Targeting Redox Signaling Through Exosomal MicroRNA: Insights into Tumor Microenvironment and Precision Oncology
Abstract
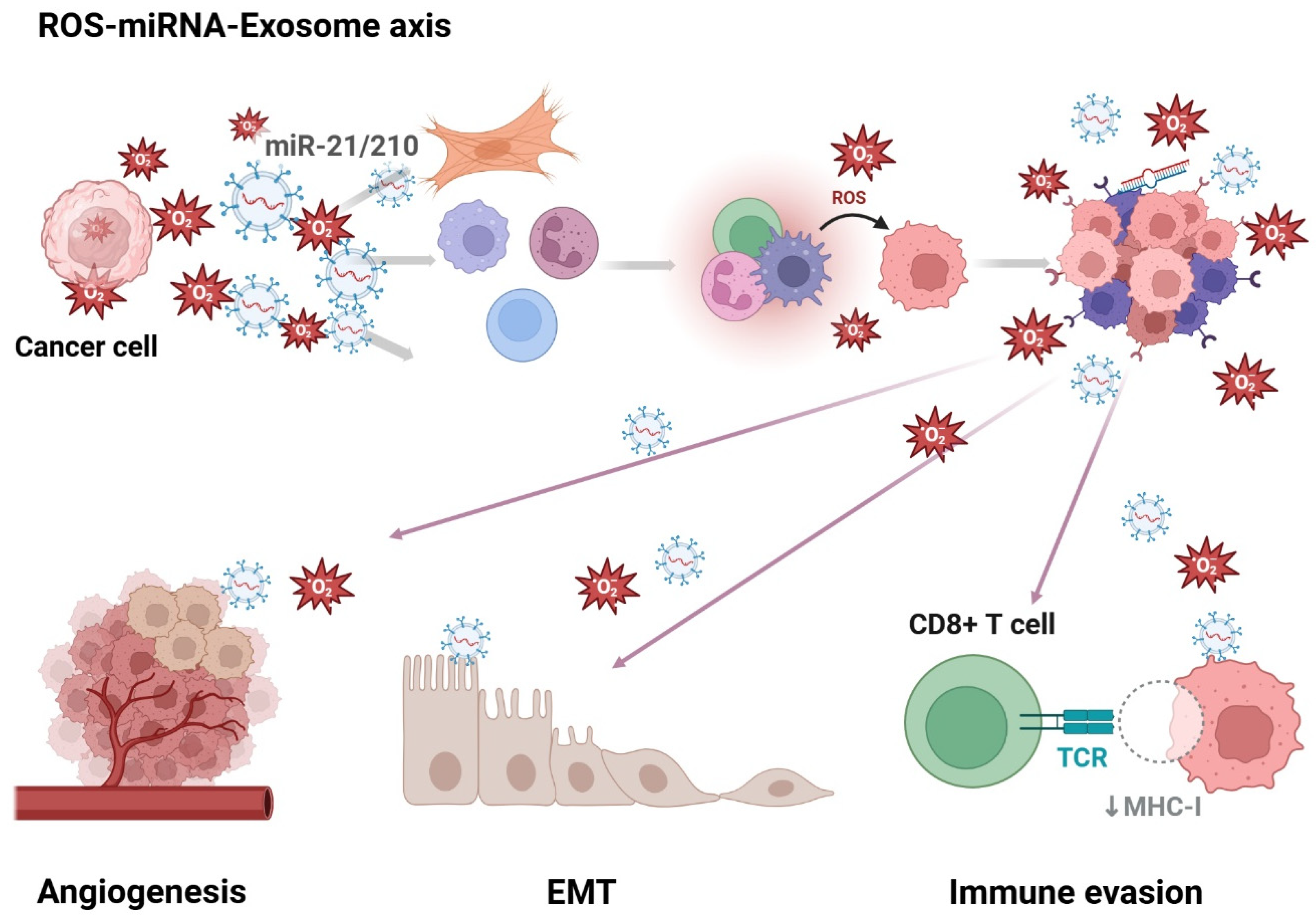
1. Introduction
1.1. Roles of Exosomes, miRNA, and Reactive Oxygen Species (ROS) in the Tumor Microenvironment (TME)
1.2. Key Redox-Associated Signaling Pathways Regulated by Exosomal miRNAs
1.3. Functional Genomics and Single-Cell Approaches in Understanding the Exosome-ROS-miRNA Axis
2. The Influence of ROS and Exosome Biogenesis and miRNA Loading
2.1. NOX1-Driven ROS Production and Its Role in Tumor Progression and Immune Modulation
2.2. Cellular Source of ROS and Exosome-Linked Immune Modulation
2.3. ROS and Selective miRNA Packing into Exosome
2.4. Mechanistic Insights into ROS-Driven Exosome Secretion and Functional Adaptation
3. Natural Compounds Modulating the ROS–miRNA Axis: A New Frontier in Exosome-Based Therapeutics
4. Clinical Implications and Therapeutic Applications
Limitations and Future Perspectives
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kretzschmar, K.; Weber, C.; Driskell, R.R.; Calonje, E.; Watt, F.M. Compartmentalized Epidermal Activation of β-Catenin Differentially Affects Lineage Reprogramming and Underlies Tumor Heterogeneity. Cell Rep. 2016, 14, 269–281. [Google Scholar] [CrossRef]
- Arina, A.; Idel, C.; Hyjek, E.M.; Alegre, M.L.; Wang, Y.; Bindokas, V.P.; Weichselbaum, R.R.; Schreiber, H. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl. Acad. Sci. USA 2016, 113, 7551–7556. [Google Scholar] [CrossRef]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef]
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Investig. 2013, 123, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Coffman, L.G.; Pearson, A.T.; Frisbie, L.G.; Freeman, Z.; Christie, E.; Bowtell, D.D.; Buckanovich, R.J. Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma. Stem Cells 2019, 37, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Bielczyk-Maczynska, E. White Adipocyte Plasticity in Physiology and Disease. Cells 2019, 8, 1570. [Google Scholar] [CrossRef]
- Zhang, Y.; Daquinag, A.; Traktuev, D.O.; Amaya-Manzanares, F.; Simmons, P.J.; March, K.L.; Pasqualini, R.; Arap, W.; Kolonin, M.G. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009, 69, 5259–5266. [Google Scholar] [CrossRef]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Dulauroy, S.; Di Carlo, S.E.; Langa, F.; Eberl, G.; Peduto, L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012, 18, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, C.; Hua, X.; Kan, A.; Mao, Y.; Sun, S.; Duan, F.; Wang, J.; Huang, P.; Li, S. Oxidative stress induces monocyte-to-myofibroblast transdifferentiation through p38 in pancreatic ductal adenocarcinoma. Clin. Transl. Med. 2020, 10, e41. [Google Scholar] [CrossRef] [PubMed]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Mori, T.; Sahoo, D.; Xu, P.X.; Bermingham, J.R., Jr.; Weissman, I.L. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat. Cell Biol. 2012, 14, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Hawinkels, L.J.; Paauwe, M.; Verspaget, H.W.; Wiercinska, E.; van der Zon, J.M.; van der Ploeg, K.; Koelink, P.J.; Lindeman, J.H.; Mesker, W.; ten Dijke, P.; et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene 2014, 33, 97–107. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Zhou, Q.; Li, P.; Yu, B.; Li, J.; Qu, Y.; Yan, J.; Yu, Y.; Yan, M.; et al. Hepatocyte growth factor activates tumor stromal fibroblasts to promote tumorigenesis in gastric cancer. Cancer Lett. 2013, 335, 128–135. [Google Scholar] [CrossRef]
- Elenbaas, B.; Weinberg, R.A. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp. Cell. Res. 2001, 264, 169–184. [Google Scholar] [CrossRef]
- Shi, Y.; Du, L.; Lin, L.; Wang, Y. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat. Rev. Drug Discov. 2017, 16, 35–52. [Google Scholar] [CrossRef]
- Sanz-Moreno, V.; Gaggioli, C.; Yeo, M.; Albrengues, J.; Wallberg, F.; Viros, A.; Hooper, S.; Mitter, R.; Féral, C.C.; Cook, M.; et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell 2011, 20, 229–245. [Google Scholar] [CrossRef]
- Costa, A.; Scholer-Dahirel, A.; Mechta-Grigoriou, F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin. Cancer Biol. 2014, 25, 23–32. [Google Scholar] [CrossRef]
- Arcucci, A.; Ruocco, M.R.; Granato, G.; Sacco, A.M.; Montagnani, S. Cancer: An Oxidative Crosstalk between Solid Tumor Cells and Cancer Associated Fibroblasts. Biomed. Res. Int. 2016, 2016, 4502846. [Google Scholar] [CrossRef]
