Intestinal Microbiota and Gene Expression Alterations in Chinese Mitten Crab (Eriocheir sinensis) Under Deltamethrin Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.1.1. Experimental Crab
2.1.2. Chemicals and Reagents
2.2. Experimental Design
2.3. 16S rRNA Gene Diversity Sequencing of Eriocheir sinensis
2.4. Real-Time Quantitative RT-PCR
2.5. Statistical Analysis
3. Results
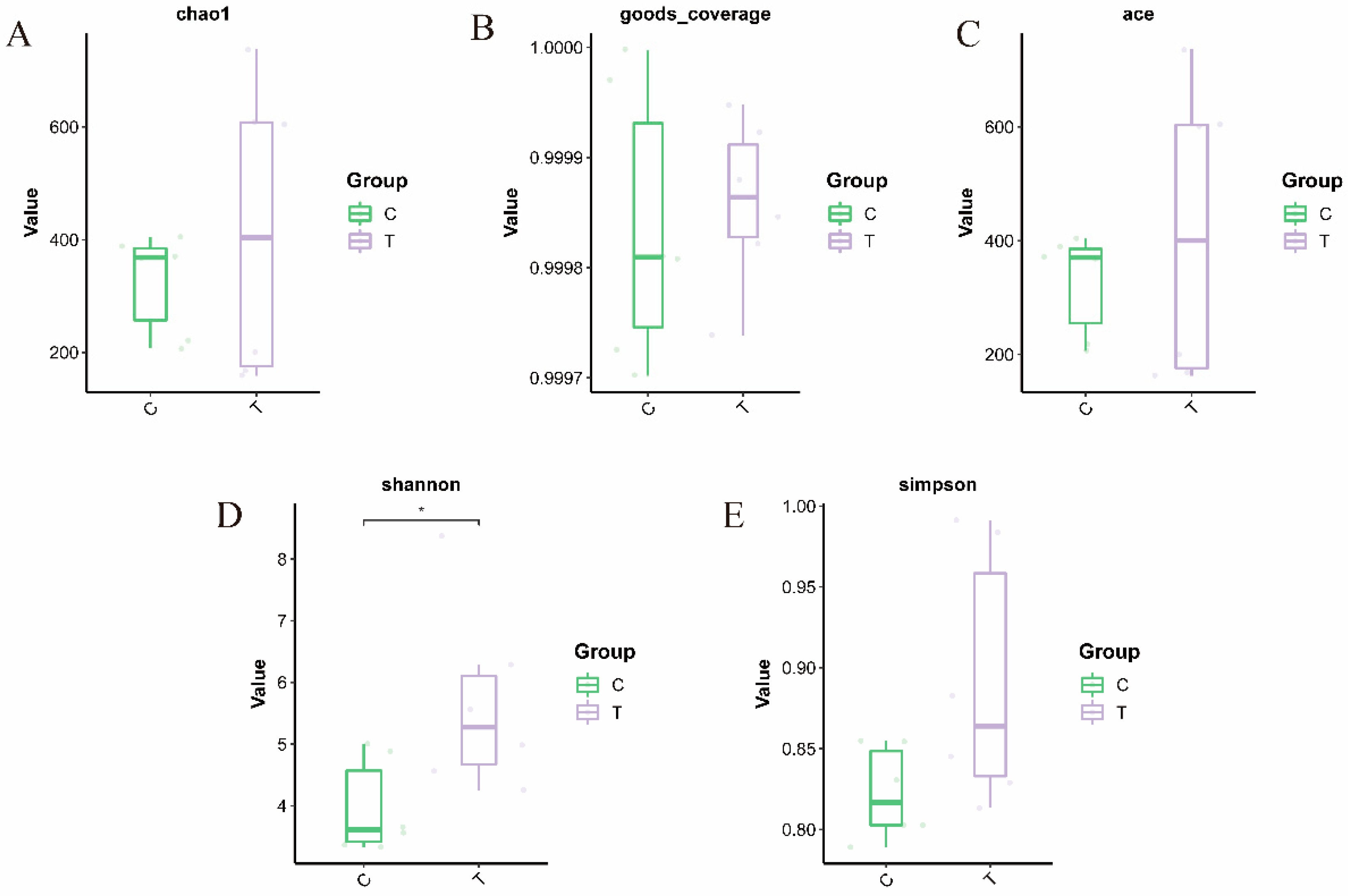
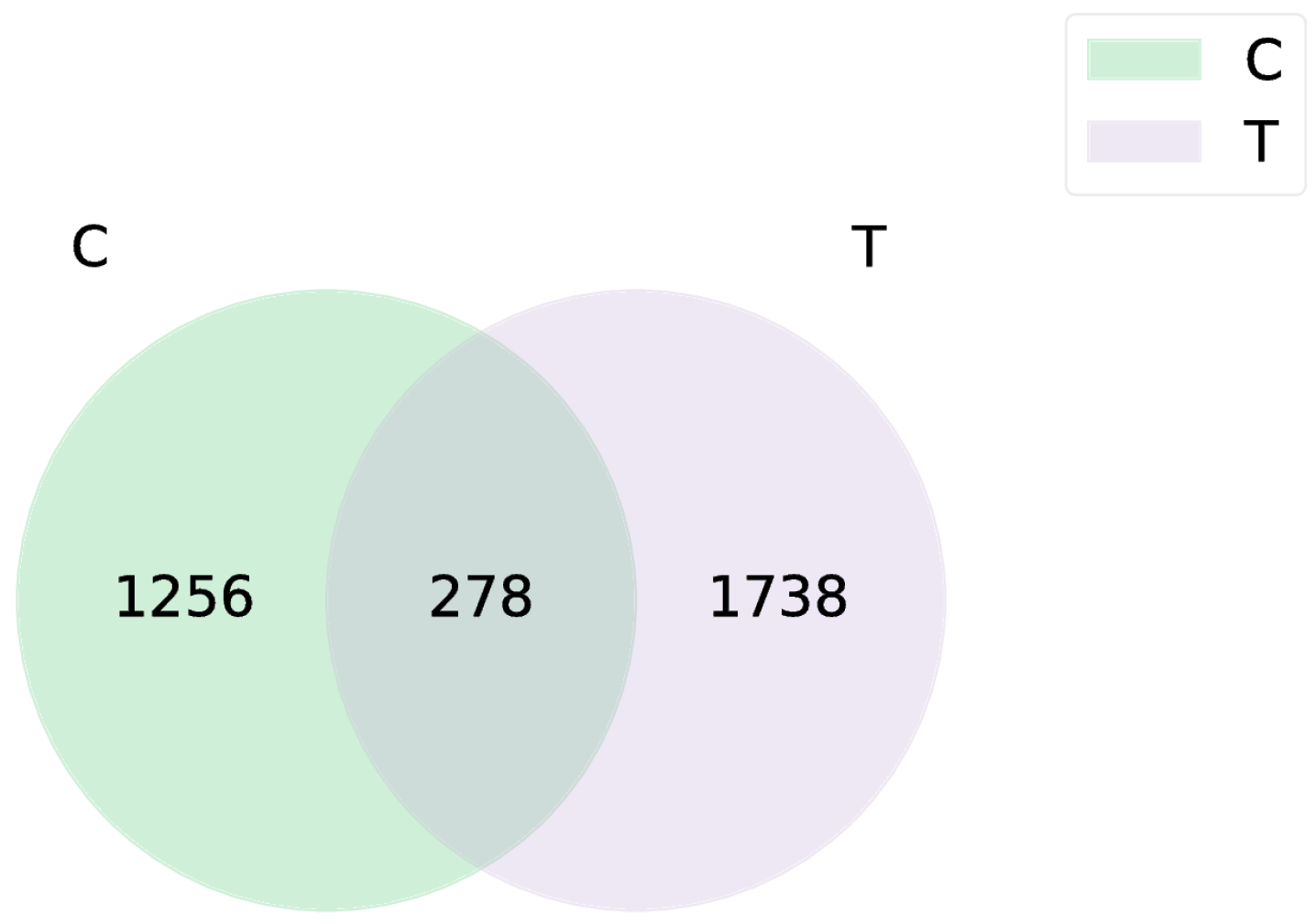
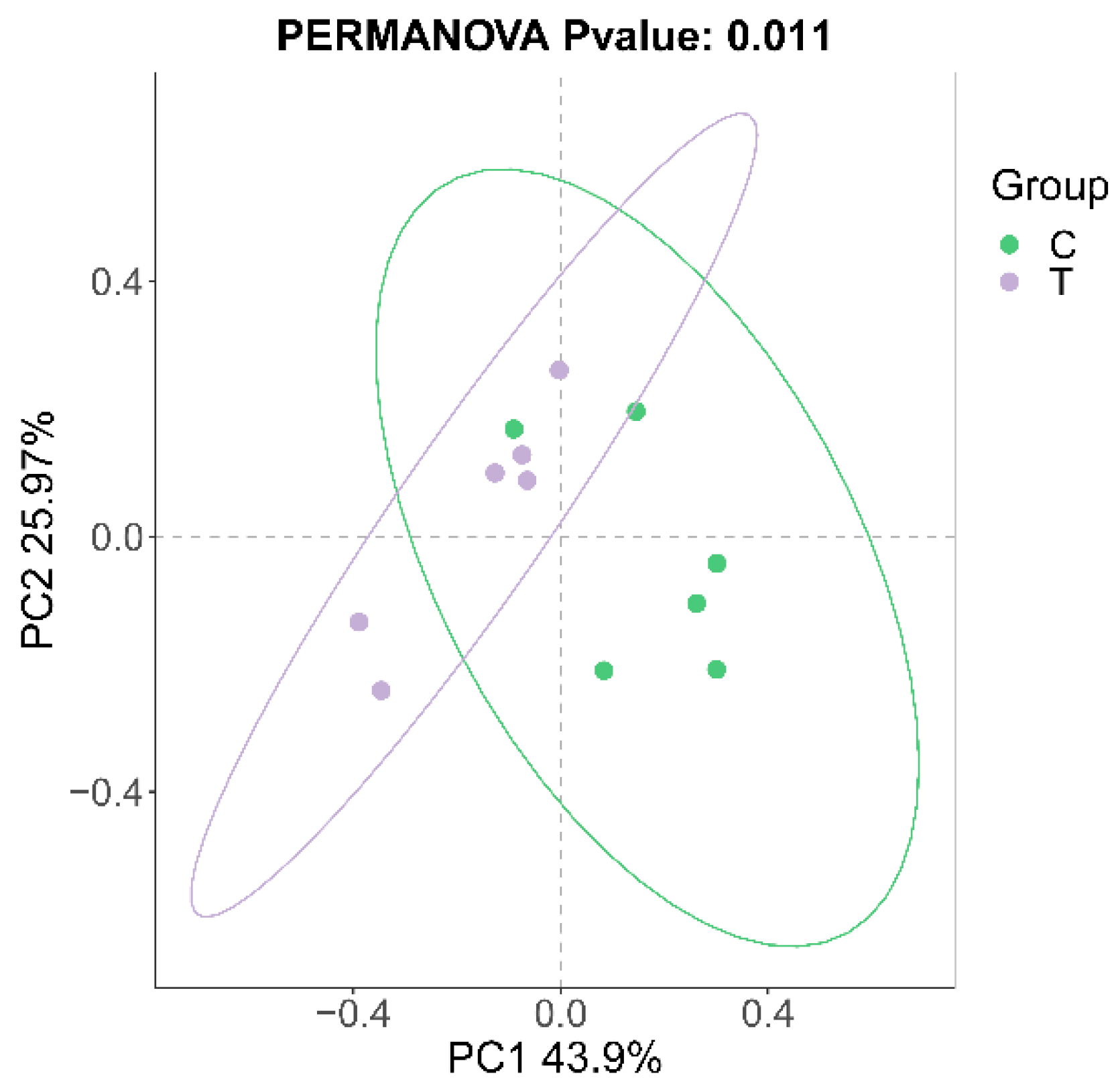
3.1. Sequencing Data
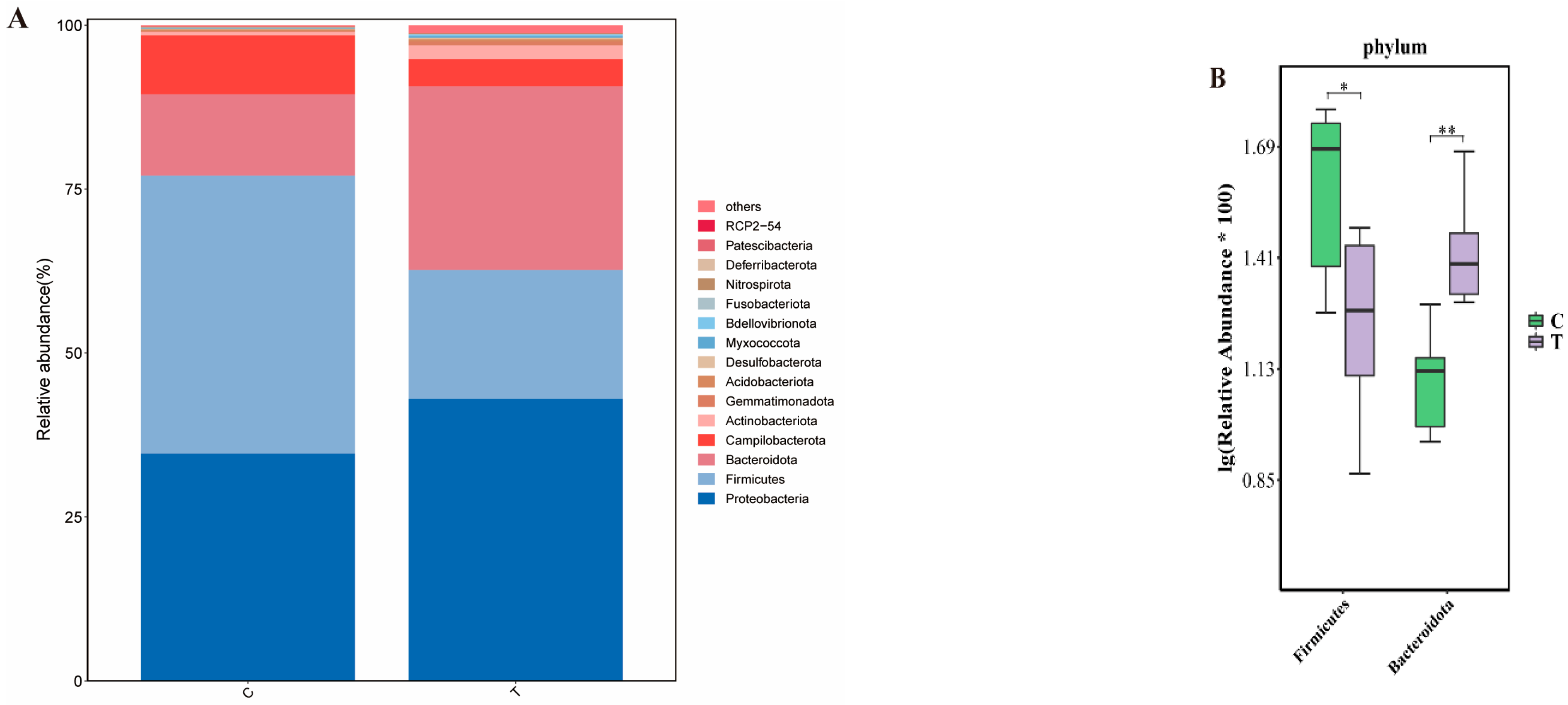
3.2. Comparative Analysis of Gut Microbial Community Composition Between Two Groups of Eriocheir sinensis at the Phylum Level
3.3. Comparative Analysis of Gut Microbial Community Composition Between Two Groups of Eriocheir sinensis at the Genus Level
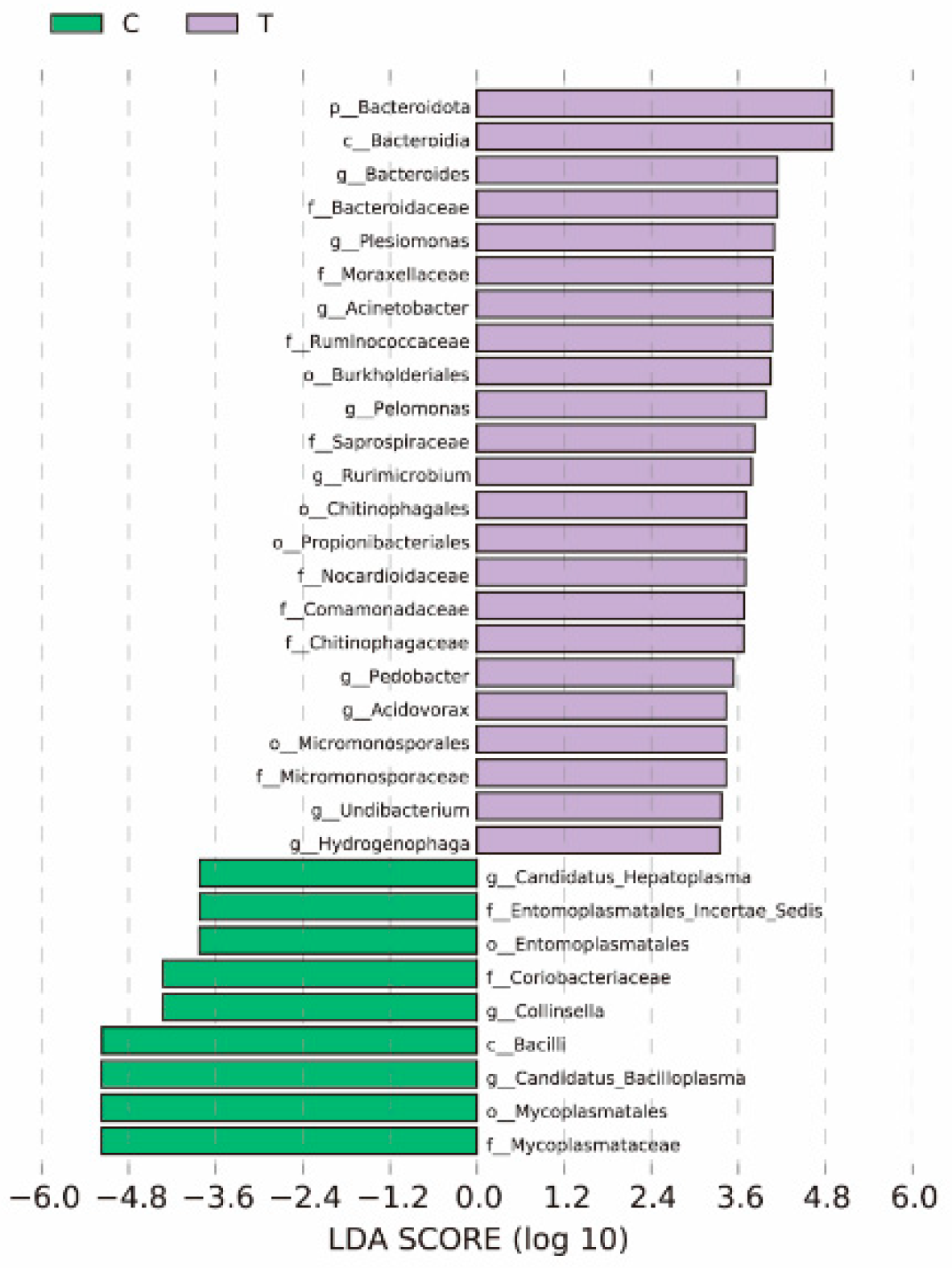
3.4. LEfSe Species Analysis
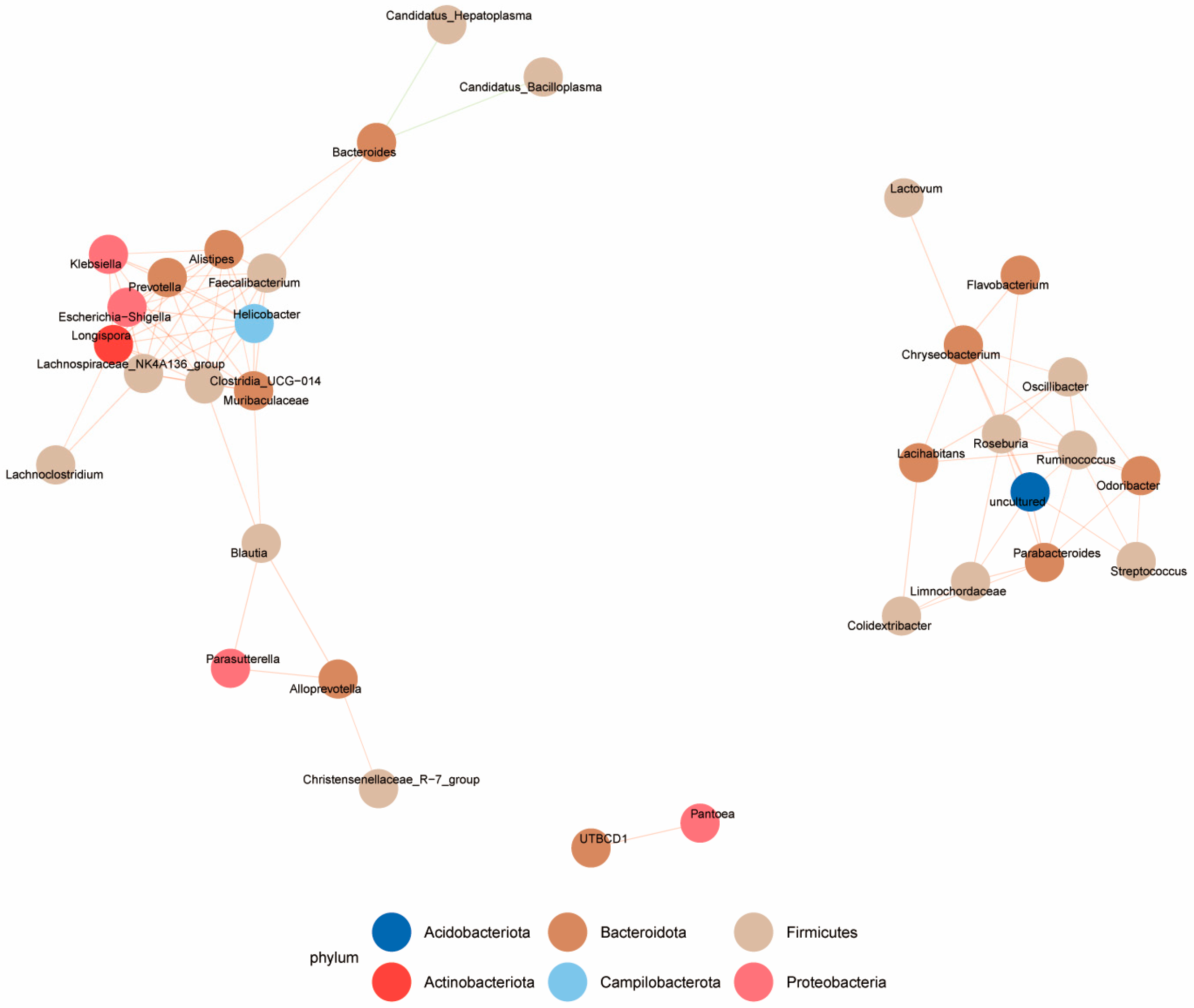
3.5. Correlation Analysis of Microbiota
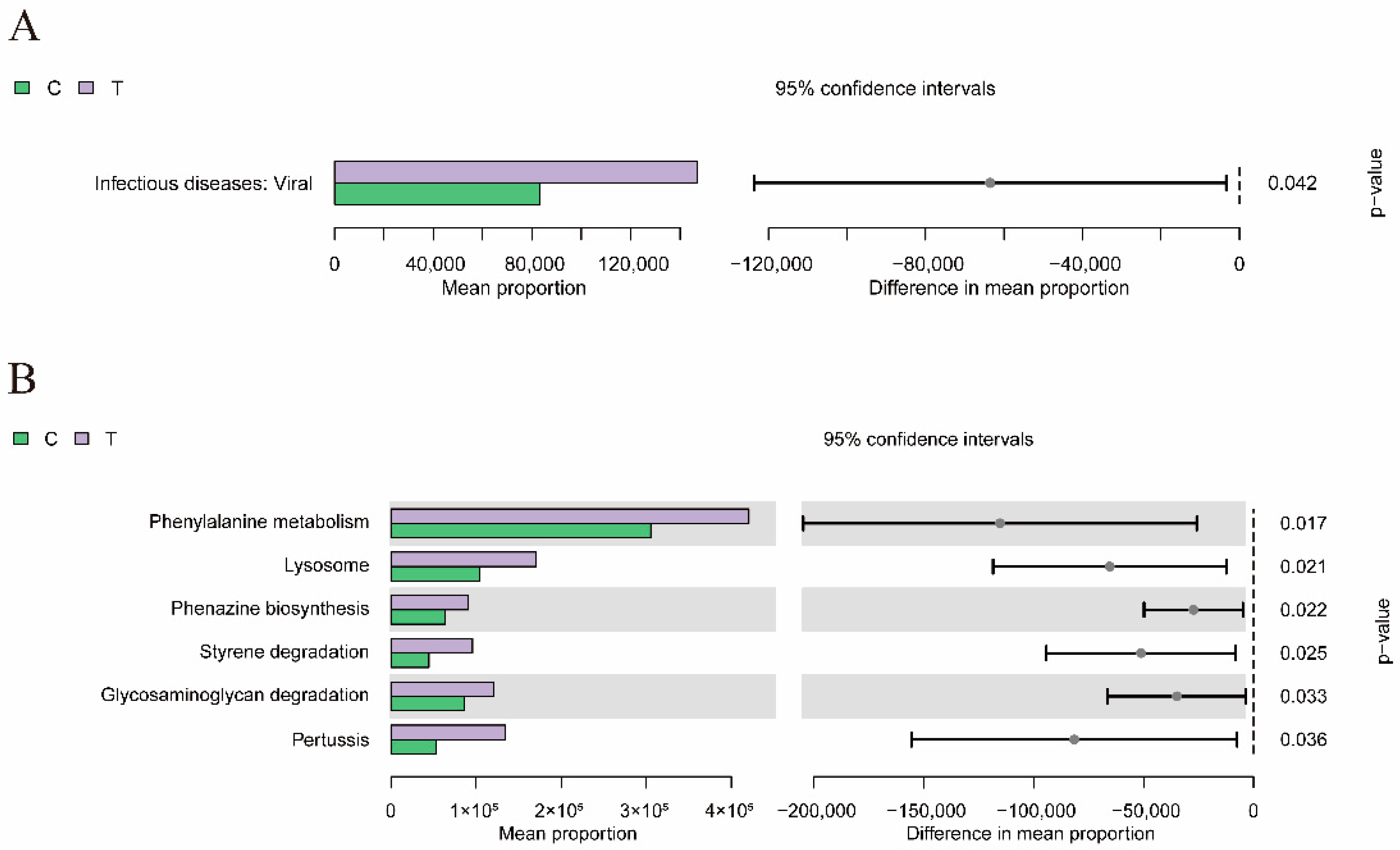
3.6. Functional Predictive Analysis
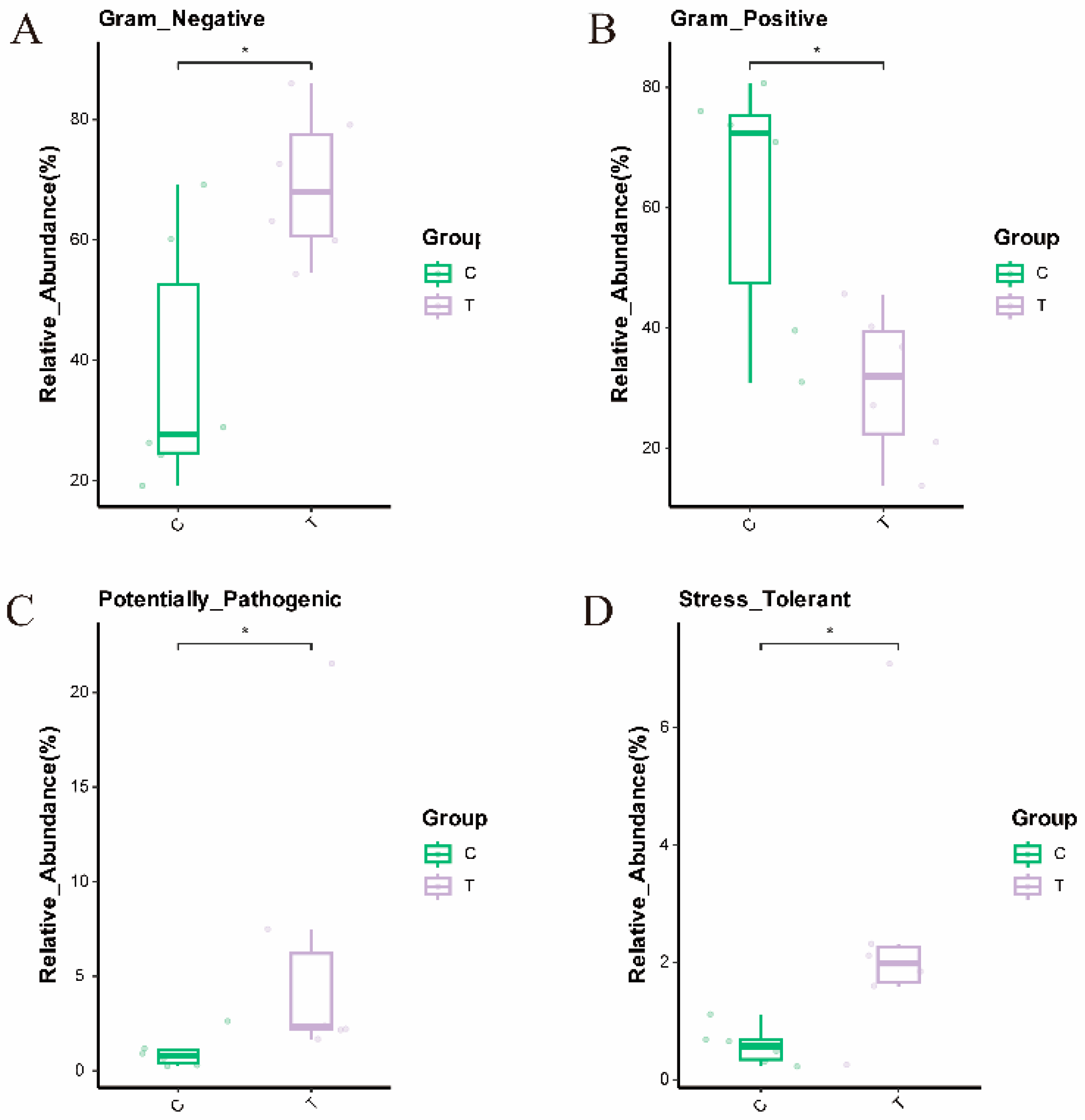
3.7. Analysis of Phenotypic Differences Among Different Groups of Microorganisms
3.8. Effects of Deltamethrin on Apoptosis and Autophagy-Related Genes in Intestinal Tissues
3.9. Effects of Deltamethrin on Genes Related to Oxidative Stress Injury in Intestinal Tissue
3.10. Effects of Deltamethrin on Immune-Related Genes in Intestinal Tissues
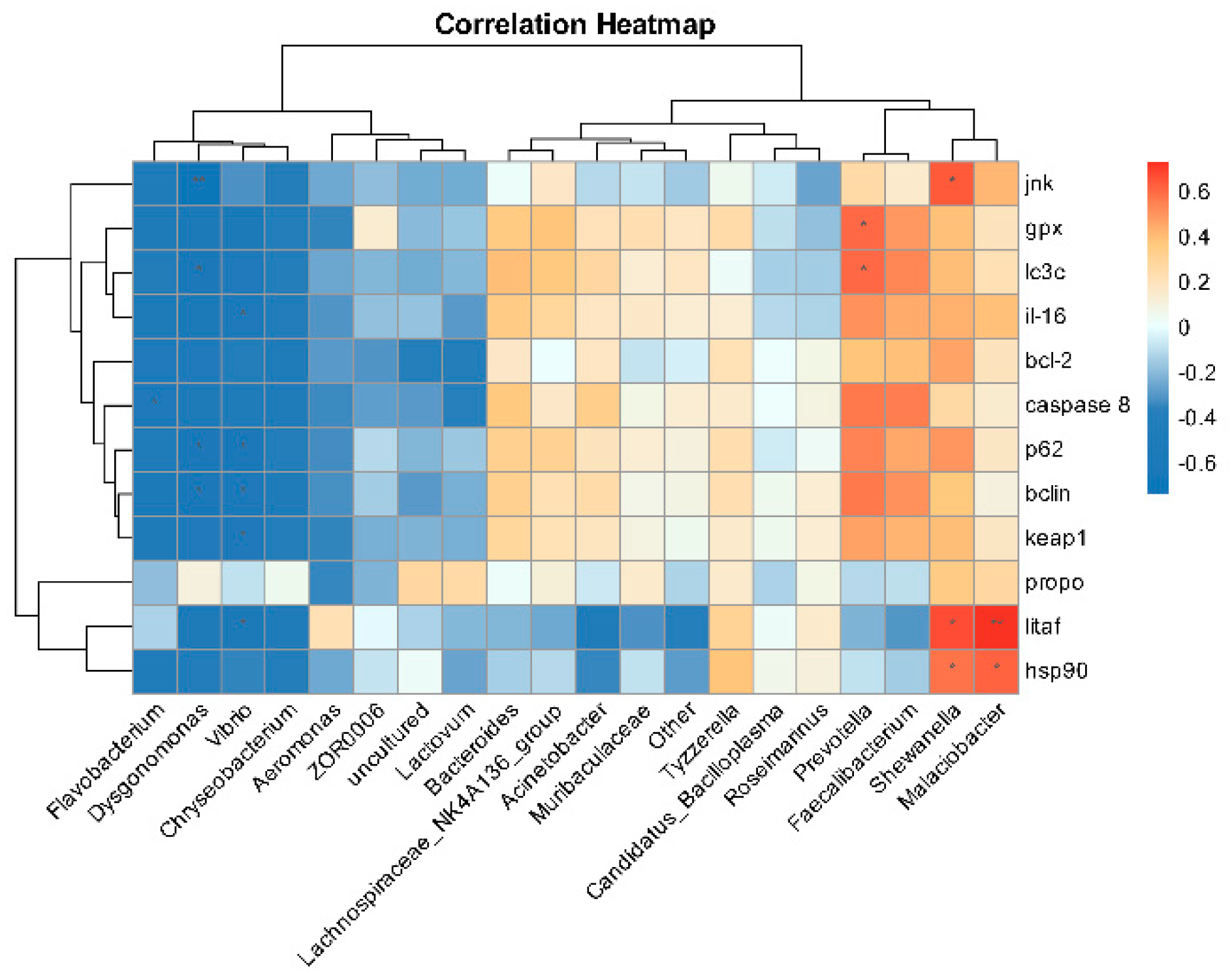
3.11. Correlation Analysis of Intestinal Microbiota and Intestinal Tissue Damage Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, B.; Gu, X.L.; Xu, Y.F.; Song, X.H. A case of diagnosis and treatment of river crab “emaciated disease” caused by expired feed. Sci. Fish Farming 2023, 5, 59–60. [Google Scholar] [CrossRef]
- Chen, L.M.; Zhou, X.W.; Zhang, Y.L.; Xu, W.Y.; Wang, J.X.; Zhang, G.D. A case analysis of “Water Collapse Disease” in Chinese mitten crabs and prevention/control recommendations. Sci. Fish Farming 2025, 1, 63. [Google Scholar] [CrossRef]
- Yang, Z.Y. Preliminary Studies on the Etiology and Pathogenic Mechanism of Hepatopancreatic Necrosis Syndrome in Chinese Mitten Crab (Eriocheir sinensis). Ph.D. Thesis, Shanghai Ocean Universiy, Shanghai, China, 2018. [Google Scholar]
