Synthesis and Health Effects of Phenolic Compounds: A Focus on Tyrosol, Hydroxytyrosol, and 3,4-Dihydroxyacetophenone
Abstract
:1. Introduction
2. Methods
3. Structure and Synthesis of Phenolic Compounds
3.1. Structure and Synthesis of Tyrosol
3.2. Structure and Synthesis of Hydroxytyrosol
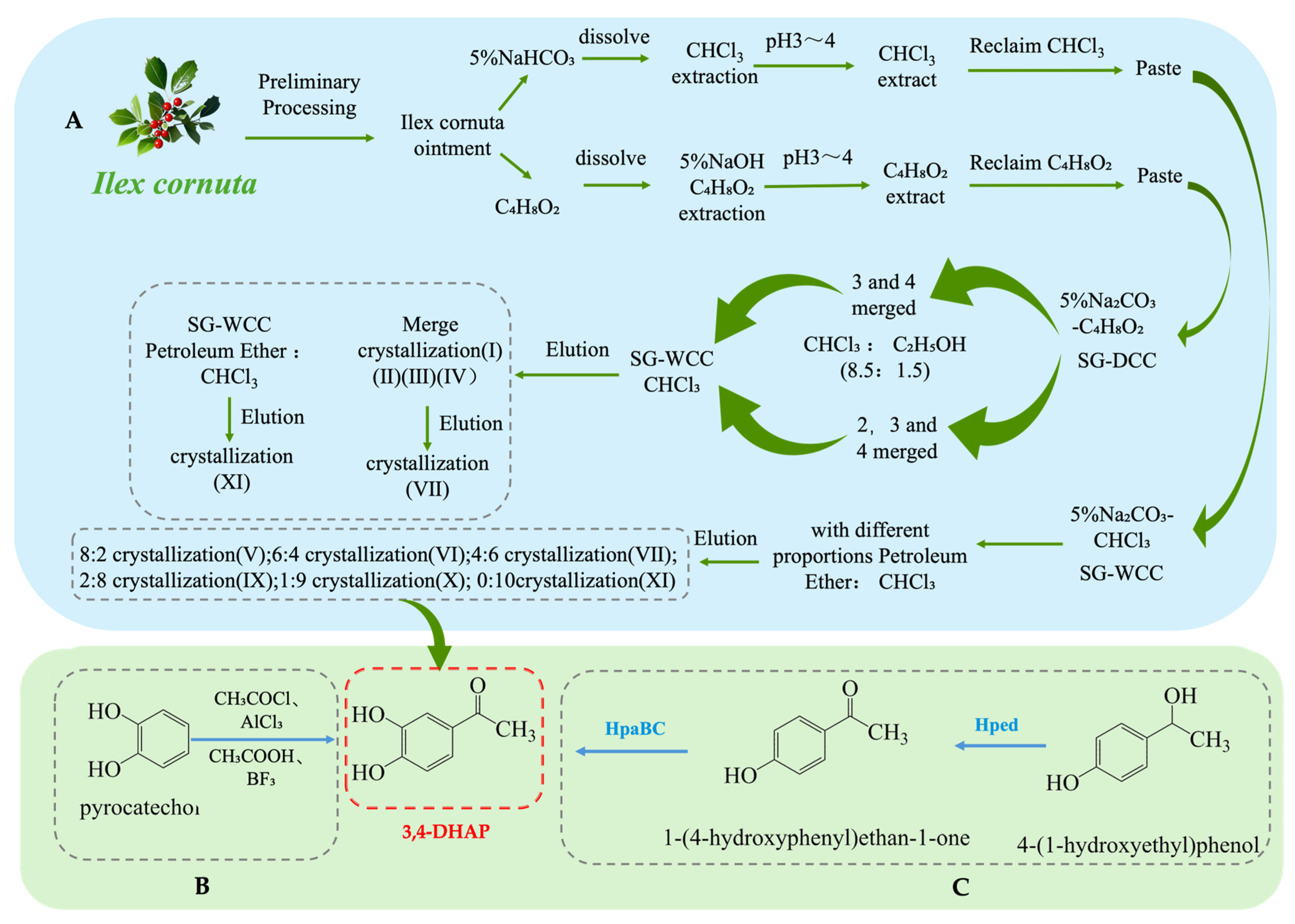
3.3. Structure and Synthesis of 3,4-Dihydroxyacetophenone
4. Health Effects of Phenolic Compounds
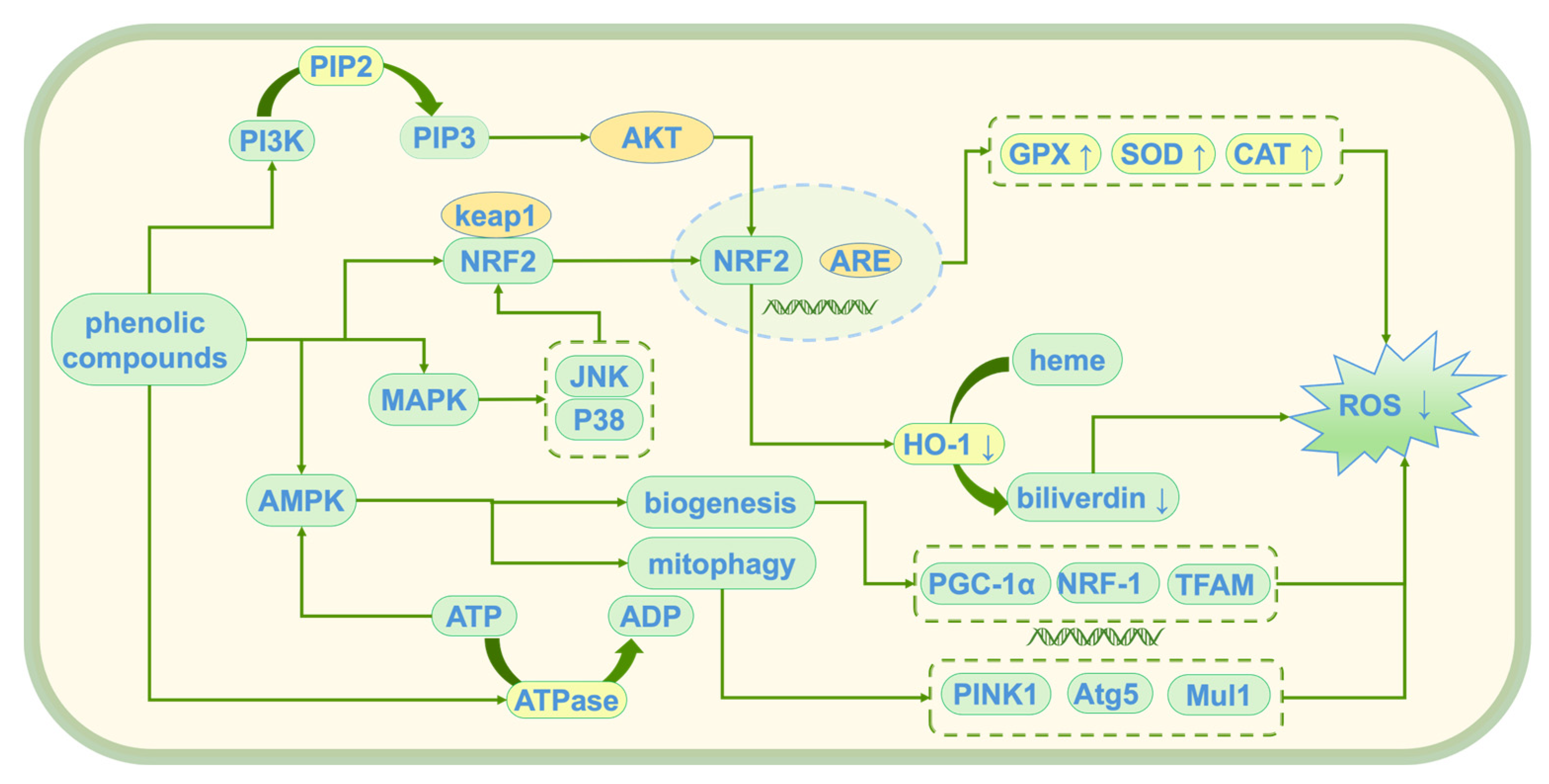
4.1. Antioxidant Activity
4.1.1. Free Radical Scavenging
4.1.2. Antioxidant Enzyme Activation
4.1.3. Inhibition of Lipid Peroxidation
4.1.4. Modulation of Oxidative Stress Response
4.1.5. Mitochondrial Protection
4.1.6. DNA and Protein Protection
4.2. Anti-Inflammatory Effects
4.2.1. Inhibition of Inflammation Initiation Triggered by Oxidative Stress
4.2.2. Regulation of the Balance Between Pro-Inflammatory and Anti-Inflammatory Factors
4.2.3. Regulation of Immune Cell Function
4.3. Cardiovascular Protective Effects
4.3.1. Protection Against Atherosclerosis
4.3.2. Protection Against Hypertension
4.3.3. Protection Against Coronary Artery Disease
4.3.4. Prevention of Thrombosis
4.4. Prevention of Neurodegenerative Diseases
4.4.1. Neuroprotective Effects
4.4.2. Anti-Apoptotic Effects on Neurons
4.4.3. Regulation of Protein Aggregation
4.5. Anticancer Effects
4.5.1. Prevention of Cancer Cells
4.5.2. Regulation of Cell Apoptosis
4.5.3. Inhibition of Cancer Cell Proliferation
4.6. Liver Protection and Metabolic Regulation
4.6.1. Hepatocyte Protection
4.6.2. Improvement of Insulin Sensitivity and Regulation of Glucose Metabolism
4.6.3. Anti-Fatty Accumulation and Improvement of Lipid Metabolism
5. Applications and Challenges for Phenolic Compounds
5.1. Application Prospects
5.2. Bioavailability and Safety
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nam, M.; Choi, J.Y.; Kim, M.S. Metabolic Profiles, Bioactive Compounds, and Antioxidant Capacity in Lentinula edodes Cultivated on Log versus Sawdust Substrates. Biomolecules 2021, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Valdés, A.; Sánchez-Martínez, J.D.; Ibáñez, E.; Bi, J.; Cifuentes, A. Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes. Foods 2022, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic Compounds of Rhodiola rosea L. as the Potential Alternative Therapy in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- Franco, C.A.; da Silva, T.I.; Dias, M.G.; Ferreira, B.W.; de Sousa, B.L.; Bousada, G.M.; Barreto, R.W.; Vaz, B.G.; Lima, G.D.S.; Dos Santos, M.H.; et al. Synthesis of Tyrosol 1,2,3-Triazole Derivatives and Their Phytotoxic Activity against Euphorbia heterophylla. J. Agric. Food Chem. 2022, 70, 2806–2816. [Google Scholar] [CrossRef]
- Vázquez-Ruiz, Z.; Toledo, E.; Vitelli-Storelli, F.; Goni, L.; de la O, V.; Bes-Rastrollo, M.; Martínez-González, M. Effect of Dietary Phenolic Compounds on Incidence of Cardiovascular Disease in the SUN Project; 10 Years of Follow-Up. Antioxidants 2022, 11, 783. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Loru, D.; Incani, A.; Deiana, M.; Corona, G.; Atzeri, A.; Melis, M.P.; Rosa, A.; Dessì, M.A. Protective effect of hydroxytyrosol and tyrosol against oxidative stress in kidney cells. Toxicol. Ind. Health 2009, 25, 301–310. [Google Scholar] [CrossRef]
- Stiuso, P.; Bagarolo, M.L.; Ilisso, C.P.; Vanacore, D.; Martino, E.; Caraglia, M.; Porcelli, M.; Cacciapuoti, G. Protective Effect of Tyrosol and S-Adenosylmethionine against Ethanol-Induced Oxidative Stress of Hepg2 Cells Involves Sirtuin 1, P53 and Erk1/2 Signaling. Int. J. Mol. Sci. 2016, 17, 622. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Navarro, C.; Montoro-García, S.; Orenes-Piñero, E. Hydroxytyrosol: Its role in the prevention of cardiovascular diseases. Heliyon 2023, 9, e12963. [Google Scholar] [CrossRef]
- Kiani, A.K.; Miggiano, G.A.D.; Aquilanti, B.; Velluti, V.; Matera, G.; Gagliardi, L.; Bertelli, M. Food supplements based on palmitoylethanolamide plus hydroxytyrosol from olive tree or Bacopa monnieri extracts for neurological diseases. Acta Biomed. 2020, 91, e2020007. [Google Scholar]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.L.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef]
- Vlachogianni, I.C.; Fragopoulou, E.; Kostakis, I.K.; Antonopoulou, S. In vitro assessment of antioxidant activity of tyrosol, resveratrol and their acetylated derivatives. Food Chem. 2015, 177, 165–173. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Q.; Zhu, J.; Xia, G.; Zang, H. Synthesis, α-Glucosidase inhibition and molecular docking studies of tyrosol derivatives. Nat. Prod. Res. 2021, 35, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.T.; Bronze, M.R.; Ventura, M.R. Phenolic Compounds from Virgin Olive Oil: Approaches for Their Synthesis and Analogues. J. Agric. Food Chem. 2022, 70, 14109–14128. [Google Scholar] [CrossRef] [PubMed]
- Strutz, H.; Frankfurt, A.M. Production of 4-Hydroxyphenethyl. U.S. Patent 5003115, 26 March 1991. [Google Scholar]
- Durrwachter, J.R. Production of Ethanol. U.S. Patent 5254753, 19 October 1993. [Google Scholar]
- Wei, Y.; Sun, R. Study on the Synthesis Process of 4-(2-Methoxyethyl)phenol. Fine Chem. 1993, 6, 28–30. [Google Scholar]
- Zhou, Y. New Synthesis Process of 4-(2′-Methoxyethyl)phenol. Liaoning Chem. Ind. 2005, 12, 515–517. [Google Scholar]
- Wang, Y.; Luo, X.; Song, J. Advances in the Synthesis of Tyrosol. Guangdong Chem. Ind. 2012, 39, 137–138+135. [Google Scholar]