- Nikolic-Paterson, D.J.; Wang, S.; Lan, H.Y. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int. Suppl. 2014, 4, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, Y.; Ho, C.; Li, L.; Jin, C.; Wang, X.; Zou, C.; Mao, Y.; Wang, X.; Li, Q.; et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut 2022, 71, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Ershaid, N.; Sharon, Y.; Doron, H.; Raz, Y.; Shani, O.; Cohen, N.; Monteran, L.; Leider-Trejo, L.; Ben-Shmuel, A.; Yassin, M.; et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat. Commun. 2019, 10, 4375. [Google Scholar] [CrossRef]
- Albrengues, J.; Bertero, T.; Grasset, E.; Bonan, S.; Maiel, M.; Bourget, I.; Philippe, C.; Herraiz Serrano, C.; Benamar, S.; Croce, O.; et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat. Commun. 2015, 6, 10204. [Google Scholar] [CrossRef]
- Albrengues, J.; Bourget, I.; Pons, C.; Butet, V.; Hofman, P.; Tartare-Deckert, S.; Feral, C.C.; Meneguzzi, G.; Gaggioli, C. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014, 7, 1664–1678. [Google Scholar] [CrossRef]
- Sanchez-Alvarez, R.; Martinez-Outschoorn, U.E.; Lin, Z.; Lamb, R.; Hulit, J.; Howell, A.; Sotgia, F.; Rubin, E.; Lisanti, M.P. Ethanol exposure induces the cancer-associated fibroblast phenotype and lethal tumor metabolism: Implications for breast cancer prevention. Cell Cycle 2013, 12, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Garufi, A.; Traversi, G.; Cirone, M.; D’Orazi, G. HIPK2 role in the tumor–host interaction: Impact on fibroblasts transdifferentiation CAF-like. IUBMB Life 2019, 71, 2055–2061. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Calvo, F.; Ranftl, R.; Hooper, S.; Farrugia, A.J.; Moeendarbary, E.; Bruckbauer, A.; Batista, F.; Charras, G.; Sahai, E. Cdc42EP3/BORG2 and septin network enables mechano-transduction and the emergence of cancer-associated fibroblasts. Cell Rep. 2015, 13, 2699–2714. [Google Scholar] [CrossRef]
- Malik, R.; Luong, T.; Cao, X.; Han, B.; Shah, N.; Franco-Barraza, J.; Han, L.; Shenoy, V.; Lelkes, P.; Cukierman, E. Rigidity controls human desmoplastic matrix anisotropy to enable pancreatic cancer cell spread via extracellular signal-regulated kinase 2. Matrix Biol. 2019, 81, 50–69. [Google Scholar] [CrossRef] [PubMed]
- Avery, D.; Govindaraju, P.; Jacob, M.; Todd, L.; Monslow, J.; Puré, E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 2018, 67, 90–106. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zeng, X.T.; Gong, Z.C.; Zhang, M.M.; Wang, K.Q.; Tang, Y.P.; Huang, Z.H. Euphorbia Pekinensis Rupr. sensitizes colorectal cancer to PD-1 blockade by remodeling the tumor microenvironment and enhancing peripheral immunity. Phytomedicine 2024, 135, 156107. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Shen, H.; Li, Z.; Wang, T.; Wang, S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J. Hematol. Oncol. 2019, 12, 84. [Google Scholar] [CrossRef]
- Xie, F.; Liu, Y.L.; Chen, X.Y.; Li, Q.; Zhong, J.; Dai, B.Y.; Shao, X.F.; Wu, G.B. Role of MicroRNA, LncRNA, and exosomes in the progression of osteoarthritis: A review of recent literature. Orthop. Surg. 2020, 12, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Chen, B.; Zhao, J.; Yu, S.; Tang, Y.; Zheng, Q.; Li, Y.; Wang, P.; He, X. exoRBase: A database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018, 46, D106–D112. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901. [Google Scholar] [CrossRef]
- Ajam-Hosseini, M.; Babashah, S. Exploring ferroptosis and miRNAs: Implications for cancer modulation and therapy. Mol. Cell. Biochem. 2025. [Google Scholar] [CrossRef]
- Masoumi-Dehghi, S.; Babashah, S.; Sadeghizadeh, M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. J. Cell Commun. Signal. 2020, 14, 233–244. [Google Scholar] [CrossRef]
- Gheytanchi, E.; Madjd, Z.; Janani, L.; Rasti, A.; Ghods, R.; Atyabi, F.; Asadi-Lari, M.H.; Babashah, S. Exosomal microRNAs as potential circulating biomarkers in gastrointestinal tract cancers: A systematic review protocol. Syst. Rev. 2017, 6, 228. [Google Scholar] [CrossRef]
- Maminezhad, H.; Ghanadian, S.; Pakravan, K.; Razmara, E.; Rouhollah, F.; Mossahebi-Mohammadi, M.; Babashah, S. A panel of six-circulating miRNA signature in serum and its potential diagnostic value in colorectal cancer. Life Sci. 2020, 258, 118226. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, M.; Razmara, E.; Mahmud Hussen, B.; Simiyari, M.; Lotfizadeh, N.; Motaghed, H.; Khazraei Monfared, A.; Montazeri, M.; Babashah, S. Identification of a six-microRNA signature as a potential diagnostic biomarker in breast cancer tissues. J. Clin. Lab. Anal. 2021, 35, e24010. [Google Scholar] [CrossRef]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar] [CrossRef]
- Eldh, M.; Ekström, K.; Valadi, H.; Sjöstrand, M.; Olsson, B.; Jernås, M.; Lötvall, J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Mu, W.; Barry, J.; Han, A.; Carpenter, R.L.; Jiang, B.H.; Peiper, S.C.; Mahoney, M.G.; Aplin, A.E.; et al. Reactive oxygen species reprogram macrophages to suppress antitumor immune response through the exosomal miR-155-5p/PD-L1 pathway. J. Exp. Clin. Cancer Res. 2022, 41, 41. [Google Scholar] [CrossRef]