- Cui, L.B.; Tang, S.L.; Qi, R.R.; Lei, Y.; Li, Y.B.; Wang, J. Pathological Study on “Shuibiezi” Disease of Crab Eriocheir sinensis. J. Yantai Univ. Nat. Sci. Eng. Ed. 2017, 30, 313–316. [Google Scholar]
- Yin, J.; Shi, X.X.; Chen, Y.; Huang, R. Distribution of Deltamethrin in Simulated Rice Field System of Chinese Mitten Crab (Eriocheir sinensis). J. Anhui Agric. Sci. 2018, 46, 9–11,21. [Google Scholar]
- Han, X.Q.; Fu, Z.R.; Chen, Y.P.; Li, T.; Zhang, L.Y.; Gao, L.N.; Chen, J.; Jiang, J.F. Acute Toxicity and Safety Assessment of Deltamethrin to Penaeus vannamei. J. Anhui Agric. Sci. 2021, 49, 122–124. [Google Scholar]
- Chen, J.Z.; Wu, W.; Qu, J.H.; Hu, G.D. Activities of Catalase and Monoamine Oxidase in Different Tissues of Tilipia Under Stress of Deltamethrin. J. Agro-Environ. Sci. 2006, 25, 1441–1445. [Google Scholar]
- Arslan, H.; Altun, S.; Özdemir, S. Acute toxication of deltamethrin results in activation of iNOS, 8-OHdG and up-regulation of caspase 3, iNOS gene expression in common carp (Cyprinus carpio L.). Aquat. Toxicol. 2017, 187, 90–99. [Google Scholar] [CrossRef]
- Yang, Z.; Fang, W.; Zhou, J.; Hu, K.; Yang, X. Study on the accumulation and elimination of deltamethrin in Eriocheir sinensis by high performance liquid chromatography/tandem mass spectrometry. Jiangsu J. Agric. Sci. 2019, 35, 709–715. [Google Scholar]
- Liu, H.Y.; Zheng, Y.; Ding, S.Y.; Liu, G.X.; Shi, Y.B.; Sun, L.S. Study on Toxicity Effect of Deltamethrin on Odontobutis Potamophilus. J. Aquac. 2022, 43, 27–32. [Google Scholar]
- Yang, Z.Y.; Zhang, Y.L.; Hu, K.; Yang, X.L.; Liu, L.S.; Zhang, F.X.; Cai, H.G. Effects of deltamethrin exposure on oxidative stress indexes and histological structure of hepatopancreas in Eriocheir sinensis. Acta Agr. Zhejiangensis 2017, 29, 1261–1270. [Google Scholar]
- Zhang, L. The Immunotoxicity of Three Pyrethroid Pesticides on Chinese Rare Minnow (Gobiocypris rarus). Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]
- Chen, J.Z.; Leng, C.M.; Hu, G.D.; Qu, J.H. Effects of continuous exposure to deitamethrin on the acetyrlcholinesterase activity in blood serum of tilapia. Chin. J. Ecol. 2008, 27, 776–779. [Google Scholar]