- Lai, Y.; Chen, H.; Liu, L.; Fu, B.; Wu, P.; Li, W.; Hu, J.; Yuan, J. Engineering a Synthetic Pathway for Tyrosol Synthesis in Escherichia coli. ACS Synth. Biol. 2022, 11, 441–447. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, M.; Deng, H.; Chen, X.; Shang, Y.; Liu, X.; Yu, X. Efficient whole cell biotransformation of tyrosol from L-tyrosine by engineered Escherichia coli. Enzyme Microb. Technol. 2022, 160, 110100. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, X.; Chang, J.; Zhang, L.; Xu, W.; Shen, W.; Fan, Y. Reconstruction of tyrosol synthetic pathways in Escherichia coli. Chin. J. Chem. Eng. 2018, 26, 2615–2621. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Katogiannis, K.; Chania, C.; Iakovis, N.; Tsoumani, M.; Christodoulou, A.; Brinia, E.; Pavlidis, G.; Thymis, J.; Tsilivarakis, D.; et al. Association of hydroxytyrosol enriched olive oil with vascular function in chronic coronary disease. Eur. J. Clin. Investig. 2023, 53, e13983. [Google Scholar] [CrossRef]
- Utami, N.D.; Nordin, A.; Katas, H.; Bt Hj Idrus, R.; Fauzi, M.B. Molecular Action of Hydroxytyrosol in Wound Healing: An In Vitro Evidence-Based Review. Biomolecules 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xia, M.; Ding, Z.; Rong, X.; Mei, X. Enhancing physical and chemical stability of hygroscopic hydroxytyrosol by cocrystal formation. Int. J. Pharm. 2023, 646, 123470. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol. Appl. Microbiol. Biotechnol. 2019, 103, 5957–5974. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Jin, R.; Liu, Q.; Xiao, C.; Yin, B.; Wu, J. Synthesis of Hydroxytyrosol. Cent. South Pharm. 2014, 12, 864–866. [Google Scholar]
- Xu, C.; Gao, J.; Zhang, J.; Jiang, S. Process Research of Hydroxytyrosol Synthesis. Fine Chem. Ind. 2010, 27, 1209–1212. [Google Scholar]
- Xiao, Y.; Zhang, Y.; Yang, Y.; Ren, F.; Cui, G.; Zhao, Y. Synthesis Methods of Hydroxytyrosol. Int. J. Pharm. Res. 2013, 40, 335–337. [Google Scholar]
- Zang, H.; Xu, Q.; Zhang, L.; Tian, D. Research on the Synthesis Process of Hydroxytyrosol. J. Tonghua Norm. Univ. 2020, 41, 44–48. [Google Scholar]
- Molina, G.G.; Peters, E.; Palmeri, R.; Awoke, Y.; Álvarez, C.M.; Blanco, R.M. Enzymatic synthesis of Hydroxytyrosol from Oleuropein for valorization of an agricultural waste. Bioengineered 2024, 15, 2396647. [Google Scholar]
- Liu, W.K.; Ren, X.X.; Xu, L.; Lin, J. Modular cascade with engineered HpaB for efficient synthesis of hydroxytyrosol. Bioorganic Chem. 2025, 155, 108125. [Google Scholar] [CrossRef]
- Zeng, B.; Lai, Y.; Liu, L.; Cheng, J.; Zhang, Y.; Yuan, J. Engineering Escherichia coli for High-Yielding Hydroxytyrosol Synthesis from Biobased l-Tyrosine. J. Agric. Food Chem. 2020, 68, 7691–7696. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jia, P.; Bai, Y.; Fan, T.P.; Zheng, X.; Cai, Y. Efficient Synthesis of Hydroxytyrosol from l-3,4-Dihydroxyphenylalanine Using Engineered Escherichia coli Whole Cells. J. Agric. Food Chem. 2019, 67, 6867–6873. [Google Scholar] [CrossRef]
- Deri-Zenaty, B.; Bachar, S.; Rebroš, M.; Fishman, A. A coupled enzymatic reaction of tyrosinase and glucose dehydrogenase for the production of hydroxytyrosol. Appl. Microbiol. Biotechnol. 2020, 104, 4945–4955. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Li, X.; Liu, W.; Yue, H.; Liu, J.; Bai, D.; Li, W.; Fan, G. Multienzyme cascade for synthesis of hydroxytyrosol via engineered Escherichia coli. Sci. Rep. 2025, 15, 471. [Google Scholar] [CrossRef]
- Feng, L.M.; Pan, H.Z.; Zhang, Z.N. Antioxidant effect of qingxintone. Acta Pharm. Sin. 1987, 241–244. [Google Scholar] [CrossRef]
- Lin, C.; Chen, X.; Zhang, D.; Fan, J.; Lin, L. Cultivation Techniques for the Domestication of Wild Mao Dongqing in Eastern Guangdong. For. Sci. Technol. Commun. 2018, 4, 9–11. [Google Scholar]
- Ruan, X.; Li, Z.H.; Wang, Q.; Pan, C.D.; Jiang, D.A.; Wang, G.G. Autotoxicity and allelopathy of 3,4-dihydroxyacetophenone isolated from Picea schrenkiana needles. Molecules 2011, 16, 8874–8893. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Dou, H.; Li, X.; Fan, X. Research Progress on the Synthesis of Aromatic Ketone Compounds. Fine Chem. Interm. 2009, 39, 1–6. [Google Scholar]
- Ding, H.; Zhang, Y.; Jiang, X. Synthesis of 3,4-Dihydroxyphenylacetone. Fine Chem. Interm. 2016, 46, 25–26+44. [Google Scholar]
- Yuan, X.; Tian, Y.; Wang, W.; Zhang, Y.; Xu, D. Research on the Synthesis of the Traditional Chinese Medicine Active Substance Qingxin Ketone via Escherichia coli Whole-Cell Transformation of 1-(4-Hydroxyphenyl)ethanol. Chin. J. Tradit. Chin. Med. 2024, 1–9. [Google Scholar] [CrossRef]
- Arangia, A.; Marino, Y.; Impellizzeri, D.; D’Amico, R.; Cuzzocrea, S.; Di Paola, R. Hydroxytyrosol and Its Potential Uses on Intestinal and Gastrointestinal Disease. Int. J. Mol. Sci. 2023, 24, 3111. [Google Scholar] [CrossRef]
- Cuffaro, D.; Pinto, D.; Silva, A.M.; Bertolini, A.; Bertini, S.; Saba, A.; Macchia, M.; Rodrigues, F.; Digiacomo, M. Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules 2023, 28, 5150. [Google Scholar] [CrossRef]
- Cañuelo, A.; Gilbert-López, B.; Pacheco-Liñán, P.; Martínez-Lara, E.; Siles, E.; Miranda-Vizuete, A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech. Ageing Dev. 2012, 133, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Casadey, R.; Challier, C.; Altamirano, M.; Spesia, M.B.; Criado, S. Antioxidant and antimicrobial properties of tyrosol and derivative-compounds in the presence of vitamin B2. Assays of synergistic antioxidant effect with commercial food additives. Food Chem. 2021, 335, 127576. [Google Scholar] [CrossRef]