- Lander, E.S. Initial impact of the sequencing of the human genome. Nature 2011, 470, 187–197. [Google Scholar] [CrossRef]
- Jacob, R.; Zander, S.; Gutschner, T. The Dark Side of the Epitranscriptome: Chemical Modifications in Long Non-Coding RNAs. Int. J. Mol. Sci. 2017, 18, 2387. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, S.; Agah, S.; Mirzajani, E.; Asghari Gharakhyli, E.; Norollahi, S.E.; Rahbar Taramsari, M.; Babaei, K.; Samadani, A.A. microRNAs, oxidative stress, and genotoxicity as the main inducers in the pathobiology of cancer development. Horm. Mol. Biol. Clin. Investig. 2024, 45, 55–73. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.; Liu, S.; Tao, Y. Chromatin remodeling factor lymphoid-specific helicase inhibits ferroptosis through lipid metabolic genes in lung cancer progression. Chin. J. Cancer 2017, 36, 82. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, C.; Dai, R.; Zeng, Y. Ferroptosis-mediated crosstalk in the tumor microenvironment implicated in cancer progression and therapy. Front. Cell Dev. Biol. 2021, 9, 739392. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Son, K.J.; Choi, K.R.; Lee, S.J.; Lee, H. Immunogenic Cell Death Induced by Ginsenoside Rg3: Significance in Dendritic Cell-based Anti-tumor Immunotherapy. Immune Netw. 2016, 16, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Y.; Ma, L.; Chen, Y.; Liu, J.; Guo, Y.; Yu, T.; Zhang, L.; Zhu, L.; Shu, Y. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol. Ther. 2022, 30, 3133–3154. [Google Scholar] [CrossRef]
- Wright, M.W.; Bruford, E.A. Naming ‘junk’: Human non-protein coding RNA (ncRNA) gene nomenclature. Hum. Genom. 2011, 5, 90–98. [Google Scholar] [CrossRef]
- Rafiee, A.; Riazi-Rad, F.; Havaskary, M.; Nuri, F. Long noncoding RNAs: Regulation, function and cancer. Biotechnol. Genet. Eng. Rev. 2018, 34, 153–180. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Lei, W.; Luo, Y.; Lei, W.; Luo, Y.; Yan, K.; Zhao, S.; Li, Y.; Qiu, X.; Zhou, Y.; Long, H.; et al. Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand. J. Rheumatol. 2009, 38, 369–374. [Google Scholar] [CrossRef]
- Cui, C.; Cui, Q. The relationship of human tissue microRNAs with those from body fluids. Sci. Rep. 2020, 10, 5644. [Google Scholar] [CrossRef] [PubMed]
- Fehlmann, T.; Ludwig, N.; Backes, C.; Meese, E.; Keller, A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 2016, 13, 1084–1088. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Ren, Y.; Jia, H.H.; Xu, Y.Q.; Zhou, X.; Zhao, X.H.; Wang, Y.F.; Song, X.; Zhu, Z.Y.; Sun, T.; Dou, Y.; et al. Paracrine and epigenetic control of CAF-induced metastasis: The role of HOTAIR stimulated by TGF-ß1 secretion. Mol. Cancer 2018, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Chen, W.Y.; Li, X.B.; Ke, H.; Zhou, X.L. Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway. Open Life Sci. 2021, 16, 961–968. [Google Scholar] [CrossRef]
- Volpini, L.; Monaco, F.; Santarelli, L.; Neuzil, J.; Tomasetti, M. Advances in RNA cancer therapeutics: New insight into exosomes as miRNA delivery. Asp. Mol. Med. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Qiu, B.; Huang, L.; Lin, J.; Li, Y.; Ze, Y.; Huang, C.; Yao, Y. Exosomes and ferroptosis: Roles in tumour regulation and new cancer therapies. PeerJ 2022, 10, e13238. [Google Scholar] [CrossRef]
- Wu, X.; Iroegbu, C.D.; Peng, J.; Guo, J.; Yang, J.; Fan, C. Cell death and exosomes regulation after myocardial infarction and ischemia-reperfusion. Front. Cell Dev. Biol. 2021, 9, 673677. [Google Scholar] [CrossRef]
- Wei, X.-b.; Jiang, W.-q.; Zeng, J.-h.; Huang, L.-q.; Ding, H.-g.; Jing, Y.-w.; Han, Y.-l.; Li, Y.-c.; Chen, S.-l. Exosome-derived lncRNA NEAT1 exacerbates sepsis-associated encephalopathy by promoting ferroptosis through regulating miR-9-5p/TFRC and GOT1 axis. Mol. Neurobiol. 2022, 59, 1954–1969. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, W.; Jiang, C.; Jiang, Z.; Tang, D. The engineered exosomes targeting ferroptosis: A novel approach to reverse immune checkpoint inhibitors resistance. Int. J. Cancer 2024, 155, 7–18. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Guo, L.; Gao, W.; Tang, T.-L.; Yan, M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Poggio, M.; Hu, T.; Pai, C.-C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019, 177, 414–427.e413. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 2019, 18, 146. [Google Scholar] [CrossRef]