- Ren, Q.; Zhang, T.; Li, S.; Ren, Z. Integrative Characterization of Toxic Response of Zebra Fish (Danio rerio) to Deltamethrin Based on AChE Activity and Behavior Strength. BioMed Res. Int. 2016, 2016, 7309184. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.K.; Terech-Majewska, E.; Grudniewska, J.; Malaczewska, J.; Kazun, K.; Lepa, A. Influence of deltamethrin on nonspecific cellular and humoral defense mechanisms in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2010, 29, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Doolgindachbaporn, S.; Suksri, A. Effects of low molecular weight agar and Lactobacillus plantarum on growth performance, immunity, and disease resistance of basa fish (Pangasius bocourti, Sauvage 1880). Fish Shellfish. Immunol. 2014, 41, 340–345. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–22. [Google Scholar] [CrossRef]
- Luo, L.; Chang, Y.; Sheng, L. Gut-liver axis in the progression of nonalcoholic fatty liver disease: From the microbial derivatives-centered perspective. Life Sci. 2023, 321, 121614. [Google Scholar] [CrossRef]
- Li, S.; Han, W.; He, Q.; Zhang, W.; Zhang, Y. Relationship between Intestinal Microflora and Hepatocellular Cancer Based on Gut-Liver Axis Theory. Contrast Media Mol. Imaging 2022, 2022, 6533628. [Google Scholar] [CrossRef]
- Wu, H.; Gao, J.; Xie, M.; Wu, J.; Song, R.; Yuan, X.; Wu, Y.; Ou, D. Chronic exposure to deltamethrin disrupts intestinal health and intestinal microbiota in juvenile crucian carp. Ecotoxicol. Environ. Saf. 2022, 241, 113732. [Google Scholar] [CrossRef]
- Sun, Y.; Li, T.; Zhou, G.; Zhou, Y.; Wu, Y.; Xu, J.; Chen, J.; Zhong, S.; Zhong, D.; Liu, R.; et al. Relationship between deltamethrin resistance and gut symbiotic bacteria of Aedes albopictus by 16S rDNA sequencing. Parasites Vectors 2024, 17, 330. [Google Scholar] [CrossRef]
- Zhang, X.J.; Lu, H.D.; Tian, Q.Q.; Jia, X.X.; Ren, F.F. Effects of deltamethrin on histopathology of Chinese mitten crab (Eriocheir sinensis). Asian J. Ecotoxicol. 2018, 13, 342–351. [Google Scholar]