- Casadey, R.; Challier, C.; Senz, A.; Criado, S. Antioxidant ability of tyrosol and derivative-compounds in the presence of O(2)((1)Δ(g))-species. Studies of synergistic antioxidant effect with commercial antioxidants. Food Chem. 2019, 285, 275–281. [Google Scholar] [CrossRef]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Zanotto, I.; Sayaf, K.; Colognesi, M.; Polini, B.; Digiacomo, M.; Macchia, M.; Nieri, P.; et al. The phenolic compounds tyrosol and hydroxytyrosol counteract liver fibrogenesis via the transcriptional modulation of NADPH oxidases and oxidative stress-related miRNAs. Biomed. Pharmacother. 2023, 157, 114014. [Google Scholar] [CrossRef]
- Begines, P.; Biedermann, D.; Valentová, K.; Petrásková, L.; Pelantová, H.; Maya, I.; Fernández-Bolaños, J.G.; Křen, V. Chemoenzymatic Synthesis and Radical Scavenging of Sulfated Hydroxytyrosol, Tyrosol, and Acetylated Derivatives. J. Agric. Food Chem. 2019, 67, 7281–7288. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.G.; Ma, J. Nutritional benefit of olive oil: The biological effects of hydroxytyrosol and its arylating quinone adducts. J. Agric. Food Chem. 2008, 56, 8774–8786. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Pan; Zhang, Z. Antioxidant Effect of Qingxin Ketone. J. Pharm. 1987, 4, 241–244. [Google Scholar]
- Cao, D.; Wang, Y.; Li, W.; Ji, J.; Guo, J.; Zhang, D.; Liu, J. 3,4-Dihydroxyacetophenone attenuates oxidative stress-induced damage to HUVECs via regulation of the Nrf2/HO-1 pathway. Mol. Med. Rep. 2022, 25, 199. [Google Scholar] [CrossRef]
- Sadauskiene, I.; Liekis, A.; Bernotiene, R.; Sulinskiene, J.; Kasauskas, A.; Zekonis, G. The Effects of Buckwheat Leaf and Flower Extracts on Antioxidant Status in Mouse Organs. Oxid. Med. Cell Longev. 2018, 2018, 6712407. [Google Scholar] [CrossRef]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, D.; Shahidi, F. Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 2018, 245, 1262–1268. [Google Scholar] [CrossRef]
- Marzocchi, S.; Anankanbil, S.; Caboni, M.F.; Guo, Z. Enzymatic alkylsuccinylation of tyrosol: Synthesis, characterization and property evaluation as a dual-functional antioxidant. Food Chem. 2018, 246, 108–114. [Google Scholar] [CrossRef]
- Aguiar, R.P.; Aldawsari, F.S.; Wiirzler, L.A.M.; Silva-Filho, S.E.; Silva-Comar, F.M.S.; Bersani-Amado, C.A.; Velazquez-Martinez, C.A.; Cuman, R.K.N. Synthesis and Biological Evaluation of New Tyrosol-Salicylate Derivatives as Potential Anti-Inflammatory Agents. Curr. Pharm. Des. 2017, 23, 6841–6848. [Google Scholar] [CrossRef]
- de Las Hazas, M.C.L.; Rubio, L.; Macia, A.; Motilva, M.J. Hydroxytyrosol: Emerging Trends in Potential Therapeutic Applications. Curr. Pharm. Des. 2018, 24, 2157–2179. [Google Scholar] [CrossRef]
- Pérez-Barrón, G.; Montes, S.; Aguirre-Vidal, Y.; Santiago, M.; Gallardo, E.; Espartero, J.L.; Ríos, C.; Monroy-Noyola, A. Antioxidant Effect of Hydroxytyrosol, Hydroxytyrosol Acetate and Nitrohydroxytyrosol in a Rat MPP(+) Model of Parkinson’s Disease. Neurochem. Res. 2021, 46, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Zhou, X.; Li, R. Effect of 3,4-dihydroxyacetophenone on endothelial dysfunction in obese rats. Pharm. Biol. 2015, 53, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M.; Brocchini, S.; Somavarapu, S.; Procopio, A. Hydroxytyrosol oleate: A promising neuroprotective nanocarrier delivery system of oleuropein and derivatives. Int. J. Pharm. 2023, 631, 122498. [Google Scholar] [CrossRef]
- Pazos, M.; Alonso, A.; Sánchez, I.; Medina, I. Hydroxytyrosol prevents oxidative deterioration in foodstuffs rich in fish lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; Bast, A.; Haenen, G.R. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxid. Med. Cell Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef]
- Marrero, A.D.; Quesada, A.R.; Martínez-Poveda, B.; Medina, M. Anti-Cancer, Anti-Angiogenic, and Anti-Atherogenic Potential of Key Phenolic Compounds from Virgin Olive Oil. Nutrients 2024, 16, 1283. [Google Scholar] [CrossRef]
- Shi, L.; Qin; Gao, S. Using Fluorescence Polarization to Investigate the Effects of Qingxin Ketone on the Microviscosity and Fluidity of Lipid Domains in Platelet Membranes. J. Suzhou Med. Coll. 1983, 2, 34–38. [Google Scholar]
- Peng, D.; Shi, L. Effect of 3,4-Dihydroxyphenylacetone on the Phospholipid Composition of Platelet Membranes in Aged Rats. J. Pharm. 1995, 5, 343–346. [Google Scholar]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Hur, J.; Lee, Y.; Yoon, B.-R.; Choi, S.Y. Protective Effects of Tyrosol Against Oxidative Damage in L6 Muscle Cells. Food Sci. Technol. Res. 2018, 24, 943–947. [Google Scholar] [CrossRef]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Li, W.B.; Qiao, X.P.; Wang, Z.X.; Wang, S.; Chen, S.W. Synthesis and antioxidant activity of conjugates of hydroxytyrosol and coumarin. Bioorg Chem. 2020, 105, 104427. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, M.; Zhou, K.; Song, T.; Huang, L. 3,4-Dihydroxyphenylacetone Alleviates Lipopolysaccharide-Induced Acute Lung Injury Through Anti-Inflammatory and Antioxidant Effects. J. Chin. Pharm. Sci. 2021, 30, 956–968. [Google Scholar]
- Vijayan, M.; Reddy, P.H. Reduced VDAC1, Maintained Mitochondrial Dynamics and Enhanced Mitochondrial Biogenesis in a Transgenic Tau Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8561. [Google Scholar] [CrossRef]
- Liu, B.Y.; Li, L.; Liu, G.L.; Ding, W.; Chang, W.G.; Xu, T.; Ji, X.Y.; Zheng, X.X.; Zhang, J.; Wang, J.X. Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol. Sin. 2021, 42, 701–714. [Google Scholar] [CrossRef]