- Efimova, I.; Catanzaro, E.; Van der Meeren, L.; Turubanova, V.D.; Hammad, H.; Mishchenko, T.A.; Vedunova, M.V.; Fimognari, C.; Bachert, C.; Coppieters, F. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer 2020, 8, e001369. [Google Scholar] [CrossRef]
- Cai, H.; Ren, Y.; Chen, S.; Wang, Y.; Chu, L. Ferroptosis and tumor immunotherapy: A promising combination therapy for tumors. Front. Oncol. 2023, 13, 1119369. [Google Scholar] [CrossRef]
- Gu, X.; Liu, Y.e.; Dai, X.; Yang, Y.-G.; Zhang, X. Deciphering the potential roles of ferroptosis in regulating tumor immunity and tumor immunotherapy. Front. Immunol. 2023, 14, 1137107. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Zhang, Z.; Zhang, R.; Sun, Q.; Zhang, Z.; Liu, Y.; Ma, B. Mesenchymal stem cell-derived exosomes ameliorate delayed neurocognitive recovery in aged mice by inhibiting hippocampus ferroptosis via activating SIRT1/Nrf2/HO-1 signaling pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3593294. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Li, X.P.; Zou, H.; Liu, L.; Liu, W.; Duan, T. C-C chemokine receptor type 2 promotes epithelial-to-mesenchymal transition by upregulating matrix metalloproteinase-2 in human liver cancer. Oncol. Rep. 2018, 40, 2734–2741. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, T.; Liang, X.; Gou, S.; Xiong, J.; Cui, J.; Peng, T. Tumor Cell Derived Exosomal GOT1 Suppresses Tumor Cell Ferroptosis to Accelerate Pancreatic Cancer Progression by Activating Nrf2/HO-1 Axis via Upregulating CCR2 Expression. Cells 2022, 11, 3893. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxid. Med. Cell. Longev. 2022, 2022, 3553617. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.A.; Ontoria-Oviedo, I.; González-King, H.; Diez-Juan, A.; Sepúlveda, P. Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. PLoS ONE 2015, 10, e0138849. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Feng, C.; Li, M.; Hong, Z.; Di, W.; Qiu, L. Exosomal NOX1 promotes tumor-associated macrophage M2 polarization-mediated cancer progression by stimulating ROS production in cervical cancer: A preliminary study. Eur. J. Med. Res. 2023, 28, 323. [Google Scholar] [CrossRef]
- Barton, M.; Meyer, M.R.; Prossnitz, E.R. Nox1 downregulators: A new class of therapeutics. Steroids 2019, 152, 108494. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Li, Y.; Shen, J.; Wei, Y.; Li, H.; Chen, S.; Yang, H.; Zeng, F.; Liu, C.; et al. DDX19A Promotes Metastasis of Cervical Squamous Cell Carcinoma by Inducing NOX1-Mediated ROS Production. Front. Oncol. 2021, 11, 629974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Li, Y.; Zhao, X. Research Progress on Tumor-Associated Macrophages and Inflammation in Cervical Cancer. Biomed Res. Int. 2020, 2020, 6842963. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, N.; Li, D.; Wu, Y.; Wang, H.; Wang, J. Circulating exosomal small RNAs are promising non-invasive diagnostic biomarkers for gastric cancer. J. Cell. Mol. Med. 2020, 24, 14502–14513. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Théry, C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017, 36, 3012–3028. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Žėkas, V.; Kurg, R.; Kurg, K.; Bironaitė, D.; Radzevičius, M.; Karčiauskaitė, D.; Matuzevičienė, R.; Kučinskienė, Z.A. Oxidative Properties of Blood-Derived Extracellular Vesicles in 15 Patients After Myocardial Infarction. Med. Sci. Monit. 2022, 28, e935291. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.S.; Ferreira, P.M.; Mitchell, J.L.; Pula, G.; Gibbins, J.M. Platelet-derived extracellular vesicles express NADPH oxidase-1 (Nox-1), generate superoxide and modulate platelet function. Free Radic. Biol. Med. 2021, 165, 395–400. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-associated neutrophils as a new prognostic factor in cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef]
- Schmidt, H.; Bastholt, L.; Geertsen, P.; Christensen, I.J.; Larsen, S.; Gehl, J.; von der Maase, H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: A prognostic model. Br. J. Cancer 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef]
- Wang, H.; Zang, J.; Zhao, Z.; Zhang, Q.; Chen, S. The advances of neutrophil-derived effective drug delivery systems: A key review of managing tumors and inflammation. Int. J. Nanomed. 2021, 16, 7663–7681. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A review of neutrophil extracellular traps (NETs) in disease: Potential anti-NETs therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Kimoto, Y.; Horiuchi, T. The complement system and ANCA associated vasculitis in the era of anti-complement drugs. Front. Immunol. 2022, 13, 926044. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.-P.; Chen, H.-Z. Extracellular vesicles in the tumor immune microenvironment. Cancer Lett. 2021, 516, 48–56. [Google Scholar] [CrossRef]