- Fang, Z.; Yao, Y.; Cao, L.; Gao, J.; Li, Q.; Nie, Z.; Sun, Y.; Xu, G.; Du, J. Integration of metabolomics and transcriptomics reveals the toxicological mechanism of deltamethrin exposure in Chinese mitten crab Eriocheir sinensis. Sci. Total Environ. 2024, 955, 176975. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, J.W.; Xie, H.Q.; Cai, M.; Yao, L.; Li, J.M.; Han, L.; Chen, W.D.; Yu, N.J.; Peng, D.Y. Dendrobium huoshanense polysaccharides ameliorate ulcerative colitis by improving intestinal mucosal barrier and regulating gut microbiota. J. Funct. Foods 2022, 96, 105231. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, H.; Zhao, Y.; Han, Z.; Xu, H.; Gao, T.; Nie, X.; Huang, X. Expression levels of serine proteases, their homologs, and prophenoloxidase in the Eriocheir sinensis with hepatopancreatic necrosis syndrome (HPNS) and their expression regulation by Runt. Fish Shellfish. Immunol. 2023, 138, 108816. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X. The gut and hemolymph microbiotas of crustacean, composition, functions and homeostatic regulation. Acta Microbiol Sin. 2018, 28, 760–772. [Google Scholar]
- Garibay-Valdez, E.; Cicala, F. Longitudinal variations in the gastrointestinal microbiome of the white shrimp, Litopenaeus vannamei. PeerJ 2021, 9, e11827. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Zheng, X.T.; Duan, Y.F.; Dong, H.B.; Zhang, J.S. Research progress of intestinal immune system in crustaceans. T. Oceanol. Limnol. 2016, 3, 83–90. [Google Scholar]
- Wang, D.; Liu, C.F.; Gu, C.; Zhao, J.W. Upregulation of Bcl2a1 in microglia of mouse induced by traumatic brain injury and LPS stimulation. Chin. J. Neuroanat. 2019, 35, 236–244. [Google Scholar]
- Li, M. Oxidative Stress Response and Intervention of Curcumin in Alcohol-Induced Liver Injury in Zebrafish; Inner Mongolia Minzu University: Tongliao, China, 2020. [Google Scholar]
- Zhang, J.; Wang, K.; Guo, J.; Huang, Y.; Wei, Y.; Jia, K.; Peng, Y.; Lu, H. Study on the mechanism of liver toxicity induced by acenaphthene in zebrafish. Ecotoxicol. Environ. Saf. 2023, 249, 114441. [Google Scholar] [CrossRef]
