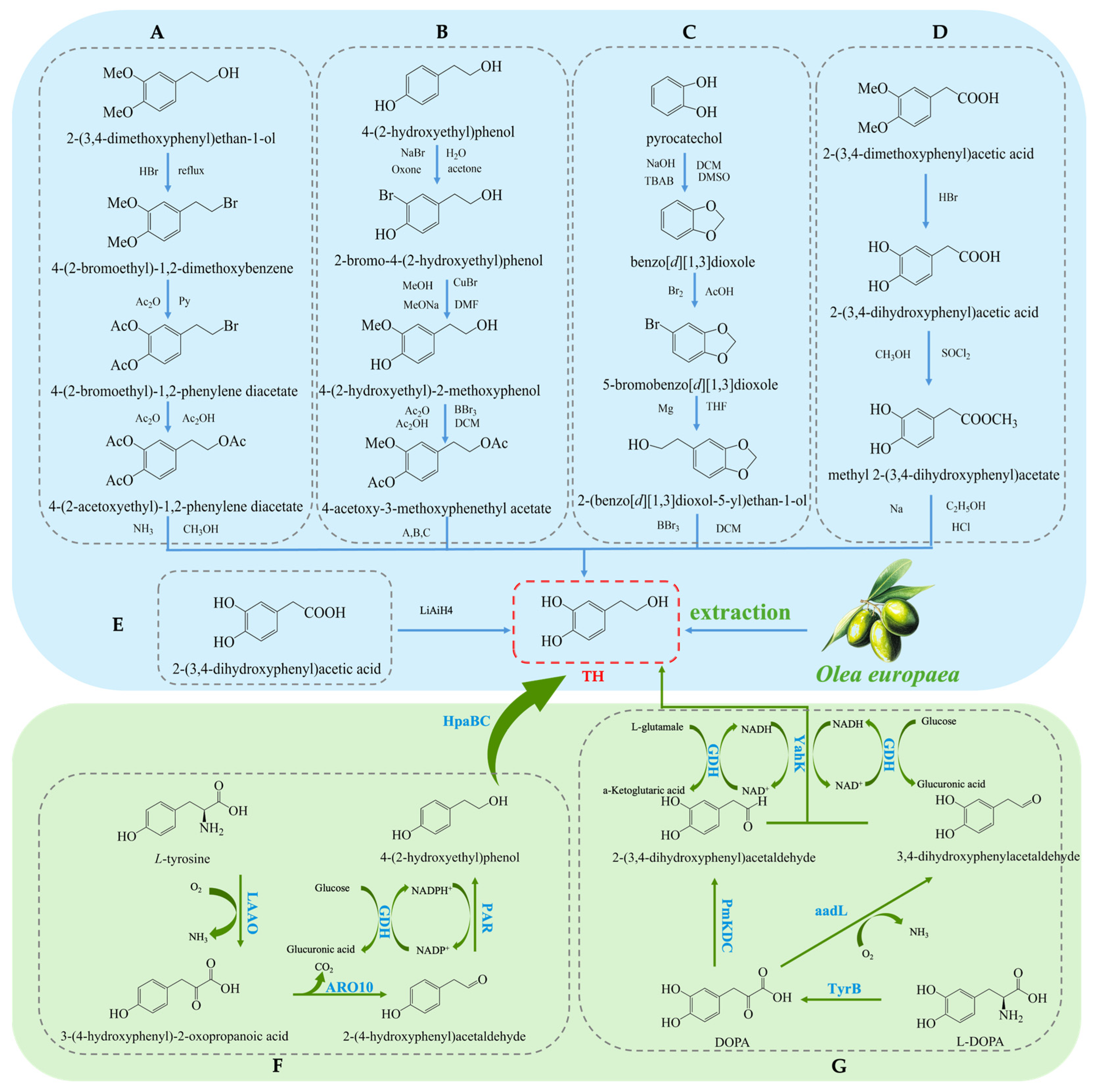
Hydroxytyrosol (TH) is a natural phenolic compound widely found in olive oil and its by-products [27,28]. Its chemical structure consists of a benzene ring, two hydroxyl groups (both located on the benzene ring), and a hydroxyethyl side chain (-CH₂CH₂OH) (see Figure 1B). Compared to Tyr, TH has an additional hydroxyl group on the benzene ring [29]. The main methods for obtaining TH include plant extraction, chemical synthesis, and biosynthesis. Each method has its advantages and drawbacks. Plant extraction yields relatively low amounts, chemical synthesis is more complex, and biosynthesis has been receiving increasing attention due to its efficiency and environmental friendliness. The plant extraction method involves extracting TH from the olive plant (Olea europaea) [30], but this method is often limited by low yield and poor extraction efficiency, and the process may require large amounts of solvents. On the other hand, chemical synthesis methods for TH have become a research hotspot, with various synthetic routes reported in the literature, each with its own strengths and weaknesses. Firstly, the synthesis method using 3,4-dimethoxyphenylethanol as the starting material (see Figure 3A) can produce the target product, but the yield of the final deprotection reaction is only 10%, and the overall steps are numerous, leading to high costs [31]. Secondly, the route using Tyr as the starting material (see Figure 3B) requires the introduction of a hydroxyl group onto the benzene ring, which involves hydroxyl protection and deprotection steps and requires complex reagents such as lithium aluminum hydride and boron tribromide, making it less commonly used in both laboratory and industrial production. Similarly, the synthesis route based on ortho-hydroquinone (see Figure 3C) involves many reaction steps and the use of the highly dangerous boron tribromide, which limits its practical application due to the intensity of the reactions involved [32]. Additionally, the route using 3,4-dimethoxyphenylacetic acid as the starting material (see Figure 3D) can yield TH but involves the use of highly corrosive hydrogen bromide and highly flammable and explosive sodium metal, which presents significant safety hazards [33]. A method using 3,4-dihydroxyphenylacetic acid as the starting material, with lithium aluminum hydride as the reducing agent, directly synthesizes TH in a single reaction step (see Figure 3E). This significant simplification of the reaction steps and the substantial increase in yield present a safer and more efficient route for the synthesis and industrial production of TH [34].
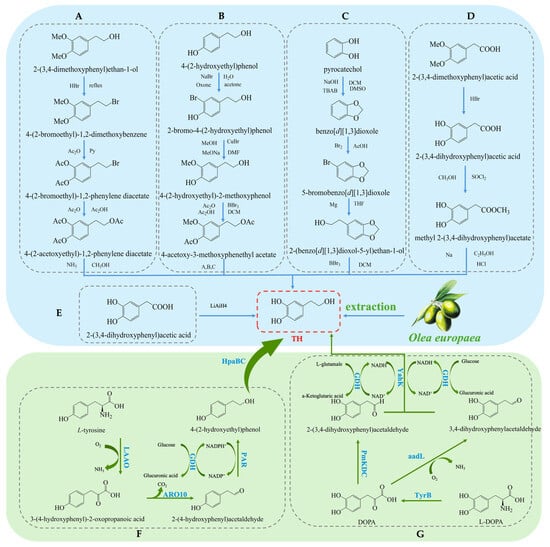
Figure 3.
Overall synthesis scheme of hydroxytyrosol: (A) synthesis method of 3,4-dimethoxyphenylethanol; (B) synthesis method of tyrosol; (C) synthesis method of ortho-hydroquinone; (D) synthesis method of 3,4-dimethoxyphenylacetic acid; (E) synthesis method of 3,4-dihydroxyphenylacetic acid; (F) glucose-based synthesis method; (G) tyrosine-based synthesis method; aadL: L-amino acid dehydrogenase; KAD: α-ketoacid decarboxylase; TyrB: aromatic amino acid aminotransferase; GDH: L-glutamate dehydrogenase; PmKDC: α-ketoacid decarboxylase; YahK: Aldehyde Reductase; HpaBC: 4-hydroxyphenylacetate 3-Monooxygenase; LAAO: L-amino acid oxidase; ARO10: phenylpyruvate decarboxylase; PAR: Phenylacetaldehyde Reductase.
In recent years, biosynthesis methods have gained increasing attention due to their high efficiency and environmental advantages. Researchers have significantly improved TH production by optimizing microorganisms and enzymatic engineering [35]. On one hand, utilizing L-tyrosine as a substrate and employing a modular cascade reaction has achieved the conversion to TH [36], as shown in (Figure 3F). For example, an enzyme cascade involving HpaBC from Escherichia coli, L-amino acid deaminase (LAAD) from Pseudomonas putida, ARO10 from Saccharomyces cerevisiae, and PAR from tomato exhibited high catalytic activity and efficiently synthesized TH. Under 50 mM L-tyrosine conditions, a TH yield of 32.35 mM was obtained [37]. On the other hand, researchers constructed a pathway for converting L-3,4-dihydroxyphenylalanine (L-DOPA) to TH in E. coli by co-expressing aromatic amino acid aminotransferase (TyrB), α-ketoacid decarboxylase (PmKDC), YahK, and L-glutamate dehydrogenase (GDH) (see Figure 3G). This system successfully synthesized 36.33 mM of TH [38,39]. This co-expression system not only improved the substrate conversion rate but also significantly increased the yield. Furthermore, by co-expressing L-amino acid dehydrogenase (aadL), YahK, and GDH, the substrate conversion rate was further increased to 89.3% [40]. This multi-enzyme co-expression system, compared to traditional chemical synthesis methods, offers not only higher yields and fewer by-products but also a more environmentally friendly production process.
3.3. Structure and Synthesis of 3,4-Dihydroxyacetophenone
3,4-Dihydroxyphenylacetone (3,4-DHAP) is a phenolic compound found in Ilex cornuta. sylvestris, with a molecular structure comprising a benzene ring attached to two hydroxyl groups, as well as an acetyl group (-COCH3) (see Figure 1C). Its structure is relatively complex, and it possesses potential biological activity. The chemical formula is C8H8O3. It has significant antioxidant, anti-inflammatory, and antitumor effects [41].
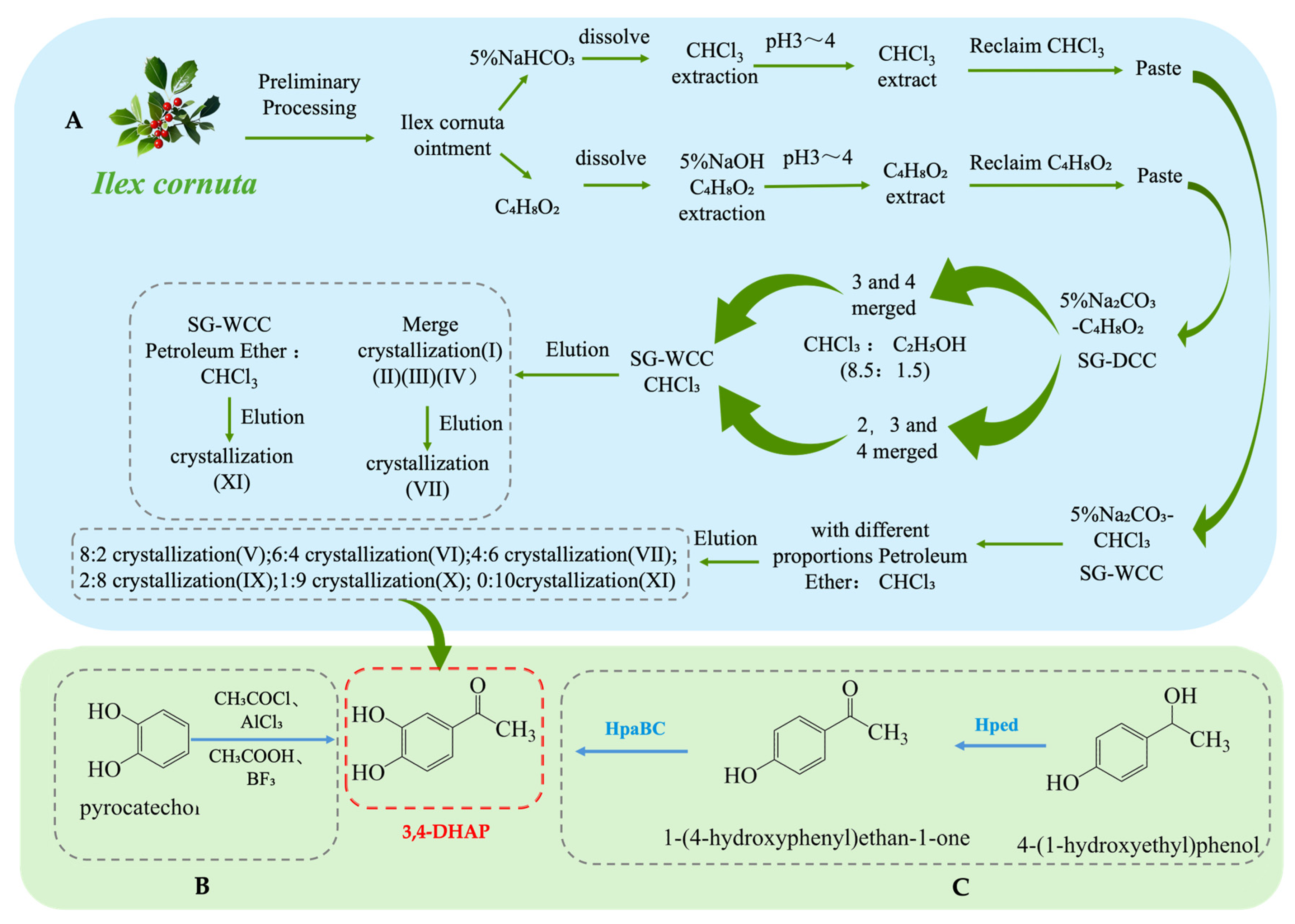
As one of the main active ingredients in Ilex cornuta. sylvestris, the extraction methods for 3,4-DHAP mainly include traditional plant extraction, chemical synthesis, and biosynthesis. Firstly, 3,4-DHAP can be extracted from Ilex cornuta. sylvestris using traditional plant extraction methods [42] (see Figure 4A). This method is relatively complex, involving multiple steps such as dissolution, extraction, separation, chromatography, elution, and crystallization, and it has the limitation of low extraction efficiency. Additionally, studies have found that 3,4-DHAP can also be isolated from the ether fraction of water extracts from Spruce needles [43]. Moreover, 3,4-DHAP can also be synthesized chemically (see Figure 4B). The first chemical synthesis method uses acetyl chloride (CH3COCl) as the acetylating reagent, anhydrous aluminum chloride (AlCl3) as the catalyst, and carbon disulfide (CS2) as the reaction medium, with the reaction carried out by heating and refluxing. However, the yield of this method is relatively low (only 37%), which may be due to suboptimal reaction conditions [44]. The second method uses acetic acid (CH3COOH) as the acetylating reagent, boron trifluoride (BF3) as the catalyst, and ether (C4H10O) as the reaction medium. The reaction is conducted at 80 °C for 4 h, achieving a yield of 66.7%, which is higher than the first method, and the reaction conditions are milder [45].