- Dutta, A.; Bhagat, S.; Paul, S.; Katz, J.P.; Sengupta, D.; Bhargava, D. Neutrophils in Cancer and Potential Therapeutic Strategies Using Neutrophil-Derived Exosomes. Vaccines 2023, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Koni, M.; Lopatina, T.; Grange, C.; Sarcinella, A.; Cedrino, M.; Bruno, S.; Buffolo, F.; Femminò, S.; Camussi, G.; Brizzi, M.F. Circulating extracellular vesicles derived from tumor endothelial cells hijack the local and systemic anti-tumor immune response: Role of mTOR/G-CSF pathway. Pharmacol. Res. 2023, 195, 106871. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef]
- Germann, M.; Zangger, N.; Sauvain, M.O.; Sempoux, C.; Bowler, A.D.; Wirapati, P.; Kandalaft, L.E.; Delorenzi, M.; Tejpar, S.; Coukos, G.; et al. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFβ. EMBO Mol. Med. 2020, 12, e10681. [Google Scholar] [CrossRef]
- Sobo-Vujanovic, A.; Munich, S.; Vujanovic, N.L. Dendritic-cell exosomes cross-present Toll-like receptor-ligands and activate bystander dendritic cells. Cell Immunol. 2014, 289, 119–127. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Mou, Z.; Zhou, Q.; Dai, X.; Ou, Y.; Chen, X.; Chen, Y.; Xu, C.; Hu, Y.; et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Mol. Ther. 2022, 30, 1054–1070. [Google Scholar] [CrossRef]
- Edwards, D.N.; Ngwa, V.M.; Raybuck, A.L.; Wang, S.; Hwang, Y.; Kim, L.C.; Cho, S.H.; Paik, Y.; Wang, Q.; Zhang, S.; et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Investig. 2021, 131, e140100. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Xue, Q.; Peng, W.; Zhou, Q. New advances of natural products in non-small cell lung cancer: From mechanisms to therapies. J. Ethnopharmacol. 2025, 346, 119636. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou, D.; Wang, Q.; Liu, W.; Yu, F.; Wu, F.; Chen, C. Crosstalk of MicroRNAs and Oxidative Stress in the Pathogenesis of Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 2415324. [Google Scholar] [CrossRef]
- Li, Y.; Tan, J.; Miao, Y.; Zhang, Q. MicroRNA in extracellular vesicles regulates inflammation through macrophages under hypoxia. Cell Death Discov. 2021, 7, 285. [Google Scholar] [CrossRef]
- Buschow, S.I.; Liefhebber, J.M.; Wubbolts, R.; Stoorvogel, W. Exosomes contain ubiquitinated proteins. Blood Cells Mol. Dis. 2005, 35, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Putz, U.; Howitt, J.; Lackovic, J.; Foot, N.; Kumar, S.; Silke, J.; Tan, S.S. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J. Biol. Chem. 2008, 283, 32621–32627. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Wubbolts, R.; Ten Broeke, T.; Buschow, S.I.; Ossendorp, F.A.; Melief, C.J.; Raposo, G.; van Balkom, B.W.; Stoorvogel, W. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 2006, 25, 885–894. [Google Scholar] [CrossRef]
- Buschow, S.I.; Nolte-‘t Hoen, E.N.; van Niel, G.; Pols, M.S.; ten Broeke, T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.; Raposo, G.; Wubbolts, R.; et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, C.; Yang, J.; Liu, P.; Jiang, K.; Shaukat, A.; Wu, H.; Deng, G. Placental exosome-mediated Bta-miR-499-Lin28B/let-7 axis regulates inflammatory bias during early pregnancy. Cell Death Dis. 2018, 9, 704. [Google Scholar] [CrossRef]
- Ross, J.W.; Ashworth, M.D.; Mathew, D.; Reagan, P.; Ritchey, J.W.; Hayashi, K.; Spencer, T.E.; Lucy, M.; Geisert, R.D. Activation of the transcription factor, nuclear factor kappa-B, during the estrous cycle and early pregnancy in the pig. Reprod. Biol. Endocrinol. 2010, 8, 39. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, L.; Lv, D.; Zhu, X.; Tang, H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J. Hematol. Oncol. 2019, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, K.; Xu, Y.; Hu, H.; Zhang, N.; Wang, Y.; Zhong, Z.; Zhao, J.; Li, Q.; Zhu, D.; et al. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of miR-125b. Circ. Res. 2018, 123, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Geng, Y.; Sepúlveda, R.; Solís, N.; Torres, J.; Arab, J.P.; Barrera, F.; Cabrera, D.; Moshage, H.; Arrese, M. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165753. [Google Scholar] [CrossRef]
- Li, G.; Huang, D.; Li, N.; Ritter, J.K.; Li, P.-L. Regulation of TRPML1 channel activity and inflammatory exosome release by endogenously produced reactive oxygen species in mouse podocytes. Redox Biol. 2021, 43, 102013. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, D.; Bhat, O.M.; Poklis, J.L.; Zhang, A.; Zou, Y.; Kidd, J.; Gehr, T.W.; Li, P.-L. Abnormal podocyte TRPML1 channel activity and exosome release in mice with podocyte-specific Asah1 gene deletion. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158856. [Google Scholar] [CrossRef]