- Manna, C.; Della Ragione, F.; Cucciolla, V.; Borriello, A.; D’Angelo, S.; Galletti, P.; Zappia, V. Biological effects of hydroxytyrosol, a polyphenol from olive oil endowed with antioxidant activity. Adv. Exp. Med. Biol. 1999, 472, 115–130. [Google Scholar]
- Visioli, F.; Rodríguez-Pérez, M.; Gómez-Torres, Ó.; Pintado-Losa, C.; Burgos-Ramos, E. Hydroxytyrosol improves mitochondrial energetics of a cellular model of Alzheimer’s disease. Nutr. Neurosci. 2022, 25, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Z.; Li, L.; Espe, M.; Lu, K.L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef]
- Lu, X.Y.; Chen, W.C. Effect of 3, 4-dihydroxyacetophenone on Na+, K+ -ATPase activity of injured mitochondria and the oxygen consumption of brain cells of rat. Yao Xue Xue Bao 2005, 40, 13–16. [Google Scholar] [PubMed]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorganic Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef]
- Zorić, N.; Kopjar, N.; Rodriguez, J.V.; Tomić, S.; Kosalec, I. Protective effects of olive oil phenolics oleuropein and hydroxytyrosol against hydrogen peroxide-induced DNA damage in human peripheral lymphocytes. Acta Pharm. 2021, 71, 131–141. [Google Scholar] [CrossRef]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. [Google Scholar] [CrossRef]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef]
- Moon, H.W.; Park, J.W.; Lee, K.W.; Jeong, H.C.; Choi, J.B.; Choi, S.W.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Administration of Goji (Lycium chinense Mill.) Extracts Improves Erectile Function in Old Aged Rat Model. World J. Mens. Health 2017, 35, 43–50. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Huang, I.T.; Shih, H.-J.; Chang, Y.-Y.; Kao, M.-C.; Shih, P.-C.; Huang, C.-J. Cluster of differentiation 14 and toll-like receptor 4 are involved in the anti-inflammatory effects of tyrosol. J. Funct. Foods 2019, 53, 93–104. [Google Scholar] [CrossRef]
- Ramírez-Tejero, J.A.; Martínez-Lara, E.; Peinado, M.; Del Moral, M.L.; Siles, E. Hydroxytyrosol as a Promising Ally in the Treatment of Fibromyalgia. Nutrients 2020, 12, 2386. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Wei, F.; Hou, G.; You, Y.; Wang, X.; Cao, S.; Yang, X.; Liu, W.; Zhang, S.; et al. Hydroxytyrosol Ameliorates Intervertebral Disc Degeneration and Neuropathic Pain by Reducing Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2022, 2022, 2240894. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wang, Y.; Zhang, D.; Liu, J. Study on the Role of 3,4-Dihydroxyphenylacetone in Regulating the JNK/Nrf2 Signaling Pathway to Alleviate ox-LDL-Induced Endothelial Cell Injury. Chinese J. Pathophysiol. 2022, 38, 788–794. [Google Scholar]
- Liu, C.; Sun, J.; Xue, F.; Yi, Y.; Han, A. Effect of 3,4-dihydroxyacetophenone on endothelial dysfunction in streptozotocin-induced rats with type 2 diabetes. J. Cardiovasc. Pharmacol. 2015, 65, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Effect of Qingxin Ketone on Vascular Smooth Muscle Cell Proliferation in Type 2 Diabetic Rats. Pract. Diabetes J. 2014, 10, 54–55. [Google Scholar]
- Bonura, A.; Vlah, S.; Longo, A.; Bulati, M.; Melis, M.R.; Cibella, F.; Colombo, P. Hydroxytyrosol modulates Par j 1-induced IL-10 production by PBMCs in healthy subjects. Immunobiology 2016, 221, 1374–1377. [Google Scholar] [CrossRef]
- Sato, K.; Mihara, Y.; Kanai, K.; Yamashita, Y.; Kimura, Y.; Itoh, N. Tyrosol ameliorates lipopolysaccharide-induced ocular inflammation in rats via inhibition of nuclear factor (NF)-κB activation. J. Vet. Med. Sci. 2016, 78, 1429–1438. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Baron, M.; Bouhlel, M.A.; Vanhoutte, J.; Copin, C.; Sebti, Y.; Derudas, B.; Mayi, T.; Bories, G.; Tailleux, A.; et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 2011, 108, 985–995. [Google Scholar] [CrossRef]
- Güvenç, M.; Cellat, M.; Özkan, H.; Tekeli, İ.O.; Uyar, A.; Gökçek, İ.; İşler, C.T.; Yakan, A. Protective Effects of Tyrosol Against DSS-Induced Ulcerative Colitis in Rats. Inflammation 2019, 42, 1680–1691. [Google Scholar] [CrossRef]
- Bertelli, A.; Migliori, M.; Bertelli, A.A.; Origlia, N.; Filippi, C.; Panichi, V.; Falchi, M.; Giovannini, L. Effect of some white wine phenols in preventing inflammatory cytokine release. Drugs Exp. Clin. Res. 2002, 28, 11–15. [Google Scholar]
- Giovannini, L.; Migliori, M.; Filippi, C.; Origlia, N.; Panichi, V.; Falchi, M.; Bertelli, A.A.; Bertelli, A. Inhibitory activity of the white wine compounds, tyrosol and caffeic acid, on lipopolysaccharide-induced tumor necrosis factor-alpha release in human peripheral blood mononuclear cells. Int. J. Tissue React. 2002, 24, 53–56. [Google Scholar]
- Bertelli, A.A.E.; Massamiliano, M.; Vincenzo, P.; Bianamaria, L.; Nicola, O.; Agnese, F.; Giuseppa, C.M.; Luca, G. Oxidative stress and inflammatory reaction modulation by white wine. Ann. N. Y. Acad. Sci. 2002, 957, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Ren, D.; Wang, P.; Song, Z.; Wu, H.; Yao, S.; Geng, L.; Su, Y.; Bai, X. Upregulation of Sirt1 by tyrosol suppresses apoptosis and inflammation and modulates extracellular matrix remodeling in interleukin-1β-stimulated human nucleus pulposus cells through activation of PI3K/Akt pathway. Int. Immunopharmacol. 2020, 88, 106904. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Kihara, T.; Ibi, K.; Senshu, M.; Nejishima, H.; Takeda, Y.; Imai, K.; Ogawa, H. Olive-Derived Hydroxytyrosol Shows Anti-inflammatory Effect without Gastric Damage in Rats. Biol. Pharm. Bull. 2019, 42, 1120–1127. [Google Scholar] [CrossRef]