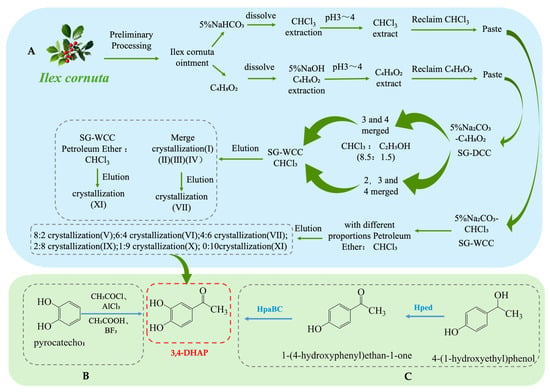
Figure 4.
Overall synthesis scheme of 3,4-Dihydroxyacetophenone: (A) plant extraction; (B) chemical synthesis; (C) biosynthesis; Hped: 1-(4-hydroxyphenyl)-ethanol dehydrogenase; HpaBC: 4-hydroxy phenylacetate 3-hydroxylase; SG-WCC: Silica Gel Wet Column Chromatography; SG-DCC: Silica Gel Dry Column Chromatography.
In addition, biosynthesis studies of 3,4-DHAP have also been reported. A whole-cell biotransformation method has been developed by constructing a recombinant E. coli strain containing the Hped gene (encoding 1-(4-hydroxyphenyl)-ethanol dehydrogenase) and the HpaBC gene (encoding 4-hydroxyphenylacetate 3-hydroxylase) (see Figure 4C). This system converts 1-(4-hydroxyphenyl)-ethanol into 3,4-DHAP, with a maximum yield of 260 mg·L−1 and a conversion rate of 17%. Although the conversion rate is relatively low, this method offers a potential green synthesis route and is more environmentally friendly compared to traditional chemical synthesis methods [46].
4. Health Effects of Phenolic Compounds
4.1. Antioxidant Activity
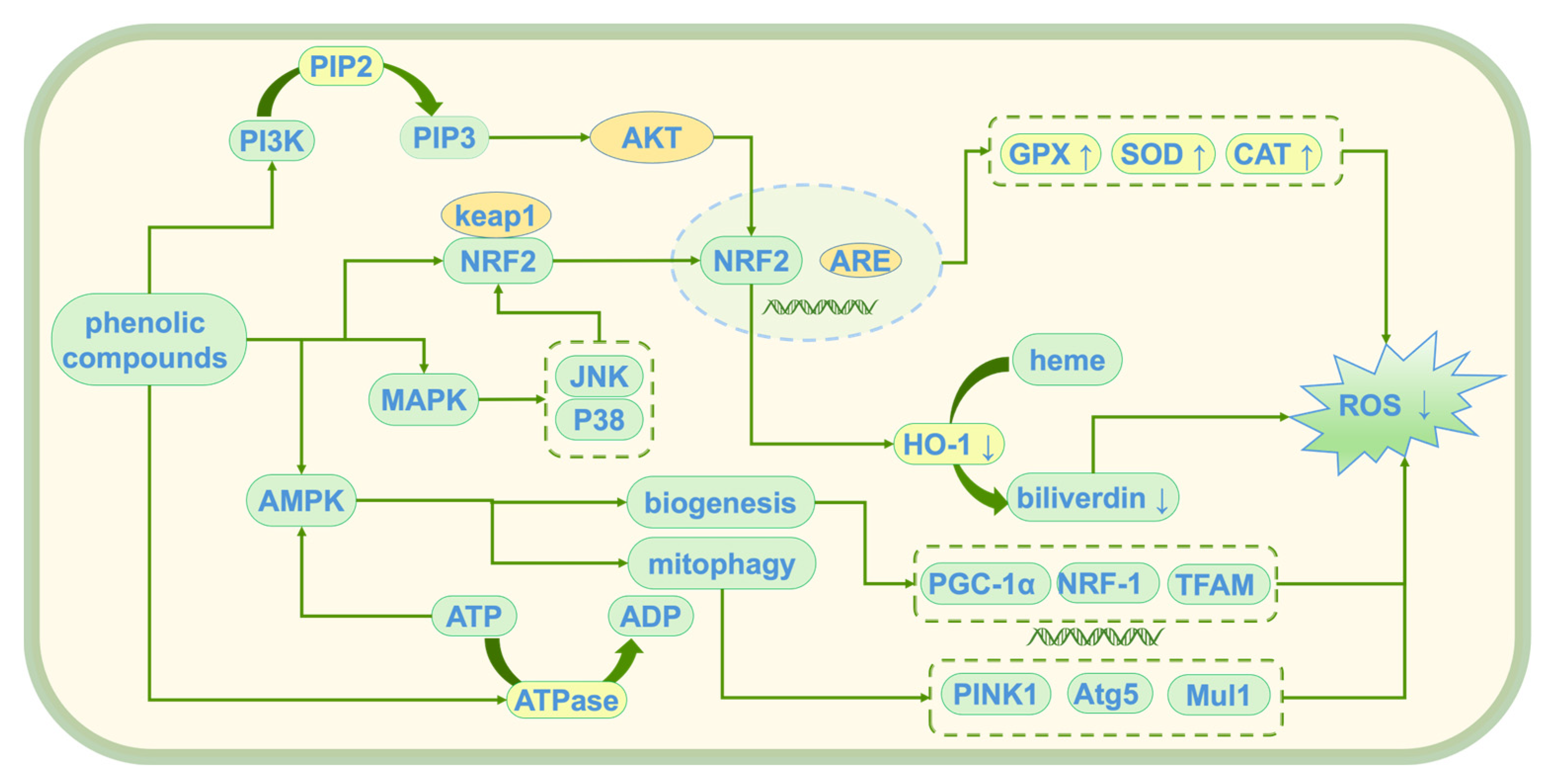
Tyr, HT, and 3,4-DHAP, as natural antioxidants, have demonstrated significant antioxidant activity [47]. These compounds alleviate oxidative stress damage induced by reactive oxygen species (ROS) through multiple mechanisms, thereby maintaining cellular homeostasis. Their main mechanisms of action (see Figure 5) include the direct scavenging of ROS, activation of endogenous antioxidant enzymes, inhibition of lipid peroxidation, modulation of oxidative stress-related pathways, and protection of mitochondrial and genomic integrity. Although the antioxidant mechanisms of these compounds are similar, their molecular structural differences result in varying targets and efficiencies. HT, due to its polyphenolic structure, exhibits a stronger free radical scavenging ability than Tyr. In contrast, 3,4-DHAP mainly exerts its antioxidant effects indirectly by modulating the Nrf2/ARE signaling pathway.
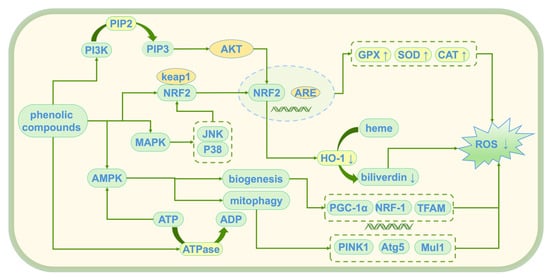
Figure 5.
Antioxidant mechanism of phenolic compounds; ↑: promote generation; ↓: Inhibit generation; PI3K: Phosphoinositide 3-Kinase; PIP2: Phosphatidylinositol 4,5-bisphosphate; PIP3: Phosphatidylinositol (3,4,5)-trisphosphate; Akt: Protein Kinase B (PKB); Keap1: Kelch-like ECH-associated Protein 1; Nrf2: Nuclear Factor Erythroid 2-Related Factor 2; ARE: antioxidant response element; MAPK: mitogen-activated protein kinase; JNK: c-Jun N-terminal Kinase; AMPK: AMP-activated protein kinase; ATP: Adenosine Triphosphate; ADP: Adenosine Diphosphate; ATPase: Adenosine Triphosphatase; HO-1: heme oxygenase-1; ROS: reactive oxygen species; GPX: glutathione peroxidase; SOD: superoxide dismutase; CAT: catalase; PINK1: PTEN-induced putative kinase 1; Atg5: Autophagy-related 5; Mul1: Mitochondrial Ubiquitin Ligase 1; PGC-1α: Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha; NRF-1: Nuclear Respiratory Factor 1; TFAM: Mitochondrial Transcription Factor A.
4.1.1. Free Radical Scavenging
ROS and other free radicals are widely considered key initiators of cellular damage and various diseases [48]. Tyr reduces cellular damage by directly reacting with superoxide anions, transforming them into stable molecules [49,50,51]. TH further enhances the antioxidant effect, not only scavenging superoxide anions but also neutralizing hydroxyl radicals and other free radicals [52,53]. Additionally, TH can inhibit the chain reaction of peroxy radicals, preventing their diffusion and significantly enhancing antioxidant capacity [54]. The mechanism of 3,4-DHAP is similar to that of TH; it can scavenge superoxide anion and hydroxyl free radicals [55]. When treated with 3,4-DHAP and exposed to high-glucose conditions, HUVEC cells showed a significant reduction in ROS generation [56].
4.1.2. Antioxidant Enzyme Activation
The antioxidant enzyme system is a key defense mechanism against oxidative damage. By activating endogenous antioxidant enzymes, it enhances the cell’s self-repair and defense capabilities, effectively responding to oxidative stress [57]. Tyr enhances the mRNA expression of superoxide dismutase (SOD) or inhibits its degradation, thereby increasing SOD activity and promoting the conversion of superoxide anions to hydrogen peroxide. This hydrogen peroxide is further cleared by glutathione peroxidase (GPx) and catalase (CAT), effectively reducing oxidative damage induced by superoxide anions [58]. TH and 3,4-DHAP enhance SOD activity through similar mechanisms, maintaining cellular redox balance. Additionally, Tyr activates GPx and CAT, accelerating the decomposition of H2O2, and preventing its accumulation and oxidative damage [59]. TH further enhances GPx and CAT activity, optimizing H2O2 clearance [60]. 3,4-DHAP also promotes GPx activity, thereby enhancing the cell’s defense against oxidative damage [61].
4.1.3. Inhibition of Lipid Peroxidation
Lipid peroxidation is an oxidative damage process initiated by free radicals, involving the reaction of lipids in the cell membrane with free radicals. The resulting lipid peroxides damage the membrane structure, directly affecting the structure and function of the cell membrane [62]. Reducing lipid peroxidation helps maintain the stability and fluidity of the cell membrane, preventing membrane damage and further protecting cellular integrity [63,64,65]. The oxidative modification of HDL decreases its antioxidant function, further exacerbating lipid peroxidation. Tyr inhibits lipid peroxidation by reducing the oxidative modification of HDL, thereby enhancing HDL function, particularly promoting cholesterol efflux and preventing the generation of lipid peroxides in the cell membrane, thus stabilizing the cell membrane [66]. TH reduces serum cholesterol, total cholesterol, and low-density lipoprotein cholesterol (LDL-C) levels while increasing high-density lipoprotein cholesterol (HDL-C) levels, slowing the lipid peroxidation process [67,68]. Studies have shown that pre-treatment of endothelial cells with TH enhances SOD activity and inhibits lipid peroxidation [69]. 3,4-DHAP reduces lipid peroxidation by decreasing free radicals, thus preventing oxidative damage to the cell membrane and maintaining its normal function [70,71,72].
4.1.4. Modulation of Oxidative Stress Response
Oxidative stress refers to the biological reactions that occur when ROS accumulate beyond the cell’s antioxidant capacity, leading to cellular damage [73]. Studies have shown that Tyr can regulate the Nrf2/ARE signaling pathway, mitogen-activated protein kinase (MAPK) pathways, c-Jun N-terminal kinase (JNK), p38 MAPK, and increase ATP production to enhance the cell’s energy supply, improving the antioxidant stress response and inhibiting H2O2-induced cell death, thereby reducing oxidative damage [74]. TH not only activates the Nrf2-ARE pathway [75] but also modulates the synthesis and activity of antioxidant enzymes via the PI3K/Akt and MAPK pathways [76]. Notably, TH can reduce the duration of JNK and p38 phosphorylation induced by H2O2, likely due to its ability to alleviate oxidative stress by influencing intracellular iron homeostasis [77]. Additionally, TH reduces the catalytic effect of metal ions (such as Fe2⁺ and Cu2⁺) in oxidative reactions by chelating these ions, thus inhibiting metal ion-induced oxidative damage [78]. Studies have shown that 3,4-DHAP modulates the Nrf2/HO-1 pathway, increases the protein and mRNA expression of antioxidant proteins Nrf-2 and HO-1, promotes Nrf2 nuclear translocation, reduces ROS production, and enhances cellular antioxidant defenses [56,79].
4.1.5. Mitochondrial Protection
Mitochondria are the energy factories of cells, and their proper function is crucial for cellular energy metabolism [80]. Oxidative damage affects mitochondrial function, disrupting the cell’s energy supply [81]. Tyr protects mitochondrial function by scavenging free radicals within the mitochondria and reducing oxidative damage to the mitochondrial membrane, thereby ensuring the stability of mitochondrial function and energy metabolism [82]. TH restores energy deficits in Alzheimer’s disease cell models (AD models) by enhancing mitochondrial activity [83]. It also reduces the production of mitochondrial superoxide, thereby protecting mitochondrial function [69]. Studies have shown that TH activates the AMPK signaling pathway; regulates the expression of the PGC-1α, NRF-1, and TFAM genes involved in mitochondrial biogenesis; and promotes mitochondrial function recovery. Additionally, it facilitates mitochondrial autophagy through the activation of PINK1, MUL1, and ATG5, improving mitochondrial function [84] and significantly reducing mitochondrial alterations and oxidative damage [85]. 3,4-DHAP increases Na+, K+, and ATPase activity; reduces peroxide generation; and inhibits ascorbate and ferrous sulfate-induced damage to brain mitochondria and cells [86]. Overall, these antioxidant compounds play an important role in reducing mitochondrial oxidative damage and maintaining energy metabolism stability.