- Martina, J.A.; Diab, H.I.; Lishu, L.; Jeong-A, L.; Patange, S.; Raben, N.; Puertollano, R. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 2014, 7, ra9. [Google Scholar] [CrossRef]
- Guitart, K.; Loers, G.; Buck, F.; Bork, U.; Schachner, M.; Kleene, R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 2016, 64, 896–910. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Ma, X.; Cheng, C.; Xiao, X.; Chen, J.; Liu, S.; Zhao, B.; Chen, Y. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid. Med. Cell. Longev. 2013, 2013, 572729. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef]
- Wang, T.; Gilkes, D.M.; Takano, N.; Xiang, L.; Luo, W.; Bishop, C.J.; Chaturvedi, P.; Green, J.J.; Semenza, G.L. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, E3234–E3242. [Google Scholar] [CrossRef]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, H.; Umezu, T.; Ohyashiki, K.; Hirano, T.; Ohyashiki, J.H. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013, 288, 34343–34351. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef]
- Aga, M.; Bentz, G.L.; Raffa, S.; Torrisi, M.R.; Kondo, S.; Wakisaka, N.; Yoshizaki, T.; Pagano, J.S.; Shackelford, J. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene 2014, 33, 4613–4622. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nakamura, Y.; Wei, M.; Wanibuchi, H.; et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2014, 445, 327–333. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, J.H.; Zhao, L.P.; Fan, G.L.; Zheng, R.R.; Qiu, X.Z.; Yu, X.Y.; Li, S.Y.; Zhang, X.Z. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials 2019, 211, 14–24. [Google Scholar] [CrossRef]
- Das, C.K.; Jena, B.C.; Banerjee, I.; Das, S.; Parekh, A.; Bhutia, S.K.; Mandal, M. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Mol. Pharm. 2019, 16, 24–40. [Google Scholar] [CrossRef]
- Aaboe Jørgensen, M.; Ugel, S.; Linder Hübbe, M.; Carretta, M.; Perez-Penco, M.; Weis-Banke, S.E.; Martinenaite, E.; Kopp, K.; Chapellier, M.; Adamo, A.; et al. Arginase 1-Based Immune Modulatory Vaccines Induce Anticancer Immunity and Synergize with Anti-PD-1 Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 1316–1326. [Google Scholar] [CrossRef]
- Tong, J.; Wu, Z.; Wang, Y.; Hao, Q.; Liu, H.; Cao, F.; Jiao, Y. Astragaloside IV Synergizing with Ferulic Acid Ameliorates Pulmonary Fibrosis by TGF-β1/Smad3 Signaling. Evid.-Based Complement. Altern. Med. 2021, 2021, 8845798. [Google Scholar] [CrossRef] [PubMed]
- Pourhanifeh, M.H.; Farrokhi-Kebria, H.; Mostanadi, P.; Farkhondeh, T.; Samarghandian, S. Anticancer Properties of Baicalin against Breast Cancer and other Gynecological Cancers: Therapeutic Opportunities based on Underlying Mechanisms. Curr. Mol. Pharmacol. 2024, 17, e18761429263063. [Google Scholar] [CrossRef]
- Hu, H.-y.; Li, K.-p.; Wang, X.-j.; Liu, Y.; Lu, Z.-g.; Dong, R.-h.; Guo, H.-b.; Zhang, M.-x. Set9, NF-κB, and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 2013, 34, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Park, M.N.; Park, H.; Rahman, M.A.; Kim, J.W.; Park, S.S.; Cho, Y.; Choi, J.; Son, S.R.; Jang, D.S.; Shim, B.S.; et al. BK002 Induces miR-192-5p-Mediated Apoptosis in Castration-Resistant Prostate Cancer Cells via Modulation of PI3K/CHOP. Front. Oncol. 2022, 12, 791365. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Jin, X.; Ma, Q.; Li, H.; Zhou, Q.; Chen, W. Bu-Shen-Ning-Xin decoction ameliorates premature ovarian insufficiency by suppressing oxidative stress through rno_circRNA_012284/rno_miR-760-3p/HBEGF pathway. Phytomedicine 2024, 133, 155920. [Google Scholar] [CrossRef]
- Cha, J.A.; Song, H.S.; Kang, B.; Park, M.N.; Park, K.S.; Kim, S.H.; Shim, B.S.; Kim, B. miR-211 Plays a Critical Role in Cnidium officinale Makino Extract-Induced, ROS/ER Stress-Mediated Apoptosis in U937 and U266 Cells. Int. J. Mol. Sci. 2018, 19, 865. [Google Scholar] [CrossRef]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Liao, L.; Li, Y.; Sun, X.; Wu, H.; Lan, Q.; Deng, Z.; Liu, P.; Xu, T.; et al. DanShen Decoction targets miR-93-5p to provide protection against MI/RI by regulating the TXNIP/NLRP3/Caspase-1 signaling pathway. Phytomedicine 2024, 135, 156225. [Google Scholar] [CrossRef]
- Park, M.N.; Jeon, H.W.; Rahman, M.A.; Park, S.S.; Jeong, S.Y.; Kim, K.H.; Kim, S.H.; Kim, W.; Kim, B. Daemonorops draco Blume Induces Apoptosis Against Acute Myeloid Leukemia Cells via Regulation of the miR-216b/c-Jun. Front. Oncol. 2022, 12, 808174. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Z.; Lin, S.; Chen, H.; Bu, H. Ginsenoside Rg3 suppresses vasculogenic mimicry by impairing DVL3-maintained stemness via PAAD cell-derived exosomal miR-204 in pancreatic adenocarcinoma. Phytomedicine 2024, 126, 155402. [Google Scholar] [CrossRef]