- Wen, X.; Tang, S.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. The PI3K/Akt-Nrf2 Signaling Pathway and Mitophagy Synergistically Mediate Hydroxytyrosol to Alleviate Intestinal Oxidative Damage. Int. J. Biol. Sci. 2024, 20, 4258–4276. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Soylu, H.; Karacor, K. The effects of hydroxytyrosol on Prdx6 and insulin expression in diabetic rat pancreases. Histochem. Cell Biol. 2023, 160, 127–134. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Abdullah; Tian, W.; Qiu, Z.; Song, M.; Cao, Y.; Xiao, J. Hydroxytyrosol Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Inflammatory Responses, Intestinal Barrier, and Microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med. 2003, 35, 1073–1081. [Google Scholar] [CrossRef]
- De Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur. J. Pharmacol. 2007, 566, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, P.; Pan, Q.; Wan, J.; Zhang, L.; Wu, T.; Zhou, X.; Ye, D. Effect of Qingxin Ketone on Heme Oxygenase-1 mRNA/Carbon Monoxide and TNF-α Expression in Activated RAW264.7 Cells. Chin. J. Integr. Med. 2004, S1, 257–260. [Google Scholar]
- Li, G.; Wu, P.; Zhang, D.; Ye, D.; Li, F. Investigation of the Mechanism by Which Qingxin Ketone Inhibits Lipopolysaccharide-Induced Apoptosis in Mouse Macrophages. Chin. Herb. Med. 2005, 12, 1835–1838. [Google Scholar]
- Zhang, D.; Wu, S.; Liu, T.; Tian, H.; Wang, Y.; Duan, W.; Ye, D. Effect of Qingxin Ketone on Soluble Toll-like Receptor 4 Expression and TNF-α in RAW264.7 Cells. J. Weifang Med. Univ. 2006, 4, 264–265+322. [Google Scholar]
- Zhang, D.; Wu, S.; Liu, T.; Tian, H.; Wang, Y.; Duan, W.; Ye, D. Effect of Qingxin Ketone on Soluble Toll-like Receptor 4 Expression and Inflammatory-Related Factors in RAW264.7 Cells. Chin. J. Clin. Rehabil. 2006, 11, 120–122. [Google Scholar]
- Wu, P.; Zhang, L.; Zhou, X.; Li, Y.; Zhang, D.; Wan, J.; Ye, D. Inflammation pro-resolving potential of 3,4-dihydroxyacetophenone through 15-deoxy-delta12,14-prostaglandin J2 in murine macrophages. Int. Immunopharmacol. 2007, 7, 1450–1459. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Wang, J. 3,4-Dihydroxyphenylacetone Inhibits NF-κB Nuclear Translocation to Alleviate Lipopolysaccharide-Induced Macrophage Inflammatory Response. J. Pract. Med. 2018, 34, 1092–1095. [Google Scholar]
- Castañer, O.; Covas, M.I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.F.; de la Torre, R.; Muñoz-Aguayo, D.; Vila, J.; Fitó, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Huang, Y.; Mo, D.; Ma, N.; Gao, F.; Song, L.; Sun, X.; Xu, X.; Liu, L.; Huo, X.; et al. Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation in in vitro oxygen glucose deprivation. Neurochem. Int. 2018, 121, 140–145. [Google Scholar] [CrossRef]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol. Pharmacol. 2018, 96, 88–96. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Haizum Abdullah, N.A.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Liu, J.; Cui, X.; Guo, J.; Wang, J.; Ye, D. Relationship between Qingxin Ketone in the Prevention and Treatment of Atherosclerosis and the TLR4 Signal Transduction Pathway. Chin. J. New Drugs Clin. Pharmacol. 2009, 20, 404–407. [Google Scholar]
- Zhang, D.; Liu, T.; Liu, J.; Cui, X.; Guo, J.; Wang, J.; Ye, D. Effect of Qingxin Ketone on Atherosclerotic Formation in ApoE-Deficient Mice and on TLR4 Expression in the Major Cells of Plaques. Chin. Mod. Appl. Pharm. 2010, 27, 1–5. [Google Scholar]
- Zhang, D.; Liu, T.; Liu, J.; Cui, X.; Guo, J.; Wang, J.; Ye, D. Effect of Qingxin Ketone on the Expression and Secretion of Lipid Factor in ApoE-Deficient Mice and Cultured Vascular Smooth Muscle and Endothelial Cells. Chin. J. Pharm. 2010, 45, 1623–1627. [Google Scholar]
- Zhang, D.; Liu, J.; Wang, L.; Wang, J.; Li, W.; Zhuang, B.; Hou, J.; Liu, T. Effects of 3,4-dihydroxyacetophenone on the hypercholesterolemia-induced atherosclerotic rabbits. Biol. Pharm. Bull. 2013, 36, 733–740. [Google Scholar] [CrossRef]
- Zhang, J.; Nugrahaningrum, D.A.; Marcelina, O.; Ariyanti, A.D.; Wang, G.; Liu, C.; Wu, S.; Kasim, V. Tyrosol Facilitates Neovascularization by Enhancing Skeletal Muscle Cells Viability and Paracrine Function in Diabetic Hindlimb Ischemia Mice. Front. Pharmacol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Aliev, O.I.; Sidekhmenova, A.V.; Shamanaev, A.Y.; Anishchenko, A.M.; Fomina, T.I.; Plotnikova, T.M.; Arkhipov, A.M. Effect of p-tyrosol on hemorheological parameters and cerebral capillary network in young spontaneously hypertensive rats. Microvasc. Res. 2018, 119, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Fukuda, D.; Ganbaatar, B.; Pham, P.T.; Aini, K.; Rahadian, A.; Suto, K.; Yagi, S.; Kusunose, K.; Yamada, H.; et al. Olive mill wastewater and hydroxytyrosol inhibits atherogenesis in apolipoprotein E-deficient mice. Heart Vessel. 2023, 38, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Wang, D.X.; Ming, Z.; Yu, S.B. Effects of 3,4-dihydroxyacetophenone (3,4-DHAP) on hypoxic pulmonary and systemic vascular response in dogs. J. Tongji Med. Univ. 1995, 15, 26–30. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Zhou, J.; Huang, Y. Effect of Qingxin Ketone on Hypoxia-Induced Expression of NF-κB and COX2 in Human Umbilical Vein Endothelial Cells. Zhejiang J. Tradit. Chin. Med. 2009, 44, 94–95. [Google Scholar]