- Li, M. Study on the mechanism of α-lipoic acid alleviating liver injury induced by Aflatoxin B1 in Ophiocephala Odontosa. Ph.D. Thesis, J. Jilin Agric. Univ., Changchun, China, 2023. [Google Scholar]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, C.; Liao, Y.; Zhou, M.; Xu, W.; Zou, Z. Caspase-8 in inflammatory diseases: A potential therapeutic target. Cell. Mol. Biol. Lett. 2024, 29, 130. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, Z.; Jia, H.; Yang, B.; Liu, C.; Yang, Y.; Zhang, S.; Wang, Z.; Yang, L.; Li, S.; et al. Hepatocyte-specific Mas activation enhances lipophagy and fatty acid oxidation to protect against acetaminophen-induced hepatotoxicity in mice. J. Hepatol. 2023, 78, 543–557. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, Z.; Niu, J.; Wang, J.; Huang, Z.; Lin, H.Z. Antioxidant activity and resistance to low-salinity stress in Litopenaeus vannamei induced by chlorogenic acid. J. Fish Sci. China 2014, 21, 340–350. [Google Scholar]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef]
- Tang, X.; Amar, S. Kavain Involvement in LPS-Induced Signaling Pathways. J. Cell. Biochem. 2016, 117, 2272–2280. [Google Scholar] [CrossRef]
- Jackson, S.E. Hsp90: Structure and function. In MolecularCahperones; Springer: Berlin/Heidelberg, Germany, 2013; pp. 155–240. [Google Scholar] [CrossRef]
- Prodromou, C. Mechanisms of Hsp90 regulation. Biochem. J. 2016, 473, 2439–2452. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellf. Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Purchiaroni, F.; Tortora, A.; Gabrielli, M.; Bertucci, F.; Gigante, G.; Ianiro, G.; Ojetti, V.; Scarpellini, E.; Gasbarrini, A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 323–333. [Google Scholar] [PubMed]
- Hong, Y.; Huang, Y.; Wu, S.; Yang, X.; Dong, Y.; Xu, D.; Huang, Z. Effects of imidacloprid on the oxidative stress, detoxification and gut microbiota of Chinese mitten crab, Eriocheir sinensis. Sci. Total Environ. 2020, 729, 138276. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, Y.; Zhang, C.; Pang, Y.; Song, X.; Wu, M.; Cheng, Y. Effects of the glyphosate-based herbicide roundup on the survival, immune response, digestive activities and gut microbiota of the Chinese mitten crab, Eriocheir sinensis. Aquat. Toxicol. 2019, 214, 105243. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.; He, Y.; Yue, H.; Wang, Q. Microbial structures and community functions of anaerobic sludge in six full-scale wastewater treatment plants as revealed by 454 high-throughput pyrosequencing. Bioresour. Technol. 