4.1.6. DNA and Protein Protection
Phenolic compounds protect cellular DNA and proteins by reducing oxidative damage, preventing gene mutations, protein denaturation, and loss of cellular function [59]. Both Tyr and TH protect DNA by scavenging ROS, reducing oxidative DNA damage, and preventing gene mutations, thereby maintaining normal protein function [87]. Studies have shown that TH significantly reduces ROS levels in human mammary epithelial cells (MCF10A) and effectively prevents oxidative DNA damage in breast cancer cell lines (such as MCF7 and MDA-MB-231) [88]. TH can effectively reduce DNA breakage, protecting DNA from H2O2-induced DNA fragmentation [89]. 3,4-DHAP promotes the formation of autophagosomes, which indirectly reduces DNA damage by clearing damaged mitochondria, thus protecting DNA from oxidative stress and maintaining protein structure and function [56].
4.2. Anti-Inflammatory Effects
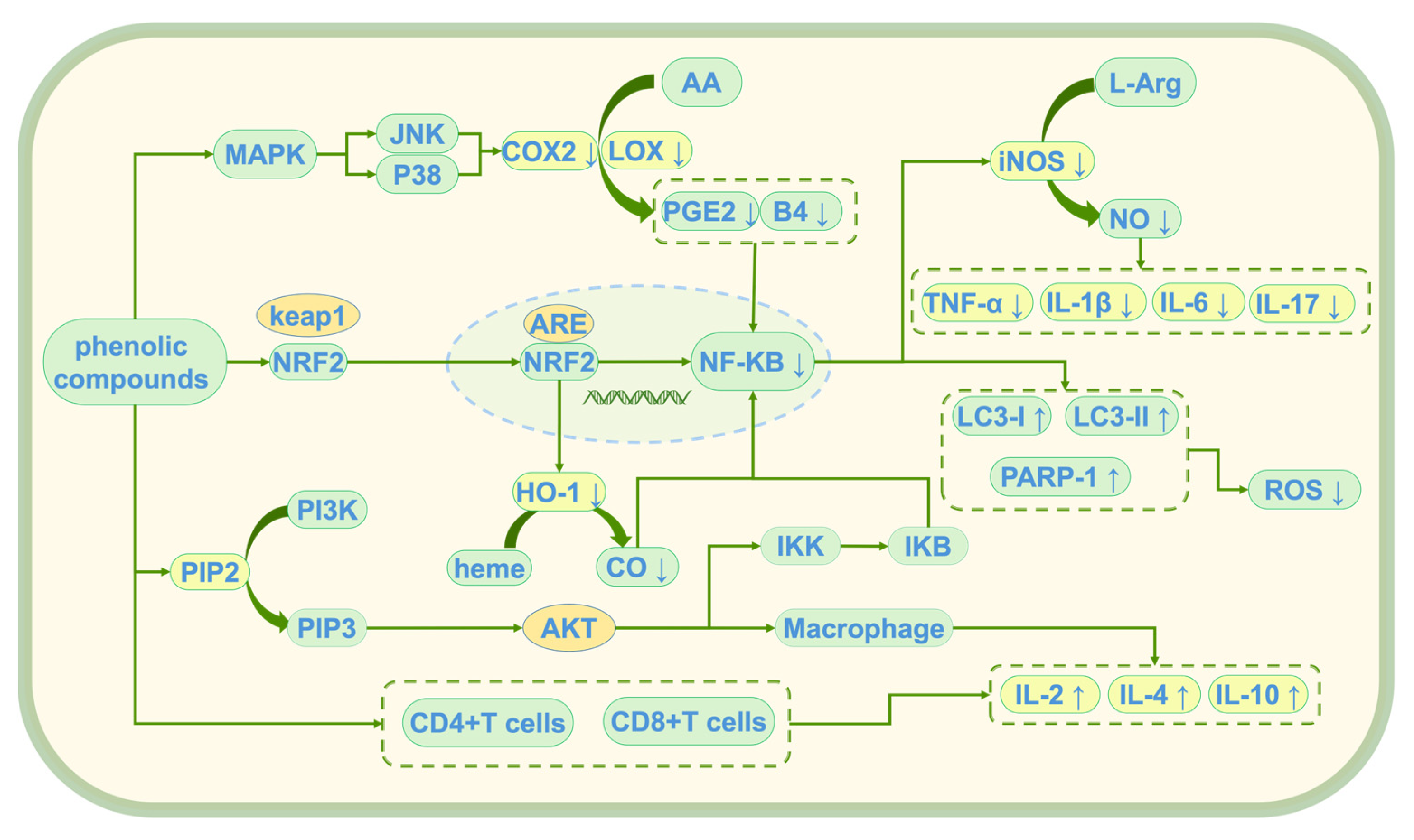
Phenolic compounds exert their anti-inflammatory effects through multiple mechanisms, collaboratively alleviating chronic inflammation induced by oxidative stress [90]. These anti-inflammatory effects are mainly achieved through three key mechanisms: the inhibition of inflammation initiation triggered by oxidative stress, modulation of the balance between pro-inflammatory and anti-inflammatory factors, and regulation of immune cell function [91]. Through these mechanisms (see Figure 6), they not only provide powerful antioxidant protection for cells but also reduce inflammatory damage by regulating immune responses, offering new therapeutic strategies for the prevention and treatment of chronic inflammation-related diseases induced by oxidative stress.
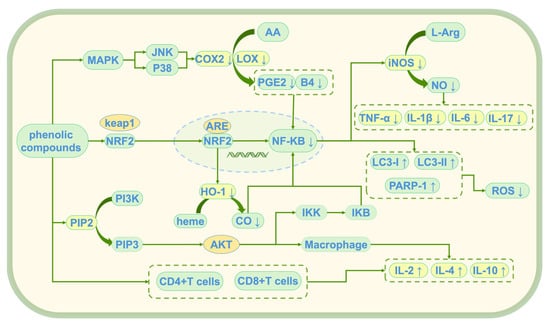
Figure 6.
Anti-inflammatory mechanism of phenolic compounds; ↑: promote generation; ↓: Inhibit generation; AA: Arachidonic Acid; L-Arg: L-Arginine; COX2: Cyclooxygenase-2; PGE2: prostaglandin E2; B4: leukotriene B4 (LTB4); iNOS: Inducible Nitric Oxide Synthase; NO: nitric oxide; TNF-α: Tumor Necrosis Factor Alpha; IL-1β: Interleukin-1 Beta; IL-6: Interleukin-6; LC3-I/LC3-II: Microtubule-Associated Protein 1 Light Chain 3 (LC3); PARP-1: Poly (ADP-Ribose) Polymerase-1; ROS: reactive oxygen species; MAPK: mitogen-activated protein kinase; JNK: c-Jun N-terminal Kinase; p38: p38 MAPK; Keap1: Kelch-like ECH-associated Protein 1; Nrf2: Nuclear Factor Erythroid 2-Related Factor 2; ARE: antioxidant response element; NF-κB: Nuclear Factor Kappa B; HO-1: heme oxygenase-1; heme: heme; CO: Carbon Monoxide; PI3K: Phosphoinositide 3-Kinase; PIP2: Phosphatidylinositol 4,5-Bisphosphate; PIP3vPhosphatidylinositol (3,4,5)-Trisphosphate; Akt: Protein Kinase B (PKB); IKK: IκB Kinase; IκB: Inhibitor of NF-κB; IL-2: Interleukin-2; IL-4: Interleukin-4; IL-10: Interleukin-10; LOX: lipoxygenase.
4.2.1. Inhibition of Inflammation Initiation Triggered by Oxidative Stress
Scavenging free radicals can reduce oxidative stress, thereby inhibiting the inflammation response initiated by oxidative stress [92]. Tyr reduces inflammation initiation by inhibiting the signaling of CD14 (a TLR4 co-receptor) downstream of the MAPK pathway, thereby attenuating the amplification of inflammation signals [93], thus reducing oxidative stress-induced inflammation [74]. TH activates the Nrf2/ARE and PI3K/Akt pathways [94], inhibiting the expression of COX-2, iNOS, NLRP3 inflammasome, ADAMTS-4, and MMP-13, thereby alleviating inflammation induced by ROS [95]. 3,4-DHAP modulates the JNK/Nrf2 pathway, upregulating eNOS-NO signaling, which synergistically enhances endothelial protection and inhibits the vicious cycle between oxidative stress and inflammation [64,96,97,98].
4.2.2. Regulation of the Balance Between Pro-Inflammatory and Anti-Inflammatory Factors
The NF-κB pathway plays a central role in the inflammatory response. Its excessive activation can lead to the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which exacerbates inflammation [99]. The inhibition of NF-κB activation can reduce the release of pro-inflammatory cytokines, thus mitigating the inflammatory response [100,101]. In a colitis-induced model, Tyr reduces inflammation by activating the MAPK pathway and regulating the gene expression of IL-6, COX-2, and NF-κB [102]. At concentrations ranging from 0.150 to 300 μmol/L, Tyr also inhibits the release of inflammatory cytokines TNF-α, IL-1β, and IL-6 [103,104,105]. TH modulates the Nrf2/ARE and PI3K/Akt pathways [106], inhibits IκB kinase (IKK) activity, and prevents NF-κB nuclear translocation, thus reducing the transcription of pro-inflammatory cytokines such as TNF-α and IL-1β [107,108]. It also inhibits the expression of pro-inflammatory iNOS and COX-2, and the activation of granulocytes and monocytes [109,110,111,112]. In colitis, TH reduces colitis biomarkers such as MPO and pro-inflammatory cytokine IL-6, thereby mitigating inflammation [113]. TH also exerts combined antioxidant and anti-inflammatory effects by downregulating pro-inflammatory cytokines and upregulating the anti-inflammatory gene Bcl2 [114]. In a study evaluating the anti-inflammatory ability and potential mechanisms of 3,4-DHAP, it was found that the compound increases Nrf2 and HO-1 protein and mRNA expression, promotes Nrf2 nuclear translocation, and enhances autophagosome formation. This significantly increases the expression of LC3 II/LC3 I, a major modification of microtubule-associated protein 1, and PARP1 proteins, promoting autophagy and DNA damage repair, while significantly reducing the expression of COX-2 and IL-17, thus promoting inflammation resolution [56].
4.2.3. Regulation of Immune Cell Function
Immune cells (macrophages, T cells, and B cells) play a crucial role in the inflammatory response. Overactive immune cells release large amounts of inflammatory cytokines, causing tissue damage. Reducing the excessive activation of immune cells can alleviate inflammation induced by immune responses. Tyr not only inhibits the production of ROS by activated macrophages but also directly scavenges ROS, inhibiting the activity of phospholipase A2 (PLA2), and reducing the release of AA from membrane phospholipids, thus blocking prostaglandin E2 (PGE2) synthesis and reducing the expression of COX-2 and iNOS. This decreases the synthesis of PGE2, leukotriene B4 (LTB4), and nitric oxide (NO) [115,116]. Tyr also modulates the function of macrophages, T cells, and B cells by activating the MAPK pathway, reducing the excessive activation of immune cells, and alleviating inflammation induced by immune responses [108]. TH increases the expression of CD4+ T cells and CD8+ T cells; promotes the mRNA expression of IL-2, IL-4, and IL-10; and enhances the activity of SOD and GSH-Px, while reducing the MDA levels, thereby alleviating inflammation [82]. 3,4-DHAP promotes the expression of HO-1 and CO in macrophages [117,118], significantly upregulates the mRNA and protein expression of soluble Toll-like receptor 4 (sTLR4), and reduces TNF-α secretion [119,120], thus regulating macrophage apoptosis and promoting the resolution of inflammation [121,122].
4.3. Cardiovascular Protective Effects
Tyr, HT, and 3,4-DHAP are natural phenolic compounds found in olives, and they exhibit significant cardiovascular protective effects. Through various mechanisms, they positively influence cardiovascular health, including antioxidant, anti-inflammatory, lipid-lowering, and vascular function-enhancing effects. These compounds can effectively alleviate the occurrence and development of cardiovascular diseases. The following summarizes their roles in different cardiovascular diseases.
4.3.1. Protection Against Atherosclerosis
Atherosclerosis is a major cause of cardiovascular diseases, often initiated by oxidized LDL, inflammatory responses, and lipid accumulation. Tyr alleviates oxidative damage by enhancing antioxidant enzyme activity and reduces the deposition of oxidized LDL in blood vessel walls [123]. It inhibits the production of leukotriene B4 (LTB4), which affects endothelial function [124], and also suppresses the degradation of IκBα, thereby inhibiting NF-κB activity and further reducing inflammation [125]. TH prevents the progression of atherosclerosis by activating the Nrf2/ARE signaling pathway, which reduces LDL oxidation [27]. It also regulates vascular inflammation by modulating the autophagy of vascular adventitial fibroblasts (VAF) through the SIRT1-mediated Akt/mTOR pathway, thereby alleviating inflammation [126]. TH metabolites bind to HDL, providing local antioxidant protection and preventing the oxidative modification of HDL proteins and apolipoprotein A-I (ApoA-I), thus reducing oxidative stress and inflammation and benefiting atherosclerosis [127]. 3,4-DHAP reduces the expression levels of TLR4, 5-lipoxygenase (5-LOX), and LTB4 in plaque macrophages while decreasing the expression of vascular cell adhesion molecule-1 (VCAM-1) in plaques, thereby slowing the progression of atherosclerosis and stabilizing plaques [128,129,130,131].
4.3.2. Protection Against Hypertension
Hypertension is an important risk factor for cardiovascular diseases. Improving vascular function, alleviating oxidative damage to the vascular endothelium, and reducing the proliferation of vascular smooth muscle cells can lower blood pressure. Tyr significantly increases the secretion of vascular endothelial growth factor-A (VEGF-A) and platelet-derived growth factor-BB (PDGF-BB) in skeletal muscle cells, enhancing the proliferation and migration of endothelial cells and smooth muscle cells (the two cell types responsible for blood vessel formation) [132]. Tyr also inhibits the development of TH syndrome, improving oxygen transport capacity and increasing cortical microvascular density [133]. TH lowers blood pressure by improving vascular relaxation responses [134]. 3,4-DHAP improves vascular health and reduces hypertension progression by enhancing antioxidant and anti-inflammatory effects [135], modulating the ratio of TXA2 to prostacyclin (PGI2) [136], and inhibiting the expression of hypoxia-inducible NF-κB and COX2 [137]. It also reduces the hypoxia-induced Ca2+ increase in smooth muscle cells (PASMCs) [138].