- Wazir, F.; Wang, D.; Hu, Q. Effects of 3,4-dihydroxyacetophenone on cytosolic calcium in pulmonary artery endothelial and smooth muscle cells during acute hypoxia. J. Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 550–551. [Google Scholar]
- Giovannini, C.; Straface, E.; Modesti, D.; Coni, E.; Cantafora, A.; De Vincenzi, M.; Malorni, W.; Masella, R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J. Nutr. 1999, 129, 1269–1277. [Google Scholar] [CrossRef]
- Covas, M.I.; Miró-Casas, E.; Fitó, M.; Farré-Albadalejo, M.; Gimeno, E.; Marrugat, J.; De La Torre, R. Bioavailability of tyrosol, an antioxidant phenolic compound present in wine and olive oil, in humans. Drugs Exp. Clin. Res. 2003, 29, 203–206. [Google Scholar]
- Covas, M.I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef]
- Giordano, E.; Davalos, A.; Nicod, N.; Visioli, F. Hydroxytyrosol attenuates tunicamycin-induced endoplasmic reticulum stress in human hepatocarcinoma cells. Mol. Nutr. Food Res. 2014, 58, 954–962. [Google Scholar] [CrossRef]
- Farman, U.; Wang, D.X.; Deng, J. Effects of 3,4-dihydroxyacetophenone on hypoxic vasoconstriction in isolated pulmonary and basilar arterial rings. J. Tongji Med. Univ. 1994, 14, 252–256. [Google Scholar] [PubMed]
- Lin, C.; Zhang, Z.; Xu, Y.; Ni, W. Effect of Qingxin Ketone on Hemodynamics and Plasma Levels of Atrial Natriuretic Peptide and Cyclic Nucleotides in Patients with Chronic Obstructive Pulmonary Disease. Chin. J. Integr. Med. 1995, 3, 131–133. [Google Scholar]
- Lin, C.; Zhang, Z.; Xu, Y.; Ni, W.; Wu, H.; Lai, S.; Ye, D. Investigation of the Therapeutic Mechanism of Qingxin Ketone in Chronic Obstructive Pulmonary Disease. Chin. J. Tuberc. Respir. Dis. 1995, 2, 97–98+128. [Google Scholar]
- Léger, C.L.; Carbonneau, M.A.; Michel, F.; Mas, E.; Monnier, L.; Cristol, J.P.; Descomps, B. A thromboxane effect of a hydroxytyrosol-rich olive oil wastewater extract in patients with uncomplicated type I diabetes. Eur. J. Clin. Nutr. 2005, 59, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Sánchez-Hidalgo, M.; Castejón, M.L.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Extra-virgin olive oil phenols hydroxytyrosol and hydroxytyrosol acetate, down-regulate the production of mediators involved in joint erosion in human synovial cells. J. Funct. Foods 2017, 36, 27–33. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef]
- Yan, M.; Fan, Q.; Wang, G.; Lu, L. Expression of COX-2, PGE₂, and 15d-PGJ₂ in Brain Tissue after Focal Cerebral Ischemic Injury in Rats and the Intervention Effect of Qingxin Ketone. Chin. J. Pharmacol. Clin. 2009, 25, 24–27. [Google Scholar]
- Wang, Z.; Huang, G.Y.; An, Y.; Qiang, Z. 3,4-Dihydroxyphenylacetone—A Cyclooxygenase Inhibitor (Brief Communication). J. Chinese Acad. Med. Sci. 1988, 1, 35. [Google Scholar]
- Wang, Z.; An, Y.; Liu, Z.; Zhu, G.Q.; Huang, R. Effect of 3,4-Dihydroxyphenylacetone on Thromboxane A₂ Release from Rabbit Platelets. J. Pharm. 1987, 5, 330–334. [Google Scholar]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, Skeletal Muscle Dysfunction and Gut Microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Bu, Y.; Rho, S.; Kim, J.; Kim, M.Y.; Lee, D.H.; Kim, S.Y.; Choi, H.; Kim, H. Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci. Lett. 2007, 414, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; García-Viñuales, S.; Tempra, C.; Bernini, R.; Zarrelli, A.; Lolicato, F.; Milardi, D.; Di Fabio, G. Modulating Aβ aggregation by tyrosol-based ligands: The crucial role of the catechol moiety. Biophys. Chem. 2020, 265, 106434. [Google Scholar] [CrossRef] [PubMed]
- Salis, C.; Papageorgiou, L.; Papakonstantinou, E.; Hagidimitriou, M.; Vlachakis, D. Olive Oil Polyphenols in Neurodegenerative Pathologies. Adv. Exp. Med. Biol. 2020, 1195, 77–91. [Google Scholar]
- St-Laurent-Thibault, C.; Arseneault, M.; Longpré, F.; Ramassamy, C. Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-β-induced toxicity. Involvement of the NF-κB signaling. Curr. Alzheimer Res. 2011, 8, 543–551. [Google Scholar] [CrossRef]
- Atochin, D.N.; Chernysheva, G.A.; Smolyakova, V.I.; Osipenko, A.N.; Logvinov, S.V.; Zhdankina, A.A.; Sysolyatin, S.V.; Kryukov, Y.A.; Anfinogenova, Y.; Plotnikova, T.M.; et al. Neuroprotective effects of p-tyrosol after the global cerebral ischemia in rats. Phytomedicine 2016, 23, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free. Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef]
- Sirangelo, I.; Borriello, M.; Vilasi, S.; Iannuzzi, C. Hydroxytyrosol Inhibits Protein Oligomerization and Amyloid Aggregation in Human Insulin. Int. J. Mol. Sci. 2020, 21, 4636. [Google Scholar] [CrossRef]
- Li, X.; Tian, X.; Liu, T.; Li, M.; Wang, W.; Wang, P.; Guo, Z. Hydroxytyrosol Alleviated Hypoxia-Mediated PC12 Cell Damage through Activating PI3K/AKT/mTOR-HIF-1α Signaling. Oxid. Med. Cell Longev. 2022, 2022, 8673728. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol and dopamine metabolites: Anti-aggregative effect and neuroprotective activity against α-synuclein-induced toxicity. Food Chem. Toxicol. 2023, 171, 113542. [Google Scholar] [CrossRef]
- Sun, L.; Isaak, C.K.; Zhou, Y.; Petkau, J.C.; O, K.; Liu, Y.; Siow, Y.L. Salidroside and tyrosol from Rhodiola protect H9c2 cells from ischemia/reperfusion-induced apoptosis. Life Sci. 2012, 91, 151–158. [Google Scholar] [CrossRef]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J.; Tang, Y.; Shi, L.; Li, Y.; Long, J.; et al. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Yamamoto, F.; Arai, T.; Yang, J.; Sakai, Y.; Itoh, M.; Mamada, N.; Sekiguchi, M.; Yamada, D.; Saitoh, A.; et al. Tyrosol Reduces Amyloid-β Oligomer Neurotoxicity and Alleviates Synaptic, Oxidative, and Cognitive Disturbances in Alzheimer’s Disease Model Mice. J. Alzheimers Dis. 2019, 70, 937–952. [Google Scholar] [CrossRef]