2015, 186, 163–172. [Google Scholar] [CrossRef]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tai, W.C.; Liang, C.M.; Wu, C.K.; Tsai, M.C.; Hu, W.H.; Huang, P.Y.; Chen, C.H.; Kuo, Y.H.; Yao, C.C.; et al. Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J. Microbiol. Immunol. Infect. 2025, 58, 62–69. [Google Scholar] [CrossRef]
- Trojanowska, D.; Zwolinska-Wcislo, M.; Tokarczyk, M.; Kosowski, K.; Mach, T.; Budak, A. The role of Candida in inflammatory bowel disease. Estimation of transmission of C. albicans fungi in gastrointestinal tract based on genetic affinity between strains. Med. Sci. Monit. 2010, 16, CR451–CR457. [Google Scholar]
- Li, S.Q.; Li, N.; Luo, Y.Q.; Zhang, B.P.; Zhang, J.P. Study on IgY Against the Pathogen of the Bacterial Gill Rot Disease in Grass Carp. Guizhou J. Anim. Husbandry Vet. Med. 2019, 43, 37–42. [Google Scholar]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1–19. [Google Scholar] [CrossRef]
- Niu, C.; Hu, X.L.; Yuan, Z.W.; Xiao, Y.; Ji, P.; Wei, Y.M.; Hua, Y.L. Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity. J. Ethnopharmacol. 2023, 300, 115741. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.; Begum, M.K.; Eshik, M.M.E.; Punom, N.J.; Ahmmed, S.; Rahman, M.S. Molecular identification and antibiotic resistance patterns of diverse bacteria associated with shrimp PL nurseries of Bangladesh: Suspecting Acinetobacter venetianus as future threat. PeerJ 2022, 10, e12808. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Wang, X.; Liu, N.; Wang, W.; Luo, Y. Acinetobacter pittii, an emerging new multi-drug resistant fish pathogen isolated from diseased blunt snout bream (Megalobrama amblycephala Yih) in China. Appl. Microbiol. Biotechnol. 2017, 101, 6459–6471. [Google Scholar] [CrossRef]
- Xiao Joe, J.T.; Tseng, Y.C. The Alteration of Intestinal Microbiota Profile and Immune Response in Epinephelus coioides during Pathogen Infection. Life 2021, 11, 99. [Google Scholar] [CrossRef]
- Gu, Z.M.; Liu, Y.; Chen, C.F.; Zhu, J.; Xie, J.; Xu, P. Isolation and Identification of Acinetobacter Baumannii from the Diseased Channel Catfish. J. Huazhong Agric. Univ. 2010, 29, 489–493. [Google Scholar]