4.3.3. Protection Against Coronary Artery Disease
Coronary artery disease is caused by the blockage or hardening of coronary arteries. Tyr improves blood lipid metabolism by increasing HDL cholesterol levels [139], reducing the oxidation of LDL cholesterol [140], decreasing plaque formation in heart vessels, and lowering the risk of coronary artery disease [141]. TH regulates endoplasmic reticulum (ER) stress in the human hepatocellular carcinoma cell line HepG2 and prevents the oxidation of LDL [142]. 3,4-DHAP regulates the COX and NO/endothelium-dependent relaxing factor (EDRF) signaling pathways, reducing vascular tension and alleviating the risk of blockage, thus slowing the progression of coronary artery disease [143]. It also increases the ratio of cyclic adenosine monophosphate (cAMP) to cyclic guanosine monophosphate (cGMP) in the blood [144], modulates Ca2+ levels [138], effectively reducing pulmonary artery pressure, pulmonary vascular resistance, and systemic vascular resistance; increasing cardiac output; and slowing the heart rate [145].
4.3.4. Prevention of Thrombosis
Platelet aggregation is the first step in thrombosis, regulated by a series of cytokines. When the blood vessel wall is damaged, the coagulation system is activated, causing the release of AA from platelet membranes, which in turn, under the catalysis of COX, forms TXA2 and promotes platelet aggregation. Early studies have shown that the addition of TH in the diet of type 1 diabetic patients significantly reduced the content of thromboxane B2 (the inactive form of TXA2) in their blood samples, suggesting that TH may reduce platelet aggregation by inhibiting TXA2 production [146]. Additionally, TH inhibits COX activity and expression [147] and may also reduce platelet aggregation through the inhibition of the cyclic adenosine monophosphate–phosphodiesterase (PDE) pathway [148]. Tyr inhibits the production of ROS by activated macrophages, suppresses AA release and COX-2 and iNOS expression, and reduces the synthesis of prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and NO [115,116]. Reducing the production of pro-thrombotic prostaglandins and thromboxanes or inhibiting excessive COX2 activity is an effective approach for thrombosis prevention and treatment. 3,4-DHAP significantly reduces the expression of COX2 and prostaglandin PGE2 while increasing the expression of prostaglandin PGJ2 [149], thus inhibiting the production of TXA2 and B2 (TXB2) [150,151].
4.4. Prevention of Neurodegenerative Diseases
Phenolic compounds, such as TH, have increasingly been studied in recent years for their potential in preventing neurodegenerative diseases [152]. These compounds exhibit significant antioxidant, anti-inflammatory, and anti-apoptotic effects, playing a key role in improving neuronal health [153] and slowing the progression of neurodegenerative diseases. Neurodegenerative diseases, including Alzheimer’s disease (AD) [154], Parkinson’s disease (PD), and Huntington’s disease (HD), are closely associated with factors such as oxidative stress, inflammation, and neuronal cell death [155].
4.4.1. Neuroprotective Effects
Oxidative stress and chronic neuroinflammation are key factors in the development of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [156]. Excessive ROS damages neuronal membranes, proteins, and DNA, ultimately leading to cell death [157]. High levels of oxidative stress and nitrosative stress compromise the integrity and function of brain tissue, particularly during aging. TH has powerful free radical scavenging properties and induces antioxidant enzymes, making it beneficial for treating some neurodegenerative diseases, such as Parkinson’s disease [158]. TH is also highly effective in inhibiting the formation of toxic amyloid proteins and reversing the toxic effects of insulin amyloid aggregates in neuroblastoma cell lines [159]. By activating the Nrf2/ARE pathway, TH reduces the accumulation of free radicals and ROS, protecting neurons from excessive oxidative stress [160]. Furthermore, it inhibits inflammation pathways such as NF-κB and COX-2, reduces the activation of microglial cells, and alleviates neuroinflammatory damage caused by chronic inflammation [161]. 3,4-DHAP increases the expression of Nrf2 and HO-1 proteins and mRNA, promotes Nrf2 nuclear translocation, and enhances autophagic activity, thereby reducing ROS production and indirectly protecting neurons [56].
4.4.2. Anti-Apoptotic Effects on Neurons
Apoptosis (programmed cell death) of neurons is a major hallmark of the progression of neurodegenerative diseases. Oxidative stress and inflammation activate apoptosis-related signaling pathways, leading to neuronal cell death. Tyr regulates the expression of apoptosis-related proteins such as p53 and Bcl-2, inhibits apoptotic signaling, and promotes neuronal cell survival, thereby slowing the progression of neurodegenerative diseases [162]. TH improves neuronal damage in AD by regulating mitochondrial oxidative stress, neuronal inflammation, and cell apoptosis, without affecting amyloid precursor protein (AβPP) processing [163]. It also increases intracellular ROS levels, p53, and γH2AX (phosphorylated H2AX) expression, and reduces AKT expression, significantly decreasing melanoma cell viability and inducing apoptosis [27].
4.4.3. Regulation of Protein Aggregation
Abnormal protein aggregation and deposition (such as β-amyloid and α-synuclein) are major causes of neuronal damage in Alzheimer’s disease and Parkinson’s disease. Tyr inhibits the enhancement of 4-hydroxy-2-nonenal-induced immune response in the hippocampal CA3 region of AD mice [164] and provides molecular insight into its protective signaling effects on endoplasmic reticulum stress-induced β-cell death [165]. Tyr effectively reduces α-synuclein inclusions, thus delaying the degeneration of α-synuclein-dependent dopaminergic neurons in vivo. Tyr treatment also reduces ROS levels and promotes the expression of specific molecular chaperones and antioxidant enzymes, showing potential as a nutritional supplement targeting key pathogenic factors in Parkinson’s disease [166]. Protein aggregation is the basis of many human diseases. Studies have shown that TH effectively inhibits the aggregation of egg white lysozyme fibrils, suggesting its potential in treating Alzheimer’s disease, Parkinson’s disease, and related conditions [167].
4.5. Anticancer Effects
In the past decade, phenolic compounds such as TH have been extensively studied for their significant anticancer effects. Numerous in vitro and in vivo studies have shown that these phenolic compounds inhibit the proliferation of various cancer cell types, induce apoptosis, suppress tumor metastasis, and regulate antioxidant and anti-inflammatory pathways, exhibiting strong anticancer bioactivity [27,168].
4.5.1. Prevention of Cancer Cells
Cancer development is often closely related to oxidative stress and chronic inflammation. Tyr not only reduces oxidative stress-induced cellular damage by activating antioxidant enzymes to scavenge ROS but also alleviates chronic inflammation in the tumor microenvironment by inhibiting the activation of the HIF-1α/NF-κB signaling pathway, thereby reducing the release of inflammatory factors such as TNF-α and IL-6, thus inhibiting cancer initiation and progression [169]. Moreover, Tyr inhibits the PI3K/Akt/mTOR/S6K signaling pathway and reduces the expression of hypoxia-inducible factor 1α (HIF-1α) and its target genes. Additionally, Tyr binds weakly to the cytoplasmic transcription factor AhR and even decreases its transcriptional activity, thereby exerting control over tumor progression in hypoxic conditions [170]. TH downregulates ROS levels and HIF-1α expression in MCF-7 breast cancer cells and can bind to the aryl hydrocarbon receptor (AhR) at high concentrations. It reduces the expression of NF-κB and COX-2, mitigating cell mutations and tumor growth caused by chronic inflammation. Furthermore, TH, when combined with paclitaxel, improves oxidative stress status, reduces chemotherapy side effects, and enhances the antitumor effects of paclitaxel, improving the overall health of patients [171].
4.5.2. Regulation of Cell Apoptosis
Apoptosis is a critical mechanism to prevent carcinogenesis by eliminating abnormally proliferating cells to maintain cellular homeostasis. By regulating apoptosis-related signaling pathways, apoptosis in cancer cells can be promoted, and tumor growth can be inhibited. Tyr induces apoptosis in cancer cells by regulating the expression of apoptosis-related proteins such as p53 and Bcl-2, inhibiting anti-apoptotic signals, and promoting cell survival, thereby reducing tumor growth. TH can also prevent UVB-induced apoptosis in HaCaT cell lines [172]. It reduces the viability of human prostate cancer cells (PC-3 and DU145) through ROS-mediated apoptosis and mitochondrial dysfunction, preventing prostate cancer, and thus providing a foundation for its potential as an anticancer agent [173]. In recent years, extensive research has shown that the antitumor activity of TH is mainly associated with its ability to scavenge ROS and regulate the antioxidant system [174]. Studies have shown that TH inhibits the activation of the AKT and NF-κB pathways, induces G1/S cell cycle arrest, and inhibits the expression of cyclin D1/E and CDK2/4, ultimately reducing androgen receptor and prostate-specific antigen (PSA) levels, supporting its potential application in prostate cancer treatment [175,176]. Cholangiocarcinoma, a digestive tumor with high mortality due to difficulties in early diagnosis and resistance to chemotherapy, has also shown potential anticancer effects from TH. It induces cell cycle arrest and apoptosis in both in vitro and in vivo models, suppressing the proliferation of the TFK-1, KMBC, and GBS-SD cell lines [177]. High doses of TH reduce thyroid cancer cell viability, downregulate cyclin D1 expression, and upregulate the key regulator P21, with Annexin V-P1 staining and DNA ladder assays showing its pro-apoptotic effects on papillary and follicular thyroid cancer cells, further confirming its ability to induce apoptosis and reduce thyroid cancer cell viability [178]. 3,4-DHAP promotes apoptosis by inhibiting NF-κB activation and TNF-α autocrine production, significantly enhancing cell death [179]. Additionally, 3,4-DHAP increases heme oxygenase-1 (HO-1) and CO levels in macrophages [117,118], significantly upregulates soluble Toll-like receptor 4 (sTLR4) mRNA and protein expression, and reduces TNF-α secretion, thus contributing to the regulation of cell apoptosis [119,120].
4.5.3. Inhibition of Cancer Cell Proliferation
The excessive proliferation of cancer cells is a key hallmark of cancer progression. By regulating signaling pathways, cancer cell proliferation can be suppressed, reducing tumor cell division and growth. The MAPK and PI3K/Akt pathways are closely related to cell growth, proliferation, and survival. Tyr significantly reduces cancer cell proliferation by inhibiting these pathways, thereby slowing tumor expansion [180]. Xue Lijun et al. suggested that Tyr induces the expression of phase II detoxifying enzyme NAD(P)H/quinone oxidoreductase-1 (NQO1) in liver cancer cells via the antioxidant response element (ARE), thereby inhibiting the proliferation of human liver cancer SMMC-7721 cells [181,182]. Studies have shown that TH inhibits the expression of chemokine CCL5 in normal human fibroblasts (NHF), thereby blocking the activation of the ERK1/2-cyclin D1 pathway, which suppresses its proliferative effect on MB231 cells. Compared to paclitaxel alone, the combined treatment significantly reduced tumor volume and improved the oxidative status without affecting chemotherapy efficacy. Furthermore, TH is an effective adjunct in paclitaxel treatment for breast cancer. The combination with TH ensures minimal oxidative damage caused by chemotherapy agents, potentially improving patient health [183]. In addition, when used in combination with cetuximab, TH downregulates epidermal growth factor receptor (EGFR) expression, thereby inhibiting colon cancer cell proliferation. The synergistic effect of this combination reduces the required drug concentration by about 10 times compared to monotherapy and enhances the chemotherapy effect [184]. TH inhibits the PI3K/Akt pathway and activates the MAPK pathway, regulating the expression of cell cycle-related proteins (such as Cyclin D1 and CDK4), slowing cancer cell proliferation, and preventing uncontrolled cell growth [27]. It also alters several genes related to cell proliferation, apoptosis, and the Wnt signaling pathway, promoting high expression of the secreted Wnt ligand-related protein 4 (Sfrp4), thus inhibiting breast tumor growth and cell proliferation [185]. Moreover, studies have found that 3,4-DHAP significantly reduces the expression of focal adhesion kinase (FAK) in human pulmonary vascular smooth muscle cells while upregulating caspase-3 expression, thereby inhibiting pathological proliferation [186].
4.6. Liver Protection and Metabolic Regulation
Tyr, HT, and 3,4-DHAP are natural phenolic compounds that have been shown in numerous studies to protect the liver from damage through various mechanisms, including antioxidant, anti-inflammatory, anti-fibrotic effects, and the regulation of lipid metabolism. The following is a summary of their mechanisms of action in liver protection.
4.6.1. Hepatocyte Protection
The liver is the body’s primary detoxifying organ. Prolonged exposure to harmful substances such as drugs, toxins, and alcohol can induce oxidative stress, leading to liver damage, inflammation, and fat accumulation. Chronic liver inflammation is one of the key factors leading to liver fibrosis and cirrhosis. By scavenging ROS, these compounds effectively reduce oxidative stress-induced liver damage. Tyr mitigates inflammation by inhibiting the activation of NF-κB and MAPK pathways, reducing the release of pro-inflammatory cytokines such as TNF-α and IL-6, thus protecting the liver from immune cell-induced damage. Additionally, Tyr lowers the expression of prooxidant enzyme NOX1 [187] as well as the mRNA expressions of transforming growth factor β1 (TGF-β1) and IL-6, reducing inflammation associated with non-alcoholic steatohepatitis (NASH) and showing protective effects against liver immune infiltration [188]. Studies have shown that the addition of Tyr to drinking water significantly alleviates chronic liver disease and liver fibrosis [189]. TH activates the Nrf2/ARE pathway, enhancing the synthesis of antioxidant enzymes and reducing ROS accumulation, thus preventing oxidative damage to liver cells. It also inhibits the activation of inflammation-related pathways such as NF-κB and COX-2, reducing chronic liver inflammation and significantly promoting liver protection [190]. Research indicates that 3,4-DHAP increases Nrf2 and HO-1 protein and mRNA expression, promotes Nrf2 nuclear translocation, and enhances autophagy, significantly reducing inflammation and indirectly contributing to liver protection [56].