- Lee, H.; Im, S.W.; Jung, C.H.; Jang, Y.J.; Ha, T.Y.; Ahn, J. Tyrosol, an olive oil polyphenol, inhibits ER stress-induced apoptosis in pancreatic β-cell through JNK signaling. Biochem. Biophys. Res. Commun. 2016, 469, 748–752. [Google Scholar] [CrossRef]
- Garcia-Moreno, J.C.; Porta de la Riva, M.; Martínez-Lara, E.; Siles, E.; Cañuelo, A. Tyrosol, a simple phenol from EVOO, targets multiple pathogenic mechanisms of neurodegeneration in a C. elegans model of Parkinson’s disease. Neurobiol. Aging 2019, 82, 60–68. [Google Scholar] [CrossRef]
- Orsini, F.; Ami, D.; Lascialfari, A.; Natalello, A. Inhibition of lysozyme fibrillogenesis by hydroxytyrosol and dopamine: An Atomic Force Microscopy study. Int. J. Biol. Macromol. 2018, 111, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Meng, F.; Hu, R.; Chen, L.; Chang, J.; Zhao, K.; Ren, H.; Liu, Z.; Hu, P.; Wang, G.; et al. Inhibition of the NF-κB/HIF-1α signaling pathway in colorectal cancer by tyrosol: A gut microbiota-derived metabolite. J. Immunother. Cancer 2024, 12, e008831. [Google Scholar] [CrossRef]
- Calahorra, J.; Araujo-Abad, S.; Granadino-Roldán, J.M.; Naranjo, Á.; Martínez-Lara, E.; Siles, E. Tyrosol: Repercussion of the Lack of a Hydroxyl-Group in the Response of MCF-7 Cells to Hypoxia. J. Med. Food 2023, 26, 511–520. [Google Scholar] [CrossRef]
- El-Azem, N.; Pulido-Moran, M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Cara, F.E.; Sanchez-Rovira, P.; Granados-Principal, S.; Ramirez-Tortosa, M. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur. J. Nutr. 2019, 58, 1203–1211. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Battistelli, M.; Buontempo, F.; Canonico, B.; Martelli, A.M.; Papa, S.; Falcieri, E. Tyrosol prevents apoptosis in irradiated keratinocytes. J. Dermatol. Sci. 2015, 80, 61–68. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; et al. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef] [PubMed]
- López de Las Hazas, M.C.; Piñol, C.; Macià, A.; Motilva, M.J. Hydroxytyrosol and the Colonic Metabolites Derived from Virgin Olive Oil Intake Induce Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol Induces Apoptosis and Cell Cycle Arrest and Suppresses Multiple Oncogenic Signaling Pathways in Prostate Cancer Cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Ma, Y.; Song, R.; Pei, T.; Zheng, T.; Wang, J.; Xu, D.; Fang, X.; Jiang, H.; et al. Hydroxytyrosol inhibits cholangiocarcinoma tumor growth: An in vivo and in vitro study. Oncol. Rep. 2014, 31, 145–152. [Google Scholar] [CrossRef]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162. [Google Scholar] [CrossRef]
- Wu, P.; Ye, D.; Zhang, D.; Zhang, L.; Wan, J.; Pan, Q. Dual effect of 3,4-dihydroxyacetophenone on LPS-induced apoptosis in RAW264.7 cells by modulating the production of TNF-alpha. J. Huazhong Univ. Sci. Technolog Med. Sci. 2005, 25, 131–134. [Google Scholar]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Xue, L.; Jin, Z. Tyrosol Induces Increased Expression of NQO1 Enzyme Gene and Inhibits Cell Proliferation in Hepatocellular Carcinoma Cells. Chin. J. Pathophysiol. 2002, 8, 5–9. [Google Scholar]
- Xue, L.; Jin; Xia, X.; Chen, W.; Li, H.; Xue, D.; Mei, R. Expression and Mechanism of Quinone Oxidoreductase 1 Gene Induced by Antioxidants and Hypoxia. Chin. J. Hyg. Toxicol. 2005, 2, 92–95. [Google Scholar]
- Sarsour, E.H.; Goswami, M.; Kalen, A.L.; Lafin, J.T.; Goswami, P.C. Hydroxytyrosol inhibits chemokine C-C motif ligand 5 mediated aged quiescent fibroblast-induced stimulation of breast cancer cell proliferation. Age 2014, 36, 9645. [Google Scholar] [CrossRef]
- Terzuoli, E.; Nannelli, G.; Frosini, M.; Giachetti, A.; Ziche, M.; Donnini, S. Inhibition of cell cycle progression by the hydroxytyrosol-cetuximab combination yields enhanced chemotherapeutic efficacy in colon cancer cells. Oncotarget 2017, 8, 83207–83224. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.; Camacho-Corencia, P.; Sanchez-Rovira, P.; Vera-Ramirez, L.; Ramirez-Tortosa, M.C. Hydroxytyrosol inhibits growth and cell proliferation and promotes high expression of sfrp4 in rat mammary tumours. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S117–S126. [Google Scholar] [CrossRef]
- Lin, C. Effect of Qingxin Ketone on the Synthesis and Secretion of FAK and Caspase-3 in In Vitro Cultured HPASMCs. Chin. J. Appl. Physiol. 2008, 4, 486–487+507. [Google Scholar]
- Serreli, G.; Le Sayec, M.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G. Conjugated Metabolites of Hydroxytyrosol and Tyrosol Contribute to the Maintenance of Nitric Oxide Balance in Human Aortic Endothelial Cells at Physiologically Relevant Concentrations. Molecules 2021, 26, 7480. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Sayaf, K.; Zanotto, I.; Colognesi, M.; Frion-Herrera, Y.; Carrara, M.; Russo, F.P.; De Martin, S. Tyrosol attenuates NASH features by reprogramming the hepatic immune milieu. Eur. J. Pharmacol. 2024, 969, 176453. [Google Scholar] [CrossRef]