- Park, M.N.; Um, E.S.; Rahman, M.A.; Kim, J.W.; Park, S.S.; Cho, Y.; Song, H.; Son, S.R.; Jang, D.S.; Kim, W.; et al. Leonurus japonicus Houttuyn induces reactive oxygen species-mediated apoptosis via regulation of miR-19a-3p/PTEN/PI3K/AKT in U937 and THP-1 cells. J. Ethnopharmacol. 2022, 291, 115129. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hu, Y.; Sun, C.; Wang, Y.; Sun, Z.; Man, Y.; Wang, Y.; Ouyang, Z.; Ge, P.; Zou, X.; et al. Qizhu Jianwei decoction triggers ferroptosis by exosome-mediated miR-199-3p/ACSL4 signaling pathways. J. Ethnopharmacol. 2025, 344, 119529. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Z.; Ni, F.; Ye, X.; Qian, W. Shikonin overcomes drug resistance and induces necroptosis by regulating the miR-92a-1-5p/MLKL axis in chronic myeloid leukemia. Aging 2020, 12, 17662–17680. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, M.N.; Kim, C.; Kang, B.; Song, H.S.; Lee, H.; Kim, S.H.; Shim, B.S.; Kim, B. MiR-657/ATF2 Signaling Pathway Has a Critical Role in Spatholobus suberectus Dunn Extract-Induced Apoptosis in U266 and U937 Cells. Cancers 2019, 11, 150. [Google Scholar] [CrossRef]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, H.; Yu, R.; Zhang, Y.; Zou, Y.; Liu, X.; Sun, S. Wogonin suppresses proliferation, invasion and migration in gastric cancer cells via targeting the JAK-STAT3 pathway. Sci. Rep. 2024, 14, 30803. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, S.; Tong, F.; Wang, Y.; Wei, L. HPV(+) HNSCC-derived exosomal miR-9-5p inhibits TGF-β signaling-mediated fibroblast phenotypic transformation through NOX4. Cancer Sci. 2022, 113, 1475–1487. [Google Scholar] [CrossRef]
- Chen, L.; Qu, J.; Mei, Q.; Chen, X.; Fang, Y.; Chen, L.; Li, Y.; Xiang, C. Small extracellular vesicles from menstrual blood-derived mesenchymal stem cells (MenSCs) as a novel therapeutic impetus in regenerative medicine. Stem Cell Res. Ther. 2021, 12, 433. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, L.; Giurato, G.; Cicchini, C.; Montaldo, C.; Mancone, C.; Tarallo, R.; Battistelli, C.; Alonzi, T.; Weisz, A.; Tripodi, M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016, 17, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G. CAFs Interacting With TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front. Oncol. 2021, 11, 668349. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Dang, K.; Myers, K.A. The role of hypoxia-induced miR-210 in cancer progression. Int. J. Mol. Sci. 2015, 16, 6353–6372. [Google Scholar] [CrossRef]
- He, G.; Peng, X.; Wei, S.; Yang, S.; Li, X.; Huang, M.; Tang, S.; Jin, H.; Liu, J.; Zhang, S.; et al. Exosomes in the hypoxic TME: From release, uptake and biofunctions to clinical applications. Mol. Cancer 2022, 21, 19. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, M.; Feng, W.; Lin, Y.; Yin, J.; Jin, J.; Wang, Y. Exosomal miRNA Let-7 from Menstrual Blood-Derived Endometrial Stem Cells Alleviates Pulmonary Fibrosis through Regulating Mitochondrial DNA Damage. Oxid. Med. Cell. Longev. 2019, 2019, 4506303. [Google Scholar] [CrossRef]
- Zhao, L.; Xiong, M.; Liu, Y. Baicalin enhances the proliferation and invasion of trophoblasts and suppresses vascular endothelial damage by modulating long non-coding RNA NEAT1/miRNA-205-5p in hypertensive disorder complicating pregnancy. J. Obstet. Gynaecol. Res. 2021, 47, 3060–3070. [Google Scholar] [CrossRef]
- Park, M.N.; Choi, J.; Maharub Hossain Fahim, M.; Asevedo, E.A.; Nurkolis, F.; Ribeiro, R.; Kang, H.N.; Kang, S.; Syahputra, R.A.; Kim, B. Phytochemical synergies in BK002: Advanced molecular docking insights for targeted prostate cancer therapy. Front. Pharmacol. 2025, 16, 1504618. [Google Scholar] [CrossRef]
- Xu, W.; Ding, J.; Li, B.; Sun, T.; You, X.; He, Q.; Sheng, W. Effects of icariin and curcumol on autophagy, ferroptosis, and lipid metabolism based on miR-7/m-TOR/SREBP1 pathway on prostate cancer. Biofactors 2023, 49, 438–456. [Google Scholar] [CrossRef]
- Abdelrahman, S.A.; El-Shal, A.S.; Abdelrahman, A.A.; Saleh, E.Z.H.; Mahmoud, A.A. Neuroprotective effects of quercetin on the cerebellum of zinc oxide nanoparticles (ZnoNps)-exposed rats. Tissue Barriers 2023, 11, 2115273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tao, Q.; Bai, L.; Qin, Z.; Liu, X.; Li, S.; Yang, Y.; Ge, W.; Li, J. MicroRNA in the Exosomes Mediated by Resveratrol to Activate Neuronal Cells. Toxics 2024, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, Y.; Li, J.; Jin, M.; Yang, H.; Huang, G. Shikonin inhibited glycolysis and sensitized cisplatin treatment in non-small cell lung cancer cells via the exosomal pyruvate kinase M2 pathway. Bioengineered 2022, 13, 13906–13918. [Google Scholar] [CrossRef]