4.6.2. Improvement of Insulin Sensitivity and Regulation of Glucose Metabolism
Disorders in glucose metabolism, such as insulin resistance and elevated blood glucose, are major characteristics of diabetes. By promoting glucose metabolism and improving insulin sensitivity, these compounds can effectively improve blood sugar levels, aiding in the prevention and treatment of diabetes. In a streptozotocin (STZ)-induced diabetic rat model treated with Tyr, the compound significantly reduced the activity of 3-hydroxy-3-methylglutaryl-CoA reductase, exhibiting notable anti-inflammatory effects on the liver and pancreas [191,192], playing an important role in diabetes treatment. Specifically, Tyr improves insulin sensitivity by increasing glucose uptake and fatty acid metabolism, thereby alleviating symptoms of diabetes. TH promotes the expression of insulin and peroxiredoxin 6 (Prdx6) [112]. Its derivative, TH nicotinate (HT-N), significantly inhibits α-glucosidase activity, demonstrating strong antioxidant activity and anti-glycation properties, thus improving insulin sensitivity [193]. TH increases the activity of AMPK, enhances glucose metabolism, improves insulin sensitivity, lowers blood glucose levels, and improves glucose and insulin tolerance [194]. 3,4-DHAP regulates the AMPK pathway, enhancing insulin sensitivity, which further helps to improve blood glucose levels and alleviate insulin resistance. It also reduces serum triglycerides, cholesterol, malondialdehyde, and tumor necrosis factor α levels, further regulating metabolism [97].
4.6.3. Anti-Fatty Accumulation and Improvement of Lipid Metabolism
Fat accumulation and lipid metabolism disorders, particularly in the liver and visceral organs, are major contributors to metabolic syndrome, obesity, and fatty liver disease [195]. Tyr intake can alleviate obesity and associated symptoms in high-fat diet (HFD)-fed mice by regulating thermogenesis mediated by peroxisome proliferator-activated receptor α (PPAR α) and the gut microbiota [196]. Additionally, TH dose-dependently inhibits intracellular triglyceride (TG) accumulation and the expression of lipogenesis stimulatory factors, promoting the lipolysis of primary visceral adipocytes and alleviating liver steatosis, demonstrating beneficial effects on obesity [197,198]. Studies show that TH alters the genes associated with adipocyte maturation and differentiation, inhibiting lipogenesis, including the synthesis of new fatty acids and cholesterol, thus benefiting hyperlipidemia [199,200]. TH regulates the transcription factors NF-κB, Nrf2, sterol regulatory element-binding protein 1c (SREBP-1c), and PPAR-γ, as well as their target genes, improving the function of white adipose tissue caused by HDL dietary intake in mice [198]. Furthermore, TH significantly improves liver steatosis and lipid deposition [201]. 3,4-DHAP not only helps maintain lipid homeostasis but also exerts positive metabolic regulatory effects by improving the balance of HDL function [202]. In disease models, 3,4-DHAP reduces total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels in plasma and liver [203]. Research by Zhang Daijuan et al. suggests that 3,4-DHAP may improve pathological lipid metabolism by enhancing the activity of AMP-activated protein kinase (AMPK) and increasing the protein levels of phosphorylated sterol regulatory element-binding protein 1c (p-SREBP-1c) and phosphorylated acetyl-CoA carboxylase (p-ACC) [204].
5. Applications and Challenges for Phenolic Compounds
5.1. Application Prospects
Functional Foods and Health Products: Owing to their natural origin and wide-ranging health benefits, phenolic compounds have become ideal ingredients for developing functional foods and health supplements. Compounds like HT and 3,4-DHAP, endowed with potent antioxidant and anti-inflammatory properties, are already incorporated into various foods and beverages to enhance nutritional value and confer health effects. As technology advances, phenolic compounds will likely be more deeply integrated into daily diets and health products to help prevent chronic diseases and delay aging.
Drug Development and Adjunct Therapies: Phenolic compounds display remarkable pharmacological activities in anticancer, cardiovascular protection, and neuroprotection, offering promise for drug discovery. For instance, 3,4-DHAP, recognized for multiple anticancer mechanisms, is already considered a candidate for novel antitumor agents. Moreover, phenolic compounds may serve as adjuncts to chemo- and radiotherapy, potentially boosting efficacy and reducing side effects.
Chronic Disease Prevention: Thanks to their antioxidant, anti-inflammatory, and metabolic-regulating effects, phenolic compounds hold broad potential in preventing chronic diseases like diabetes, cardiovascular disorders, and neurodegenerative conditions. Ongoing research continues to underscore their significant role in managing chronic disease and safeguarding public health.
5.2. Bioavailability and Safety
Low bioavailability remains a major obstacle to phenolic compound utilization [205]. Due to their structural features, these compounds are poorly absorbed in the gastrointestinal tract and rapidly metabolized, restricting their in vivo activity [206]. Developing methods to enhance bioavailability—such as nano-drug delivery systems or other advanced transport technologies—will be crucial in future studies. Although phenolic compounds occur naturally in various foods and are associated with numerous health benefits, their safety in long-term, high-dose use requires further investigation.
HT is actively being developed as a potential supplement or preservative for the nutraceutical, cosmeceutical, and food industries. Toxicological assessments indicate a no-observed-adverse-effect-level (NOAEL) of 500 mg/kg/day [207]. Nanoparticle formulations (average size of ~260.90 nm) of 3,4-DHAP derivatives improve their oral bioavailability, offering new strategies to address the low bioavailability of phenolic drugs [208]. Ensuring safety and minimizing potential toxicity remain major challenges, especially when used at higher concentrations or as pharmaceutical agents [209,210].
The mechanisms of phenolic compounds’ bioactivity are diverse and complex. While substantial research has elucidated their antioxidant, anti-inflammatory, and metabolic-regulation effects, some of the key molecular signaling pathways remain incompletely understood. Further in-depth studies are necessary to clarify these compounds’ functions in different biological systems, thereby guiding more effective applications.
Moreover, phenolic compounds exhibit variable efficacy depending on their source, purity, extraction method, and mode of administration. Establishing unified quality standards and dosage protocols is essential for their clinical application [211] and for functional food development. Future work will focus on standardized extraction processes and product specifications to ensure consistency and effectiveness. By overcoming these hurdles, phenolic compounds will find broader applications in health promotion, facilitating innovation in functional foods, health products, and drug development for chronic disease prevention and treatment [212].
6. Conclusions and Outlook
In conclusion, tyrosol (Tyr), hydroxytyrosol (HT), and 3,4-Dihydroxyacetophenone (3,4-DHAP) are promising phenolic compounds with significant antioxidant, anti-inflammatory, anticancer, and cardiovascular protective properties. These compounds hold considerable potential for the prevention and treatment of chronic diseases, including cardiovascular conditions, neurodegenerative diseases, and cancer. Their mechanisms of action, such as free radical scavenging, oxidative stress reduction, and anti-inflammatory effects, highlight their therapeutic relevance.
However, challenges remain, particularly regarding their low bioavailability, which limits their clinical effectiveness. Advanced delivery systems, such as nanoparticle formulations or lipid-based carriers, are needed to enhance their absorption and bioavailability. Additionally, although these compounds show promise in short-term use, further research on their long-term safety, including toxicity and drug interactions, is essential before widespread clinical application.
Future research should focus on better understanding the molecular mechanisms underlying their therapeutic effects, including their influence on redox homeostasis, immune response, and cancer cell apoptosis. Furthermore, exploring combination therapies involving Tyr, HT, and 3,4-DHAP with conventional treatments may provide synergistic benefits, particularly for complex diseases like cancer and cardiovascular diseases.
In summary, while Tyr, HT, and 3,4-DHAP show great promise as therapeutic agents, overcoming challenges related to bioavailability, safety, and mechanistic understanding will be critical for their successful clinical application in treating chronic diseases.
Author Contributions
All the authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; and have agreed on the journal to which the article has been submitted. All authors have read and agreed to the published version of the manuscript.
Funding
Key Discipline Project on Chinese Pharmacology of Hunan University of Chinese Medicine (202302), Scientific Research Topics of Hunan Provincial Health and Wellness Commission (D202303017861), National Natural Science Foundation of China Project (82104324) and Hunan Provincial Natural Science Foundation General Project (2025JJ50100).
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nam, M.; Choi, J.Y.; Kim, M.S. Metabolic Profiles, Bioactive Compounds, and Antioxidant Capacity in Lentinula edodes Cultivated on Log versus Sawdust Substrates. Biomolecules 2021, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Valdés, A.; Sánchez-Martínez, J.D.; Ibáñez, E.; Bi, J.; Cifuentes, A. Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes. Foods 2022, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic Compounds of Rhodiola rosea L. as the Potential Alternative Therapy in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- Franco, C.A.; da Silva, T.I.; Dias, M.G.; Ferreira, B.W.; de Sousa, B.L.; Bousada, G.M.; Barreto, R.W.; Vaz, B.G.; Lima, G.D.S.; Dos Santos, M.H.; et al. Synthesis of Tyrosol 1,2,3-Triazole Derivatives and Their Phytotoxic Activity against Euphorbia heterophylla. J. Agric. Food Chem. 2022, 70, 2806–2816. [Google Scholar] [CrossRef]
- Vázquez-Ruiz, Z.; Toledo, E.; Vitelli-Storelli, F.; Goni, L.; de la O, V.; Bes-Rastrollo, M.; Martínez-González, M. Effect of Dietary Phenolic Compounds on Incidence of Cardiovascular Disease in the SUN Project; 10 Years of Follow-Up. Antioxidants 2022, 11, 783. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Loru, D.; Incani, A.; Deiana, M.; Corona, G.; Atzeri, A.; Melis, M.P.; Rosa, A.; Dessì, M.A. Protective effect of hydroxytyrosol and tyrosol against oxidative stress in kidney cells. Toxicol. Ind. Health 2009, 25, 301–310. [Google Scholar] [CrossRef]
- Stiuso, P.; Bagarolo, M.L.; Ilisso, C.P.; Vanacore, D.; Martino, E.; Caraglia, M.; Porcelli, M.; Cacciapuoti, G. Protective Effect of Tyrosol and S-Adenosylmethionine against Ethanol-Induced Oxidative Stress of Hepg2 Cells Involves Sirtuin 1, P53 and Erk1/2 Signaling. Int. J. Mol. Sci. 2016, 17, 622. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Navarro, C.; Montoro-García, S.; Orenes-Piñero, E. Hydroxytyrosol: Its role in the prevention of cardiovascular diseases. Heliyon 2023, 9, e12963. [Google Scholar] [CrossRef]
- Kiani, A.K.; Miggiano, G.A.D.; Aquilanti, B.; Velluti, V.; Matera, G.; Gagliardi, L.; Bertelli, M. Food supplements based on palmitoylethanolamide plus hydroxytyrosol from olive tree or Bacopa monnieri extracts for neurological diseases. Acta Biomed. 2020, 91, e2020007. [Google Scholar]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.L.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef]
- Vlachogianni, I.C.; Fragopoulou, E.; Kostakis, I.K.; Antonopoulou, S. In vitro assessment of antioxidant activity of tyrosol, resveratrol and their acetylated derivatives. Food Chem. 2015, 177, 165–173. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Q.; Zhu, J.; Xia, G.; Zang, H. Synthesis, α-Glucosidase inhibition and molecular docking studies of tyrosol derivatives. Nat. Prod. Res. 2021, 35, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.T.; Bronze, M.R.; Ventura, M.R. Phenolic Compounds from Virgin Olive Oil: Approaches for Their Synthesis and Analogues. J. Agric. Food Chem. 2022, 70, 14109–14128. [Google Scholar] [CrossRef] [PubMed]