- Cai, J.; Wu, J.Y.; Pan, Y.X.; Hu, G.W.; Zhang, L.Z. Isolation, Identification and Drug Resistance Analysis of Chryseobacterium sp. from Pseudosciaena crocea. Chin. Anim. Hus. Vet. Med. 2022, 49, 1135–1143. [Google Scholar]
- Li, C.Y.; Wang, L.; Wu, K.N. Isolation, physiological and biochemical identification and molecular biological identification of Bacillus aureus crucian. HLJ Anim. Sci. Vet. Med. 2018, 08, 127–129. [Google Scholar]
- Zhao, S.; Gao, N.; Zhang, Q.; Xiao, W.; Chen, D.; Huang, M.; Ye, X. Cultivar-specific rhizosphere microbial community responses to cadmium-NaHCO3 stress in relation to cadmium accumulation in rice. J. Hazard. Mater. 2025, 488, 137531. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Won, K.; Yang, J.E.; Akter, S.; Kim, K.Y.; Yin, C.; Yi, T.H. Taibaiella yonginensis sp. nov., a bacterium isolated from soil of Yongin city. Antonie Van Leeuwenhoek 2015, 108, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.; Kim, H.; Kang, H.; Lee, B.I.; Ahn, T.S.; Joh, K. Lacihabitans soyangensis gen. nov., sp. nov., a new member of the family Cytophagaceae, isolated from a freshwater reservoir. Int. J. Syst. Evol. Microbiol. 2014, 64, 3188–3194. [Google Scholar] [CrossRef]
- Xie, M.Q.; Zhang, S.Y.; Xu, L.L.; Jiang, F.; Yuan, J.F.; Wu, Z.X.; Chen, X.X. The intestinal microbiota diversities of procambarus clarkia at diffrent sexes and growth stages. Acta Hydrobiol. Sin. 2021, 45, 1243–1254. [Google Scholar]
- Shetty, A.; Barnes, R.A.; Healy, B.; Groves, P. A case of sepsis caused by Acidovorax. J. Infect. 2005, 51, e171. [Google Scholar] [CrossRef]
- Liu, C.; Pan, K.; Xu, H.; Song, Y.; Qi, X.; Lu, Y.; Jiang, X.; Liu, H. The effects of enrofloxacin exposure on responses to oxidative stress, intestinal structure and intestinal microbiome community of largemouth bass (Micropterus salmoides). Chemosphere 2024, 348, 140751. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.H.; Wu, F.; Yu, Z.Y.; Xie, P.; Ke, H.J.; Li, H.W.; Yu, Y.Y.; Guo, J.H. Study on screening and antagonistic mechanisms of Bacillus amyloliquefaciens 54 against bacterial fruit blotch (BFB) caused by Acidovorax avenae subsp. citrulli. Microbiol. Res. 2015, 170, 95–104. [Google Scholar] [CrossRef]