- Kutlu, T.; Özkan, H.; Güvenç, M. Tyrosol retards induction of fibrosis in rats. J. Food Biochem. 2021, 45, e13965. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D. Beneficial Effects of Tyrosol and Oleocanthal from Extra Virgin Olive Oil on Liver Health: Insights into Their Mechanisms of Action. Biology 2024, 13, 760. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, R.; Pari, L. Antihyperlipidemic effect of tyrosol, a phenolic compound in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2021, 31, 507–516. [Google Scholar] [CrossRef]
- Chandramohan, R.; Pari, L. Anti-inflammatory effects of tyrosol in streptozotocin-induced diabetic Wistar rats. J. Funct. Foods 2016, 27, 17–28. [Google Scholar] [CrossRef]
- Xie, Y.D.; Chen, Z.Z.; Li, N.; Lu, W.F.; Xu, Y.H.; Lin, Y.Y.; Shao, L.H.; Wang, Q.T.; Guo, L.Y.; Gao, Y.Q.; et al. Hydroxytyrosol nicotinate, a new multifunctional hypolipidemic and hypoglycemic agent. Biomed. Pharmacother. 2018, 99, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Lemonakis, N.; Efentakis, P.; Gikas, E.; Halabalaki, M.; Andreadou, I.; Skaltsounis, L.; Brown, L. Hydroxytyrosol ameliorates metabolic, cardiovascular and liver changes in a rat model of diet-induced metabolic syndrome: Pharmacological and metabolism-based investigation. Pharmacol. Res. 2017, 117, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Caruso, D.; Plasmati, E.; Patelli, R.; Mulinacci, N.; Romani, A.; Galli, G.; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001, 34, 301–305. [Google Scholar] [CrossRef]
- Hou, J.; Li, X.; Wei, T.; Li, J.; Yuan, Y.; Wu, M.; Chen, F.; Deng, Z.; Luo, T. Tyrosol Improves Obesity Symptoms in HFD-Fed Mice, Promotes Adipose Thermogenesis, and Regulates the Composition of the Gut Microbiota. In Proceedings of the 20th Annual Meeting of the Chinese Institute of Food Science and Technology, Changsha, China, 24 October 2023; p. 2. [Google Scholar]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-γ and NF-κB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Illesca, P.; Echeverría, F.; Espinosa, A.; Rincón-Cervera, M.; Ortiz, M.; Hernandez-Rodas, M.C.; Valenzuela, A.; Videla, L.A. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef]
- Warnke, I.; Goralczyk, R.; Fuhrer, E.; Schwager, J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr. Metab. 2011, 8, 30. [Google Scholar] [CrossRef]
- Dagla, I.; Benaki, D.; Baira, E.; Lemonakis, N.; Poudyal, H.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.-L.; Mikros, E.; Gikas, E. Alteration in the liver metabolome of rats with metabolic syndrome after treatment with Hydroxytyrosol. A Mass Spectrometry And Nuclear Magnetic Resonance—based metabolomics study. Talanta 2018, 178, 246–257. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. Biomed. Res. Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Rosta, V.; Zuliani, G.; Sega, F.V.D.; Fortini, F.; Rizzo, P.; Cimaglia, P.; Campo, G. A Nutraceutical Compound Containing a Low Dose of Monacolin K, Polymethoxyflavones, Phenolic Acids, Flavonoids, and Hydroxytyrosol Improves HDL Functionality. Curr. Vasc. Pharmacol. 2023, 21, 433–442. [Google Scholar] [CrossRef]
- Qu, M.; Sun, N.; Cui, Y.; Lü, Y.; Duan, Y. Research on Qingxin Ketone in the Field of Medicine. Pharm. Res. 2020, 39, 176–180. [Google Scholar]
- Zhang, D.; Liu, J.; Wang, J. 3,4-Dihydroxyphenylacetone Reduces the Triglyceride Content in Hepatocytes and Liver Tissue via the AMPK Pathway. Chin. J. Pathophysiol. 2018, 34, 1855–1860. [Google Scholar]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Annaji, M.; Poudel, I.; Rangari, S.; Boddu, S.H.S.; Tiwari, A.K.; Babu, R.J. Niosomal formulation of hydroxytyrosol, a polyphenolic antioxidant, for enhancing transdermal delivery across human cadaver skin. Pharm. Dev. Technol. 2022, 27, 155–163. [Google Scholar] [CrossRef]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef]
- Sun, N.; Qu, M.; Wang, M.; Lv, Y.; Wei, L.; Duan, Y. Synthesis, oral bioavailability evaluation and antiplatelet aggregation activity of three derivatives of 3,4-dihydroxyacetophenone. Int. J. Mol. Med. 2020, 45, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Çömez, M.; Cellat, M.; Kuzu, M.; Uyar, A.; Türk, E.; Kaya, Y.S.; Etyemez, M.; Gökçek, İ.; Güvenç, M. The effect of tyrosol on diclofenac sodium-induced acute nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2024, 38, e23582. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef]
- Li, W.; Chountoulesi, M.; Antoniadi, L.; Angelis, A.; Lei, J.; Halabalaki, M.; Demetzos, C.; Mitakou, S.; Skaltsounis, L.A.; Wang, C. Development and physicochemical characterization of nanoliposomes with incorporated oleocanthal, oleacein, oleuropein and hydroxytyrosol. Food Chem. 2022, 384, 132470. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Du, L.; Wei, Q.; Lu, M.; Xu, D.; Li, Y. Synthesis and Health Effects of Phenolic Compounds: A Focus on Tyrosol, Hydroxytyrosol, and 3,4-Dihydroxyacetophenone. Antioxidants 2025, 14, 476. https://doi.org/10.3390/antiox14040476
Wang W, Du L, Wei Q, Lu M, Xu D, Li Y. Synthesis and Health Effects of Phenolic Compounds: A Focus on Tyrosol, Hydroxytyrosol, and 3,4-Dihydroxyacetophenone. Antioxidants. 2025; 14(4):476. https://doi.org/10.3390/antiox14040476
Chicago/Turabian StyleWang, Wenyu, Lixin Du, Qidong Wei, Mengyao Lu, Dehong Xu, and Ya Li. 2025. "Synthesis and Health Effects of Phenolic Compounds: A Focus on Tyrosol, Hydroxytyrosol, and 3,4-Dihydroxyacetophenone" Antioxidants 14, no. 4: 476. https://doi.org/10.3390/antiox14040476
APA StyleWang, W., Du, L., Wei, Q., Lu, M., Xu, D., & Li, Y. (2025). Synthesis and Health Effects of Phenolic Compounds: A Focus on Tyrosol, Hydroxytyrosol, and 3,4-Dihydroxyacetophenone. Antioxidants, 14(4), 476. https://doi.org/10.3390/antiox14040476