- Strutz, H.; Frankfurt, A.M. Production of 4-Hydroxyphenethyl. U.S. Patent 5003115, 26 March 1991. [Google Scholar]
- Durrwachter, J.R. Production of Ethanol. U.S. Patent 5254753, 19 October 1993. [Google Scholar]
- Wei, Y.; Sun, R. Study on the Synthesis Process of 4-(2-Methoxyethyl)phenol. Fine Chem. 1993, 6, 28–30. [Google Scholar]
- Zhou, Y. New Synthesis Process of 4-(2′-Methoxyethyl)phenol. Liaoning Chem. Ind. 2005, 12, 515–517. [Google Scholar]
- Wang, Y.; Luo, X.; Song, J. Advances in the Synthesis of Tyrosol. Guangdong Chem. Ind. 2012, 39, 137–138+135. [Google Scholar]
- Lai, Y.; Chen, H.; Liu, L.; Fu, B.; Wu, P.; Li, W.; Hu, J.; Yuan, J. Engineering a Synthetic Pathway for Tyrosol Synthesis in Escherichia coli. ACS Synth. Biol. 2022, 11, 441–447. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, M.; Deng, H.; Chen, X.; Shang, Y.; Liu, X.; Yu, X. Efficient whole cell biotransformation of tyrosol from L-tyrosine by engineered Escherichia coli. Enzyme Microb. Technol. 2022, 160, 110100. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, X.; Chang, J.; Zhang, L.; Xu, W.; Shen, W.; Fan, Y. Reconstruction of tyrosol synthetic pathways in Escherichia coli. Chin. J. Chem. Eng. 2018, 26, 2615–2621. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Katogiannis, K.; Chania, C.; Iakovis, N.; Tsoumani, M.; Christodoulou, A.; Brinia, E.; Pavlidis, G.; Thymis, J.; Tsilivarakis, D.; et al. Association of hydroxytyrosol enriched olive oil with vascular function in chronic coronary disease. Eur. J. Clin. Investig. 2023, 53, e13983. [Google Scholar] [CrossRef]
- Utami, N.D.; Nordin, A.; Katas, H.; Bt Hj Idrus, R.; Fauzi, M.B. Molecular Action of Hydroxytyrosol in Wound Healing: An In Vitro Evidence-Based Review. Biomolecules 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xia, M.; Ding, Z.; Rong, X.; Mei, X. Enhancing physical and chemical stability of hygroscopic hydroxytyrosol by cocrystal formation. Int. J. Pharm. 2023, 646, 123470. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol. Appl. Microbiol. Biotechnol. 2019, 103, 5957–5974. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Jin, R.; Liu, Q.; Xiao, C.; Yin, B.; Wu, J. Synthesis of Hydroxytyrosol. Cent. South Pharm. 2014, 12, 864–866. [Google Scholar]
- Xu, C.; Gao, J.; Zhang, J.; Jiang, S. Process Research of Hydroxytyrosol Synthesis. Fine Chem. Ind. 2010, 27, 1209–1212. [Google Scholar]
- Xiao, Y.; Zhang, Y.; Yang, Y.; Ren, F.; Cui, G.; Zhao, Y. Synthesis Methods of Hydroxytyrosol. Int. J. Pharm. Res. 2013, 40, 335–337. [Google Scholar]
- Zang, H.; Xu, Q.; Zhang, L.; Tian, D. Research on the Synthesis Process of Hydroxytyrosol. J. Tonghua Norm. Univ. 2020, 41, 44–48. [Google Scholar]
- Molina, G.G.; Peters, E.; Palmeri, R.; Awoke, Y.; Álvarez, C.M.; Blanco, R.M. Enzymatic synthesis of Hydroxytyrosol from Oleuropein for valorization of an agricultural waste. Bioengineered 2024, 15, 2396647. [Google Scholar]
- Liu, W.K.; Ren, X.X.; Xu, L.; Lin, J. Modular cascade with engineered HpaB for efficient synthesis of hydroxytyrosol. Bioorganic Chem. 2025, 155, 108125. [Google Scholar] [CrossRef]
- Zeng, B.; Lai, Y.; Liu, L.; Cheng, J.; Zhang, Y.; Yuan, J. Engineering Escherichia coli for High-Yielding Hydroxytyrosol Synthesis from Biobased l-Tyrosine. J. Agric. Food Chem. 2020, 68, 7691–7696. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jia, P.; Bai, Y.; Fan, T.P.; Zheng, X.; Cai, Y. Efficient Synthesis of Hydroxytyrosol from l-3,4-Dihydroxyphenylalanine Using Engineered Escherichia coli Whole Cells. J. Agric. Food Chem. 2019, 67, 6867–6873. [Google Scholar] [CrossRef]
- Deri-Zenaty, B.; Bachar, S.; Rebroš, M.; Fishman, A. A coupled enzymatic reaction of tyrosinase and glucose dehydrogenase for the production of hydroxytyrosol. Appl. Microbiol. Biotechnol. 2020, 104, 4945–4955. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Li, X.; Liu, W.; Yue, H.; Liu, J.; Bai, D.; Li, W.; Fan, G. Multienzyme cascade for synthesis of hydroxytyrosol via engineered Escherichia coli. Sci. Rep. 2025, 15, 471. [Google Scholar] [CrossRef]
- Feng, L.M.; Pan, H.Z.; Zhang, Z.N. Antioxidant effect of qingxintone. Acta Pharm. Sin. 1987, 241–244. [Google Scholar] [CrossRef]
- Lin, C.; Chen, X.; Zhang, D.; Fan, J.; Lin, L. Cultivation Techniques for the Domestication of Wild Mao Dongqing in Eastern Guangdong. For. Sci. Technol. Commun. 2018, 4, 9–11. [Google Scholar]
- Ruan, X.; Li, Z.H.; Wang, Q.; Pan, C.D.; Jiang, D.A.; Wang, G.G. Autotoxicity and allelopathy of 3,4-dihydroxyacetophenone isolated from Picea schrenkiana needles. Molecules 2011, 16, 8874–8893. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Dou, H.; Li, X.; Fan, X. Research Progress on the Synthesis of Aromatic Ketone Compounds. Fine Chem. Interm. 2009, 39, 1–6. [Google Scholar]
- Ding, H.; Zhang, Y.; Jiang, X. Synthesis of 3,4-Dihydroxyphenylacetone. Fine Chem. Interm. 2016, 46, 25–26+44. [Google Scholar]
- Yuan, X.; Tian, Y.; Wang, W.; Zhang, Y.; Xu, D. Research on the Synthesis of the Traditional Chinese Medicine Active Substance Qingxin Ketone via Escherichia coli Whole-Cell Transformation of 1-(4-Hydroxyphenyl)ethanol. Chin. J. Tradit. Chin. Med. 2024, 1–9. [Google Scholar] [CrossRef]
- Arangia, A.; Marino, Y.; Impellizzeri, D.; D’Amico, R.; Cuzzocrea, S.; Di Paola, R. Hydroxytyrosol and Its Potential Uses on Intestinal and Gastrointestinal Disease. Int. J. Mol. Sci. 2023, 24, 3111. [Google Scholar] [CrossRef]
- Cuffaro, D.; Pinto, D.; Silva, A.M.; Bertolini, A.; Bertini, S.; Saba, A.; Macchia, M.; Rodrigues, F.; Digiacomo, M. Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules 2023, 28, 5150. [Google Scholar] [CrossRef]
- Cañuelo, A.; Gilbert-López, B.; Pacheco-Liñán, P.; Martínez-Lara, E.; Siles, E.; Miranda-Vizuete, A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech. Ageing Dev. 2012, 133, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Casadey, R.; Challier, C.; Altamirano, M.; Spesia, M.B.; Criado, S. Antioxidant and antimicrobial properties of tyrosol and derivative-compounds in the presence of vitamin B2. Assays of synergistic antioxidant effect with commercial food additives. Food Chem. 2021, 335, 127576. [Google Scholar] [CrossRef]
- Casadey, R.; Challier, C.; Senz, A.; Criado, S. Antioxidant ability of tyrosol and derivative-compounds in the presence of O(2)((1)Δ(g))-species. Studies of synergistic antioxidant effect with commercial antioxidants. Food Chem. 2019, 285, 275–281. [Google Scholar] [CrossRef]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Zanotto, I.; Sayaf, K.; Colognesi, M.; Polini, B.; Digiacomo, M.; Macchia, M.; Nieri, P.; et al. The phenolic compounds tyrosol and hydroxytyrosol counteract liver fibrogenesis via the transcriptional modulation of NADPH oxidases and oxidative stress-related miRNAs. Biomed. Pharmacother. 2023, 157, 114014. [Google Scholar] [CrossRef]
- Begines, P.; Biedermann, D.; Valentová, K.; Petrásková, L.; Pelantová, H.; Maya, I.; Fernández-Bolaños, J.G.; Křen, V. Chemoenzymatic Synthesis and Radical Scavenging of Sulfated Hydroxytyrosol, Tyrosol, and Acetylated Derivatives. J. Agric. Food Chem. 2019, 67, 7281–7288. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.G.; Ma, J. Nutritional benefit of olive oil: The biological effects of hydroxytyrosol and its arylating quinone adducts. J. Agric. Food Chem. 2008, 56, 8774–8786. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Pan; Zhang, Z. Antioxidant Effect of Qingxin Ketone. J. Pharm. 1987, 4, 241–244. [Google Scholar]
- Cao, D.; Wang, Y.; Li, W.; Ji, J.; Guo, J.; Zhang, D.; Liu, J. 3,4-Dihydroxyacetophenone attenuates oxidative stress-induced damage to HUVECs via regulation of the Nrf2/HO-1 pathway. Mol. Med. Rep. 2022, 25, 199. [Google Scholar] [CrossRef]
- Sadauskiene, I.; Liekis, A.; Bernotiene, R.; Sulinskiene, J.; Kasauskas, A.; Zekonis, G. The Effects of Buckwheat Leaf and Flower Extracts on Antioxidant Status in Mouse Organs. Oxid. Med. Cell Longev. 2018, 2018, 6712407. [Google Scholar] [CrossRef]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, D.; Shahidi, F. Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 2018, 245, 1262–1268. [Google Scholar] [CrossRef]
- Marzocchi, S.; Anankanbil, S.; Caboni, M.F.; Guo, Z. Enzymatic alkylsuccinylation of tyrosol: Synthesis, characterization and property evaluation as a dual-functional antioxidant. Food Chem. 2018, 246, 108–114. [Google Scholar] [CrossRef]
- Aguiar, R.P.; Aldawsari, F.S.; Wiirzler, L.A.M.; Silva-Filho, S.E.; Silva-Comar, F.M.S.; Bersani-Amado, C.A.; Velazquez-Martinez, C.A.; Cuman, R.K.N. Synthesis and Biological Evaluation of New Tyrosol-Salicylate Derivatives as Potential Anti-Inflammatory Agents. Curr. Pharm. Des. 2017, 23, 6841–6848. [Google Scholar] [CrossRef]
- de Las Hazas, M.C.L.; Rubio, L.; Macia, A.; Motilva, M.J. Hydroxytyrosol: Emerging Trends in Potential Therapeutic Applications. Curr. Pharm. Des. 2018, 24, 2157–2179. [Google Scholar] [CrossRef]
- Pérez-Barrón, G.; Montes, S.; Aguirre-Vidal, Y.; Santiago, M.; Gallardo, E.; Espartero, J.L.; Ríos, C.; Monroy-Noyola, A. Antioxidant Effect of Hydroxytyrosol, Hydroxytyrosol Acetate and Nitrohydroxytyrosol in a Rat MPP(+) Model of Parkinson’s Disease. Neurochem. Res. 2021, 46, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Zhou, X.; Li, R. Effect of 3,4-dihydroxyacetophenone on endothelial dysfunction in obese rats. Pharm. Biol. 2015, 53, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M.; Brocchini, S.; Somavarapu, S.; Procopio, A. Hydroxytyrosol oleate: A promising neuroprotective nanocarrier delivery system of oleuropein and derivatives. Int. J. Pharm. 2023, 631, 122498. [Google Scholar] [CrossRef]
- Pazos, M.; Alonso, A.; Sánchez, I.; Medina, I. Hydroxytyrosol prevents oxidative deterioration in foodstuffs rich in fish lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; Bast, A.; Haenen, G.R. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxid. Med. Cell Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef]
- Marrero, A.D.; Quesada, A.R.; Martínez-Poveda, B.; Medina, M. Anti-Cancer, Anti-Angiogenic, and Anti-Atherogenic Potential of Key Phenolic Compounds from Virgin Olive Oil. Nutrients 2024, 16, 1283. [Google Scholar] [CrossRef]
- Shi, L.; Qin; Gao, S. Using Fluorescence Polarization to Investigate the Effects of Qingxin Ketone on the Microviscosity and Fluidity of Lipid Domains in Platelet Membranes. J. Suzhou Med. Coll. 1983, 2, 34–38. [Google Scholar]
- Peng, D.; Shi, L. Effect of 3,4-Dihydroxyphenylacetone on the Phospholipid Composition of Platelet Membranes in Aged Rats. J. Pharm. 1995, 5, 343–346. [Google Scholar]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Hur, J.; Lee, Y.; Yoon, B.-R.; Choi, S.Y. Protective Effects of Tyrosol Against Oxidative Damage in L6 Muscle Cells. Food Sci. Technol. Res. 2018, 24, 943–947. [Google Scholar] [CrossRef]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Li, W.B.; Qiao, X.P.; Wang, Z.X.; Wang, S.; Chen, S.W. Synthesis and antioxidant activity of conjugates of hydroxytyrosol and coumarin. Bioorg Chem. 2020, 105, 104427. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, M.; Zhou, K.; Song, T.; Huang, L. 3,4-Dihydroxyphenylacetone Alleviates Lipopolysaccharide-Induced Acute Lung Injury Through Anti-Inflammatory and Antioxidant Effects. J. Chin. Pharm. Sci. 2021, 30, 956–968. [Google Scholar]
- Vijayan, M.; Reddy, P.H. Reduced VDAC1, Maintained Mitochondrial Dynamics and Enhanced Mitochondrial Biogenesis in a Transgenic Tau Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8561. [Google Scholar] [CrossRef]
- Liu, B.Y.; Li, L.; Liu, G.L.; Ding, W.; Chang, W.G.; Xu, T.; Ji, X.Y.; Zheng, X.X.; Zhang, J.; Wang, J.X. Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol. Sin. 2021, 42, 701–714. [Google Scholar] [CrossRef]
- Manna, C.; Della Ragione, F.; Cucciolla, V.; Borriello, A.; D’Angelo, S.; Galletti, P.; Zappia, V. Biological effects of hydroxytyrosol, a polyphenol from olive oil endowed with antioxidant activity. Adv. Exp. Med. Biol. 1999, 472, 115–130. [Google Scholar]
- Visioli, F.; Rodríguez-Pérez, M.; Gómez-Torres, Ó.; Pintado-Losa, C.; Burgos-Ramos, E. Hydroxytyrosol improves mitochondrial energetics of a cellular model of Alzheimer’s disease. Nutr. Neurosci. 2022, 25, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Z.; Li, L.; Espe, M.; Lu, K.L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef]
- Lu, X.Y.; Chen, W.C. Effect of 3, 4-dihydroxyacetophenone on Na+, K+ -ATPase activity of injured mitochondria and the oxygen consumption of brain cells of rat. Yao Xue Xue Bao 2005, 40, 13–16. [Google Scholar] [PubMed]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorganic Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef]