- Ma, M.Y.; Wang, Q.; Wang, S.M.; Feng, X.J.; Xian, Z.H.; Zhang, S.H. Wogonin inhibits hepatoma cell proliferation by targeting miR-27b-5p/YWHAZ axis. J. Biochem. Mol. Toxicol. 2023, 37, e23508. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | ROS Role | Key miRNA(s) | Exosomal Impact | Representative Pathway | Reference |
|---|---|---|---|---|---|
| CAFs | ↑ ROS | miR-522 | Inhibit ferroptosis via exosomal transfer | TGF-β/SMAD, NLRP3 inflammasome | [107] |
| DCs | Moderate ROS producers | miR-155 | Enhanced antigen presentation, enhanced T cell activation | CRT, HSP, TNF-α, IL-12, CD80, CD86 | [112] |
| Suppress antigen presentation via exosomes | IL-10, MHC-II | [107] | |||
| TANs | ↑ ROS via NOX1 | miR-210, miR-451a | Promote autophagy and N2 polarization | HMGB1/TLR4/NF-κB | [105] |
| TAMs | ↑ ROS via NOX2 | miR-21, miR-155 | Drive M2 polarization and immunosuppression | JAK/STAT3, NF-κB | [84,110,111] |
| Tumor cells | ↑ ROS via metabolism and hypoxia | miR-135, miR-210, miR-23a-3p | Regulate angiogenesis and ferroptosis | HIF-1α, Nrf2/Keap1, GPX4 | [113] |
| Compound/Extract | Source Plant | Target miRNA | ROS Efficacy | Exosome Study | Reference |
|---|---|---|---|---|---|
| Astragaloside IV + Ferulic Acid | Astragalus membranaceus + Angelica sinensis | miR-29b | ↓ ROS, ↑ SOD, ↓ MDA via Nrf2 and TGF-β1/Smad3 | Not studied | [141] |
| Baicalin | Scutellaria baicalensis | miR-338-3p, let-7c | ↑ ROS-mediated apoptosis, ↓ NF-κB, ↓ Bcl-2 | Not studied | [142] |
| Berberine | Coptis chinensis | miR-21 | ↑ ROS, ↓ Bcl-2, ↑ apoptosis | Not Studied | [143] |
| BK002 | Achyranthes japonica + Melandrium firmum | miR-192-5p | ↑ ROS-mediated apoptosis via miRNA modulation | Not studied | [144] |
| Bu-Shen-Ning-Xin Decoction (BSNXD) | Multi-herbal formula | miR-760-3p (via circRNA_012284) | ↓ ROS in ovarian granulosa cells via HBEGF modulation | Not Studied | [145] |
| Cnidium officinale Makino (COM) | Cnidium officinale Makino | miR-211 | ↑ ROS→ ↑ER stress-mediated Apoptosis | Not studied | [146] |
| Curcumin | Curcuma longa | miR-34a, miR-34b/c | ↑ ROS → activates NRF2 → ↑ miR-34a/b/c | Not studied | [147] |
| DanShen Decoction (DSD) | Salvia miltiorrhiza, Santalum album, Amomum villosum | miR-93-5p, miR-15b-5p, miR-16-5p | ↓ROS, ↓ Pyroptosis, ↑ SOD | BMSC-derived exosomes | [148] |
| Daemonorops draco (DD) | Daemonorops draco Blume | miR-216b | ↑ ROS, ↑ Apoptosis | Not studied | [149] |
| Ginsenoside Rg3 | Panax ginseng | miR-204 | ↓ ROS, ↓ EMT and Stemness | Exosomal miR-204 | [150] |
| Icariin | Epimedium spp. | miR-21 | ↓ ROS via PTEN/RECK, ↑ Apoptosis | Not Studied | [152] |
| Leonurus japonicus Houttuyn | Leonurus japonicus | miR-19a-3p | ↑ ROS → ↑ ER stress-mediated Apoptosis | Not studied | [151] |
| Qizhu Jianwei decoction (QZJWD) | Astragalus membranaceus, Polygonatum odoratum, Atractylodis macrocephala, Curcuma phaeocaulis, | miR-199–3p | ↑ ROS, ↑ MDA, ↑ Fe2+, ↓ GPx → ↑ Ferroptosis | Exosomal miR-199–3p | [153] |
| Quercetin | Various plants | Dual role depending on dose | Emerging evidence | [157] | |
| Resveratrol | Vitis vinifera, Polygonum cuspidatum | miR-21, miR-22, miR-34a, miR-145, miR-200c | ↓ ROS, anti-aging, ↑ apoptosis | Not studied | [154] |
| Shikonin | Lithospermum erythrorhizon | miR-92a-1-5p | ↑ ROS; necroptosis via MLKL pathway | Not studied | [155] |
| Spatholobus suberectus Dunn (SSD) | Spatholobus suberectus | miR-657 | ↑ ROS → ↑ER stress-mediated apoptosis | Not studied | [156] |
| Wogonin | Scutellaria baicalensis | miR-155, miR-145 (indirect) | Slight ↑ ROS, apoptosis induction, mitochondrial dysfunction | Not studied | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.N.; Kim, M.; Lee, S.; Kang, S.; Ahn, C.-H.; Tallei, T.E.; Kim, W.; Kim, B. Targeting Redox Signaling Through Exosomal MicroRNA: Insights into Tumor Microenvironment and Precision Oncology. Antioxidants 2025, 14, 501. https://doi.org/10.3390/antiox14050501
Park MN, Kim M, Lee S, Kang S, Ahn C-H, Tallei TE, Kim W, Kim B. Targeting Redox Signaling Through Exosomal MicroRNA: Insights into Tumor Microenvironment and Precision Oncology. Antioxidants. 2025; 14(5):501. https://doi.org/10.3390/antiox14050501
Chicago/Turabian StylePark, Moon Nyeo, Myoungchan Kim, Soojin Lee, Sojin Kang, Chi-Hoon Ahn, Trina Ekawati Tallei, Woojin Kim, and Bonglee Kim. 2025. "Targeting Redox Signaling Through Exosomal MicroRNA: Insights into Tumor Microenvironment and Precision Oncology" Antioxidants 14, no. 5: 501. https://doi.org/10.3390/antiox14050501
APA StylePark, M. N., Kim, M., Lee, S., Kang, S., Ahn, C.-H., Tallei, T. E., Kim, W., & Kim, B. (2025). Targeting Redox Signaling Through Exosomal MicroRNA: Insights into Tumor Microenvironment and Precision Oncology. Antioxidants, 14(5), 501. https://doi.org/10.3390/antiox14050501