- Zorić, N.; Kopjar, N.; Rodriguez, J.V.; Tomić, S.; Kosalec, I. Protective effects of olive oil phenolics oleuropein and hydroxytyrosol against hydrogen peroxide-induced DNA damage in human peripheral lymphocytes. Acta Pharm. 2021, 71, 131–141. [Google Scholar] [CrossRef]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. [Google Scholar] [CrossRef]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef]
- Moon, H.W.; Park, J.W.; Lee, K.W.; Jeong, H.C.; Choi, J.B.; Choi, S.W.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Administration of Goji (Lycium chinense Mill.) Extracts Improves Erectile Function in Old Aged Rat Model. World J. Mens. Health 2017, 35, 43–50. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Huang, I.T.; Shih, H.-J.; Chang, Y.-Y.; Kao, M.-C.; Shih, P.-C.; Huang, C.-J. Cluster of differentiation 14 and toll-like receptor 4 are involved in the anti-inflammatory effects of tyrosol. J. Funct. Foods 2019, 53, 93–104. [Google Scholar] [CrossRef]
- Ramírez-Tejero, J.A.; Martínez-Lara, E.; Peinado, M.; Del Moral, M.L.; Siles, E. Hydroxytyrosol as a Promising Ally in the Treatment of Fibromyalgia. Nutrients 2020, 12, 2386. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Wei, F.; Hou, G.; You, Y.; Wang, X.; Cao, S.; Yang, X.; Liu, W.; Zhang, S.; et al. Hydroxytyrosol Ameliorates Intervertebral Disc Degeneration and Neuropathic Pain by Reducing Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2022, 2022, 2240894. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wang, Y.; Zhang, D.; Liu, J. Study on the Role of 3,4-Dihydroxyphenylacetone in Regulating the JNK/Nrf2 Signaling Pathway to Alleviate ox-LDL-Induced Endothelial Cell Injury. Chinese J. Pathophysiol. 2022, 38, 788–794. [Google Scholar]
- Liu, C.; Sun, J.; Xue, F.; Yi, Y.; Han, A. Effect of 3,4-dihydroxyacetophenone on endothelial dysfunction in streptozotocin-induced rats with type 2 diabetes. J. Cardiovasc. Pharmacol. 2015, 65, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Effect of Qingxin Ketone on Vascular Smooth Muscle Cell Proliferation in Type 2 Diabetic Rats. Pract. Diabetes J. 2014, 10, 54–55. [Google Scholar]
- Bonura, A.; Vlah, S.; Longo, A.; Bulati, M.; Melis, M.R.; Cibella, F.; Colombo, P. Hydroxytyrosol modulates Par j 1-induced IL-10 production by PBMCs in healthy subjects. Immunobiology 2016, 221, 1374–1377. [Google Scholar] [CrossRef]
- Sato, K.; Mihara, Y.; Kanai, K.; Yamashita, Y.; Kimura, Y.; Itoh, N. Tyrosol ameliorates lipopolysaccharide-induced ocular inflammation in rats via inhibition of nuclear factor (NF)-κB activation. J. Vet. Med. Sci. 2016, 78, 1429–1438. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Baron, M.; Bouhlel, M.A.; Vanhoutte, J.; Copin, C.; Sebti, Y.; Derudas, B.; Mayi, T.; Bories, G.; Tailleux, A.; et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 2011, 108, 985–995. [Google Scholar] [CrossRef]
- Güvenç, M.; Cellat, M.; Özkan, H.; Tekeli, İ.O.; Uyar, A.; Gökçek, İ.; İşler, C.T.; Yakan, A. Protective Effects of Tyrosol Against DSS-Induced Ulcerative Colitis in Rats. Inflammation 2019, 42, 1680–1691. [Google Scholar] [CrossRef]
- Bertelli, A.; Migliori, M.; Bertelli, A.A.; Origlia, N.; Filippi, C.; Panichi, V.; Falchi, M.; Giovannini, L. Effect of some white wine phenols in preventing inflammatory cytokine release. Drugs Exp. Clin. Res. 2002, 28, 11–15. [Google Scholar]
- Giovannini, L.; Migliori, M.; Filippi, C.; Origlia, N.; Panichi, V.; Falchi, M.; Bertelli, A.A.; Bertelli, A. Inhibitory activity of the white wine compounds, tyrosol and caffeic acid, on lipopolysaccharide-induced tumor necrosis factor-alpha release in human peripheral blood mononuclear cells. Int. J. Tissue React. 2002, 24, 53–56. [Google Scholar]
- Bertelli, A.A.E.; Massamiliano, M.; Vincenzo, P.; Bianamaria, L.; Nicola, O.; Agnese, F.; Giuseppa, C.M.; Luca, G. Oxidative stress and inflammatory reaction modulation by white wine. Ann. N. Y. Acad. Sci. 2002, 957, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Ren, D.; Wang, P.; Song, Z.; Wu, H.; Yao, S.; Geng, L.; Su, Y.; Bai, X. Upregulation of Sirt1 by tyrosol suppresses apoptosis and inflammation and modulates extracellular matrix remodeling in interleukin-1β-stimulated human nucleus pulposus cells through activation of PI3K/Akt pathway. Int. Immunopharmacol. 2020, 88, 106904. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Kihara, T.; Ibi, K.; Senshu, M.; Nejishima, H.; Takeda, Y.; Imai, K.; Ogawa, H. Olive-Derived Hydroxytyrosol Shows Anti-inflammatory Effect without Gastric Damage in Rats. Biol. Pharm. Bull. 2019, 42, 1120–1127. [Google Scholar] [CrossRef]
- Wen, X.; Tang, S.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. The PI3K/Akt-Nrf2 Signaling Pathway and Mitophagy Synergistically Mediate Hydroxytyrosol to Alleviate Intestinal Oxidative Damage. Int. J. Biol. Sci. 2024, 20, 4258–4276. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Soylu, H.; Karacor, K. The effects of hydroxytyrosol on Prdx6 and insulin expression in diabetic rat pancreases. Histochem. Cell Biol. 2023, 160, 127–134. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Abdullah; Tian, W.; Qiu, Z.; Song, M.; Cao, Y.; Xiao, J. Hydroxytyrosol Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Inflammatory Responses, Intestinal Barrier, and Microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med. 2003, 35, 1073–1081. [Google Scholar] [CrossRef]
- De Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur. J. Pharmacol. 2007, 566, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, P.; Pan, Q.; Wan, J.; Zhang, L.; Wu, T.; Zhou, X.; Ye, D. Effect of Qingxin Ketone on Heme Oxygenase-1 mRNA/Carbon Monoxide and TNF-α Expression in Activated RAW264.7 Cells. Chin. J. Integr. Med. 2004, S1, 257–260. [Google Scholar]
- Li, G.; Wu, P.; Zhang, D.; Ye, D.; Li, F. Investigation of the Mechanism by Which Qingxin Ketone Inhibits Lipopolysaccharide-Induced Apoptosis in Mouse Macrophages. Chin. Herb. Med. 2005, 12, 1835–1838. [Google Scholar]
- Zhang, D.; Wu, S.; Liu, T.; Tian, H.; Wang, Y.; Duan, W.; Ye, D. Effect of Qingxin Ketone on Soluble Toll-like Receptor 4 Expression and TNF-α in RAW264.7 Cells. J. Weifang Med. Univ. 2006, 4, 264–265+322. [Google Scholar]
- Zhang, D.; Wu, S.; Liu, T.; Tian, H.; Wang, Y.; Duan, W.; Ye, D. Effect of Qingxin Ketone on Soluble Toll-like Receptor 4 Expression and Inflammatory-Related Factors in RAW264.7 Cells. Chin. J. Clin. Rehabil. 2006, 11, 120–122. [Google Scholar]
- Wu, P.; Zhang, L.; Zhou, X.; Li, Y.; Zhang, D.; Wan, J.; Ye, D. Inflammation pro-resolving potential of 3,4-dihydroxyacetophenone through 15-deoxy-delta12,14-prostaglandin J2 in murine macrophages. Int. Immunopharmacol. 2007, 7, 1450–1459. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Wang, J. 3,4-Dihydroxyphenylacetone Inhibits NF-κB Nuclear Translocation to Alleviate Lipopolysaccharide-Induced Macrophage Inflammatory Response. J. Pract. Med. 2018, 34, 1092–1095. [Google Scholar]
- Castañer, O.; Covas, M.I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.F.; de la Torre, R.; Muñoz-Aguayo, D.; Vila, J.; Fitó, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Huang, Y.; Mo, D.; Ma, N.; Gao, F.; Song, L.; Sun, X.; Xu, X.; Liu, L.; Huo, X.; et al. Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation in in vitro oxygen glucose deprivation. Neurochem. Int. 2018, 121, 140–145. [Google Scholar] [CrossRef]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol. Pharmacol. 2018, 96, 88–96. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Haizum Abdullah, N.A.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Liu, J.; Cui, X.; Guo, J.; Wang, J.; Ye, D. Relationship between Qingxin Ketone in the Prevention and Treatment of Atherosclerosis and the TLR4 Signal Transduction Pathway. Chin. J. New Drugs Clin. Pharmacol. 2009, 20, 404–407. [Google Scholar]
- Zhang, D.; Liu, T.; Liu, J.; Cui, X.; Guo, J.; Wang, J.; Ye, D. Effect of Qingxin Ketone on Atherosclerotic Formation in ApoE-Deficient Mice and on TLR4 Expression in the Major Cells of Plaques. Chin. Mod. Appl. Pharm. 2010, 27, 1–5. [Google Scholar]
- Zhang, D.; Liu, T.; Liu, J.; Cui, X.; Guo, J.; Wang, J.; Ye, D. Effect of Qingxin Ketone on the Expression and Secretion of Lipid Factor in ApoE-Deficient Mice and Cultured Vascular Smooth Muscle and Endothelial Cells. Chin. J. Pharm. 2010, 45, 1623–1627. [Google Scholar]
- Zhang, D.; Liu, J.; Wang, L.; Wang, J.; Li, W.; Zhuang, B.; Hou, J.; Liu, T. Effects of 3,4-dihydroxyacetophenone on the hypercholesterolemia-induced atherosclerotic rabbits. Biol. Pharm. Bull. 2013, 36, 733–740. [Google Scholar] [CrossRef]
- Zhang, J.; Nugrahaningrum, D.A.; Marcelina, O.; Ariyanti, A.D.; Wang, G.; Liu, C.; Wu, S.; Kasim, V. Tyrosol Facilitates Neovascularization by Enhancing Skeletal Muscle Cells Viability and Paracrine Function in Diabetic Hindlimb Ischemia Mice. Front. Pharmacol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Aliev, O.I.; Sidekhmenova, A.V.; Shamanaev, A.Y.; Anishchenko, A.M.; Fomina, T.I.; Plotnikova, T.M.; Arkhipov, A.M. Effect of p-tyrosol on hemorheological parameters and cerebral capillary network in young spontaneously hypertensive rats. Microvasc. Res. 2018, 119, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Fukuda, D.; Ganbaatar, B.; Pham, P.T.; Aini, K.; Rahadian, A.; Suto, K.; Yagi, S.; Kusunose, K.; Yamada, H.; et al. Olive mill wastewater and hydroxytyrosol inhibits atherogenesis in apolipoprotein E-deficient mice. Heart Vessel. 2023, 38, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Wang, D.X.; Ming, Z.; Yu, S.B. Effects of 3,4-dihydroxyacetophenone (3,4-DHAP) on hypoxic pulmonary and systemic vascular response in dogs. J. Tongji Med. Univ. 1995, 15, 26–30. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Zhou, J.; Huang, Y. Effect of Qingxin Ketone on Hypoxia-Induced Expression of NF-κB and COX2 in Human Umbilical Vein Endothelial Cells. Zhejiang J. Tradit. Chin. Med. 2009, 44, 94–95. [Google Scholar]
- Wazir, F.; Wang, D.; Hu, Q. Effects of 3,4-dihydroxyacetophenone on cytosolic calcium in pulmonary artery endothelial and smooth muscle cells during acute hypoxia. J. Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 550–551. [Google Scholar]
- Giovannini, C.; Straface, E.; Modesti, D.; Coni, E.; Cantafora, A.; De Vincenzi, M.; Malorni, W.; Masella, R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J. Nutr. 1999, 129, 1269–1277. [Google Scholar] [CrossRef]
- Covas, M.I.; Miró-Casas, E.; Fitó, M.; Farré-Albadalejo, M.; Gimeno, E.; Marrugat, J.; De La Torre, R. Bioavailability of tyrosol, an antioxidant phenolic compound present in wine and olive oil, in humans. Drugs Exp. Clin. Res. 2003, 29, 203–206. [Google Scholar]
- Covas, M.I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef]
- Giordano, E.; Davalos, A.; Nicod, N.; Visioli, F. Hydroxytyrosol attenuates tunicamycin-induced endoplasmic reticulum stress in human hepatocarcinoma cells. Mol. Nutr. Food Res. 2014, 58, 954–962. [Google Scholar] [CrossRef]
- Farman, U.; Wang, D.X.; Deng, J. Effects of 3,4-dihydroxyacetophenone on hypoxic vasoconstriction in isolated pulmonary and basilar arterial rings. J. Tongji Med. Univ. 1994, 14, 252–256. [Google Scholar] [PubMed]
- Lin, C.; Zhang, Z.; Xu, Y.; Ni, W. Effect of Qingxin Ketone on Hemodynamics and Plasma Levels of Atrial Natriuretic Peptide and Cyclic Nucleotides in Patients with Chronic Obstructive Pulmonary Disease. Chin. J. Integr. Med. 1995, 3, 131–133. [Google Scholar]
- Lin, C.; Zhang, Z.; Xu, Y.; Ni, W.; Wu, H.; Lai, S.; Ye, D. Investigation of the Therapeutic Mechanism of Qingxin Ketone in Chronic Obstructive Pulmonary Disease. Chin. J. Tuberc. Respir. Dis. 1995, 2, 97–98+128. [Google Scholar]
- Léger, C.L.; Carbonneau, M.A.; Michel, F.; Mas, E.; Monnier, L.; Cristol, J.P.; Descomps, B. A thromboxane effect of a hydroxytyrosol-rich olive oil wastewater extract in patients with uncomplicated type I diabetes. Eur. J. Clin. Nutr. 2005, 59, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Sánchez-Hidalgo, M.; Castejón, M.L.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Extra-virgin olive oil phenols hydroxytyrosol and hydroxytyrosol acetate, down-regulate the production of mediators involved in joint erosion in human synovial cells. J. Funct. Foods 2017, 36, 27–33. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef]
- Yan, M.; Fan, Q.; Wang, G.; Lu, L. Expression of COX-2, PGE₂, and 15d-PGJ₂ in Brain Tissue after Focal Cerebral Ischemic Injury in Rats and the Intervention Effect of Qingxin Ketone. Chin. J. Pharmacol. Clin. 2009, 25, 24–27. [Google Scholar]
- Wang, Z.; Huang, G.Y.; An, Y.; Qiang, Z. 3,4-Dihydroxyphenylacetone—A Cyclooxygenase Inhibitor (Brief Communication). J. Chinese Acad. Med. Sci. 1988, 1, 35. [Google Scholar]
- Wang, Z.; An, Y.; Liu, Z.; Zhu, G.Q.; Huang, R. Effect of 3,4-Dihydroxyphenylacetone on Thromboxane A₂ Release from Rabbit Platelets. J. Pharm. 1987, 5, 330–334. [Google Scholar]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, Skeletal Muscle Dysfunction and Gut Microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Bu, Y.; Rho, S.; Kim, J.; Kim, M.Y.; Lee, D.H.; Kim, S.Y.; Choi, H.; Kim, H. Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci. Lett. 2007, 414, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; García-Viñuales, S.; Tempra, C.; Bernini, R.; Zarrelli, A.; Lolicato, F.; Milardi, D.; Di Fabio, G. Modulating Aβ aggregation by tyrosol-based ligands: The crucial role of the catechol moiety. Biophys. Chem. 2020, 265, 106434. [Google Scholar] [CrossRef] [PubMed]
- Salis, C.; Papageorgiou, L.; Papakonstantinou, E.; Hagidimitriou, M.; Vlachakis, D. Olive Oil Polyphenols in Neurodegenerative Pathologies. Adv. Exp. Med. Biol. 2020, 1195, 77–91. [Google Scholar]
- St-Laurent-Thibault, C.; Arseneault, M.; Longpré, F.; Ramassamy, C. Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-β-induced toxicity. Involvement of the NF-κB signaling. Curr. Alzheimer Res. 2011, 8, 543–551. [Google Scholar] [CrossRef]
- Atochin, D.N.; Chernysheva, G.A.; Smolyakova, V.I.; Osipenko, A.N.; Logvinov, S.V.; Zhdankina, A.A.; Sysolyatin, S.V.; Kryukov, Y.A.; Anfinogenova, Y.; Plotnikova, T.M.; et al. Neuroprotective effects of p-tyrosol after the global cerebral ischemia in rats. Phytomedicine 2016, 23, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free. Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef]
- Sirangelo, I.; Borriello, M.; Vilasi, S.; Iannuzzi, C. Hydroxytyrosol Inhibits Protein Oligomerization and Amyloid Aggregation in Human Insulin. Int. J. Mol. Sci. 2020, 21, 4636. [Google Scholar] [CrossRef]
- Li, X.; Tian, X.; Liu, T.; Li, M.; Wang, W.; Wang, P.; Guo, Z. Hydroxytyrosol Alleviated Hypoxia-Mediated PC12 Cell Damage through Activating PI3K/AKT/mTOR-HIF-1α Signaling. Oxid. Med. Cell Longev. 2022, 2022, 8673728. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol and dopamine metabolites: Anti-aggregative effect and neuroprotective activity against α-synuclein-induced toxicity. Food Chem. Toxicol. 2023, 171, 113542. [Google Scholar] [CrossRef]
- Sun, L.; Isaak, C.K.; Zhou, Y.; Petkau, J.C.; O, K.; Liu, Y.; Siow, Y.L. Salidroside and tyrosol from Rhodiola protect H9c2 cells from ischemia/reperfusion-induced apoptosis. Life Sci. 2012, 91, 151–158. [Google Scholar] [CrossRef]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J.; Tang, Y.; Shi, L.; Li, Y.; Long, J.; et al. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Yamamoto, F.; Arai, T.; Yang, J.; Sakai, Y.; Itoh, M.; Mamada, N.; Sekiguchi, M.; Yamada, D.; Saitoh, A.; et al. Tyrosol Reduces Amyloid-β Oligomer Neurotoxicity and Alleviates Synaptic, Oxidative, and Cognitive Disturbances in Alzheimer’s Disease Model Mice. J. Alzheimers Dis. 2019, 70, 937–952. [Google Scholar] [CrossRef]
- Lee, H.; Im, S.W.; Jung, C.H.; Jang, Y.J.; Ha, T.Y.; Ahn, J. Tyrosol, an olive oil polyphenol, inhibits ER stress-induced apoptosis in pancreatic β-cell through JNK signaling. Biochem. Biophys. Res. Commun. 2016, 469, 748–752. [Google Scholar] [CrossRef]
- Garcia-Moreno, J.C.; Porta de la Riva, M.; Martínez-Lara, E.; Siles, E.; Cañuelo, A. Tyrosol, a simple phenol from EVOO, targets multiple pathogenic mechanisms of neurodegeneration in a C. elegans model of Parkinson’s disease. Neurobiol. Aging 2019, 82, 60–68. [Google Scholar] [CrossRef]
- Orsini, F.; Ami, D.; Lascialfari, A.; Natalello, A. Inhibition of lysozyme fibrillogenesis by hydroxytyrosol and dopamine: An Atomic Force Microscopy study. Int. J. Biol. Macromol. 2018, 111, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Meng, F.; Hu, R.; Chen, L.; Chang, J.; Zhao, K.; Ren, H.; Liu, Z.; Hu, P.; Wang, G.; et al. Inhibition of the NF-κB/HIF-1α signaling pathway in colorectal cancer by tyrosol: A gut microbiota-derived metabolite. J. Immunother. Cancer 2024, 12, e008831. [Google Scholar] [CrossRef]
- Calahorra, J.; Araujo-Abad, S.; Granadino-Roldán, J.M.; Naranjo, Á.; Martínez-Lara, E.; Siles, E. Tyrosol: Repercussion of the Lack of a Hydroxyl-Group in the Response of MCF-7 Cells to Hypoxia. J. Med. Food 2023, 26, 511–520. [Google Scholar] [CrossRef]
- El-Azem, N.; Pulido-Moran, M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Cara, F.E.; Sanchez-Rovira, P.; Granados-Principal, S.; Ramirez-Tortosa, M. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur. J. Nutr. 2019, 58, 1203–1211. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Battistelli, M.; Buontempo, F.; Canonico, B.; Martelli, A.M.; Papa, S.; Falcieri, E. Tyrosol prevents apoptosis in irradiated keratinocytes. J. Dermatol. Sci. 2015, 80, 61–68. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; et al. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef] [PubMed]
- López de Las Hazas, M.C.; Piñol, C.; Macià, A.; Motilva, M.J. Hydroxytyrosol and the Colonic Metabolites Derived from Virgin Olive Oil Intake Induce Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol Induces Apoptosis and Cell Cycle Arrest and Suppresses Multiple Oncogenic Signaling Pathways in Prostate Cancer Cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Ma, Y.; Song, R.; Pei, T.; Zheng, T.; Wang, J.; Xu, D.; Fang, X.; Jiang, H.; et al. Hydroxytyrosol inhibits cholangiocarcinoma tumor growth: An in vivo and in vitro study. Oncol. Rep. 2014, 31, 145–152. [Google Scholar] [CrossRef]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162. [Google Scholar] [CrossRef]
- Wu, P.; Ye, D.; Zhang, D.; Zhang, L.; Wan, J.; Pan, Q. Dual effect of 3,4-dihydroxyacetophenone on LPS-induced apoptosis in RAW264.7 cells by modulating the production of TNF-alpha. J. Huazhong Univ. Sci. Technolog Med. Sci. 2005, 25, 131–134. [Google Scholar]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Xue, L.; Jin, Z. Tyrosol Induces Increased Expression of NQO1 Enzyme Gene and Inhibits Cell Proliferation in Hepatocellular Carcinoma Cells. Chin. J. Pathophysiol. 2002, 8, 5–9. [Google Scholar]
- Xue, L.; Jin; Xia, X.; Chen, W.; Li, H.; Xue, D.; Mei, R. Expression and Mechanism of Quinone Oxidoreductase 1 Gene Induced by Antioxidants and Hypoxia. Chin. J. Hyg. Toxicol. 2005, 2, 92–95. [Google Scholar]
- Sarsour, E.H.; Goswami, M.; Kalen, A.L.; Lafin, J.T.; Goswami, P.C. Hydroxytyrosol inhibits chemokine C-C motif ligand 5 mediated aged quiescent fibroblast-induced stimulation of breast cancer cell proliferation. Age 2014, 36, 9645. [Google Scholar] [CrossRef]
- Terzuoli, E.; Nannelli, G.; Frosini, M.; Giachetti, A.; Ziche, M.; Donnini, S. Inhibition of cell cycle progression by the hydroxytyrosol-cetuximab combination yields enhanced chemotherapeutic efficacy in colon cancer cells. Oncotarget 2017, 8, 83207–83224. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.; Camacho-Corencia, P.; Sanchez-Rovira, P.; Vera-Ramirez, L.; Ramirez-Tortosa, M.C. Hydroxytyrosol inhibits growth and cell proliferation and promotes high expression of sfrp4 in rat mammary tumours. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S117–S126. [Google Scholar] [CrossRef]
- Lin, C. Effect of Qingxin Ketone on the Synthesis and Secretion of FAK and Caspase-3 in In Vitro Cultured HPASMCs. Chin. J. Appl. Physiol. 2008, 4, 486–487+507. [Google Scholar]
- Serreli, G.; Le Sayec, M.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G. Conjugated Metabolites of Hydroxytyrosol and Tyrosol Contribute to the Maintenance of Nitric Oxide Balance in Human Aortic Endothelial Cells at Physiologically Relevant Concentrations. Molecules 2021, 26, 7480. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Sayaf, K.; Zanotto, I.; Colognesi, M.; Frion-Herrera, Y.; Carrara, M.; Russo, F.P.; De Martin, S. Tyrosol attenuates NASH features by reprogramming the hepatic immune milieu. Eur. J. Pharmacol. 2024, 969, 176453. [Google Scholar] [CrossRef]
- Kutlu, T.; Özkan, H.; Güvenç, M. Tyrosol retards induction of fibrosis in rats. J. Food Biochem. 2021, 45, e13965. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D. Beneficial Effects of Tyrosol and Oleocanthal from Extra Virgin Olive Oil on Liver Health: Insights into Their Mechanisms of Action. Biology 2024, 13, 760. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, R.; Pari, L. Antihyperlipidemic effect of tyrosol, a phenolic compound in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2021, 31, 507–516. [Google Scholar] [CrossRef]
- Chandramohan, R.; Pari, L. Anti-inflammatory effects of tyrosol in streptozotocin-induced diabetic Wistar rats. J. Funct. Foods 2016, 27, 17–28. [Google Scholar] [CrossRef]
- Xie, Y.D.; Chen, Z.Z.; Li, N.; Lu, W.F.; Xu, Y.H.; Lin, Y.Y.; Shao, L.H.; Wang, Q.T.; Guo, L.Y.; Gao, Y.Q.; et al. Hydroxytyrosol nicotinate, a new multifunctional hypolipidemic and hypoglycemic agent. Biomed. Pharmacother. 2018, 99, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Lemonakis, N.; Efentakis, P.; Gikas, E.; Halabalaki, M.; Andreadou, I.; Skaltsounis, L.; Brown, L. Hydroxytyrosol ameliorates metabolic, cardiovascular and liver changes in a rat model of diet-induced metabolic syndrome: Pharmacological and metabolism-based investigation. Pharmacol. Res. 2017, 117, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Caruso, D.; Plasmati, E.; Patelli, R.; Mulinacci, N.; Romani, A.; Galli, G.; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001, 34, 301–305. [Google Scholar] [CrossRef]
- Hou, J.; Li, X.; Wei, T.; Li, J.; Yuan, Y.; Wu, M.; Chen, F.; Deng, Z.; Luo, T. Tyrosol Improves Obesity Symptoms in HFD-Fed Mice, Promotes Adipose Thermogenesis, and Regulates the Composition of the Gut Microbiota. In Proceedings of the 20th Annual Meeting of the Chinese Institute of Food Science and Technology, Changsha, China, 24 October 2023; p. 2. [Google Scholar]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-γ and NF-κB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Illesca, P.; Echeverría, F.; Espinosa, A.; Rincón-Cervera, M.; Ortiz, M.; Hernandez-Rodas, M.C.; Valenzuela, A.; Videla, L.A. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef]
- Warnke, I.; Goralczyk, R.; Fuhrer, E.; Schwager, J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr. Metab. 2011, 8, 30. [Google Scholar] [CrossRef]
- Dagla, I.; Benaki, D.; Baira, E.; Lemonakis, N.; Poudyal, H.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.-L.; Mikros, E.; Gikas, E. Alteration in the liver metabolome of rats with metabolic syndrome after treatment with Hydroxytyrosol. A Mass Spectrometry And Nuclear Magnetic Resonance—based metabolomics study. Talanta 2018, 178, 246–257. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. Biomed. Res. Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Rosta, V.; Zuliani, G.; Sega, F.V.D.; Fortini, F.; Rizzo, P.; Cimaglia, P.; Campo, G. A Nutraceutical Compound Containing a Low Dose of Monacolin K, Polymethoxyflavones, Phenolic Acids, Flavonoids, and Hydroxytyrosol Improves HDL Functionality. Curr. Vasc. Pharmacol. 2023, 21, 433–442. [Google Scholar] [CrossRef]
- Qu, M.; Sun, N.; Cui, Y.; Lü, Y.; Duan, Y. Research on Qingxin Ketone in the Field of Medicine. Pharm. Res. 2020, 39, 176–180. [Google Scholar]
- Zhang, D.; Liu, J.; Wang, J. 3,4-Dihydroxyphenylacetone Reduces the Triglyceride Content in Hepatocytes and Liver Tissue via the AMPK Pathway. Chin. J. Pathophysiol. 2018, 34, 1855–1860. [Google Scholar]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Annaji, M.; Poudel, I.; Rangari, S.; Boddu, S.H.S.; Tiwari, A.K.; Babu, R.J. Niosomal formulation of hydroxytyrosol, a polyphenolic antioxidant, for enhancing transdermal delivery across human cadaver skin. Pharm. Dev. Technol. 2022, 27, 155–163. [Google Scholar] [CrossRef]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef]
- Sun, N.; Qu, M.; Wang, M.; Lv, Y.; Wei, L.; Duan, Y. Synthesis, oral bioavailability evaluation and antiplatelet aggregation activity of three derivatives of 3,4-dihydroxyacetophenone. Int. J. Mol. Med. 2020, 45, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Çömez, M.; Cellat, M.; Kuzu, M.; Uyar, A.; Türk, E.; Kaya, Y.S.; Etyemez, M.; Gökçek, İ.; Güvenç, M. The effect of tyrosol on diclofenac sodium-induced acute nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2024, 38, e23582. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef]
- Li, W.; Chountoulesi, M.; Antoniadi, L.; Angelis, A.; Lei, J.; Halabalaki, M.; Demetzos, C.; Mitakou, S.; Skaltsounis, L.A.; Wang, C. Development and physicochemical characterization of nanoliposomes with incorporated oleocanthal, oleacein, oleuropein and hydroxytyrosol. Food Chem. 2022, 384, 132470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).