- Van Le, V.; Ko, S.R.; Nguyen, L.T.T.; Kim, J.C.; Shin, Y.; Kim, K.; Ahn, C.Y. Undibacterium cyanobacteriorum sp. nov., an auxin-producing bacterium isolated from fresh water during cyanobacterial bloom period. Antonie Van Leeuwenhoek 2024, 117, 99. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, N.; Huang, Y.Y.; Huang, Y.Y.; Zou, A.H.; Zhao, Y.L. Effects of Lambda-Cyhalothrin/Polydopamine—Microcapsule Suspension on Innate Immunity and Intestinal Microflorain Eriocheir sinensis. J. Fudan Univ. Nat. Sci. 2021, 60, 462–472. [Google Scholar]
- Auguste, M.; Rahman, F.U.; Balbi, T.; Leonessi, M.; Oliveri, C.; Bellese, G.; Vezzulli, L.; Furones, D.; Canesi, L. Responses of Mytilus galloprovincialis to challenge with environmental isolates of the potential emerging pathogen Malaciobacter marinus. Fish Shellf. Immunol. 2022, 131, 1–9. [Google Scholar] [CrossRef]
- Ferreira, S.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Insights in the pathogenesis and resistance of Arcobacter: A review. Crit. Rev. Microbiol. 2016, 42, 364–383. [Google Scholar] [CrossRef]
- Liu, J.; Teng, C.; Zheng, X.; Xu, L.; Cao, H.; Gai, C. Shewanella putrefaciens as an emerging pathogen of hepatopancreas necrosis disease in Chinese mitten crab Eriocheir sinensis. Dis. Aquat. Org. 2024, 159, 143–152. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Sun, M.Y.; Xiang, X.X. Role of gut microbiota and bile acid pathway in nonalcoholic fatty liver disease. J. Clin. Hepatol. 2020, 36, 2831–2834. [Google Scholar]
- Xu, H.S. Identification of Common Pathogens Isolated from Cultured Eriocheir Sinensis, Their Pathogenesis and lmmunological Mechanisms. Ph.D. Thesis, Zhejiang Universty, Hangzhou, China, 2004. [Google Scholar]
- Decostere, A. Flavobacterium columnare infections in fish: The agent and its adhesion to the gill tissue. Verh. K. Acad. Geneeskd. Belg. 2002, 64, 421–430. [Google Scholar] [PubMed]
- Bader, J.A.; Nusbaum, K.E.; Shoemaker, C.A. Comparative challenge model of Flavobacterium columnare using abraded and unabraded channel catfish, Ictalurus punctatus (Rafinesque). J. Fish Dis. 2003, 26, 461–467. [Google Scholar] [CrossRef] [PubMed]

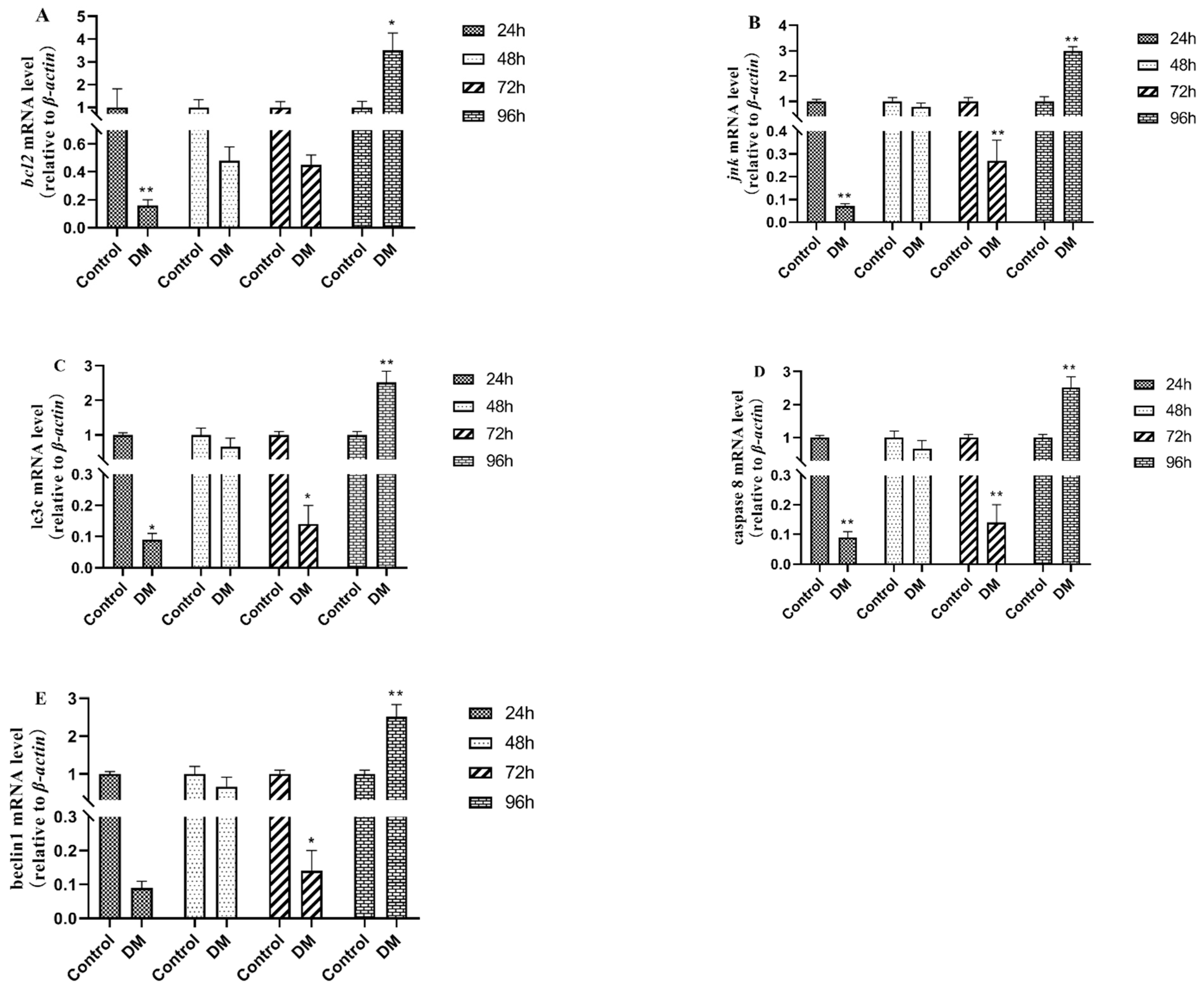
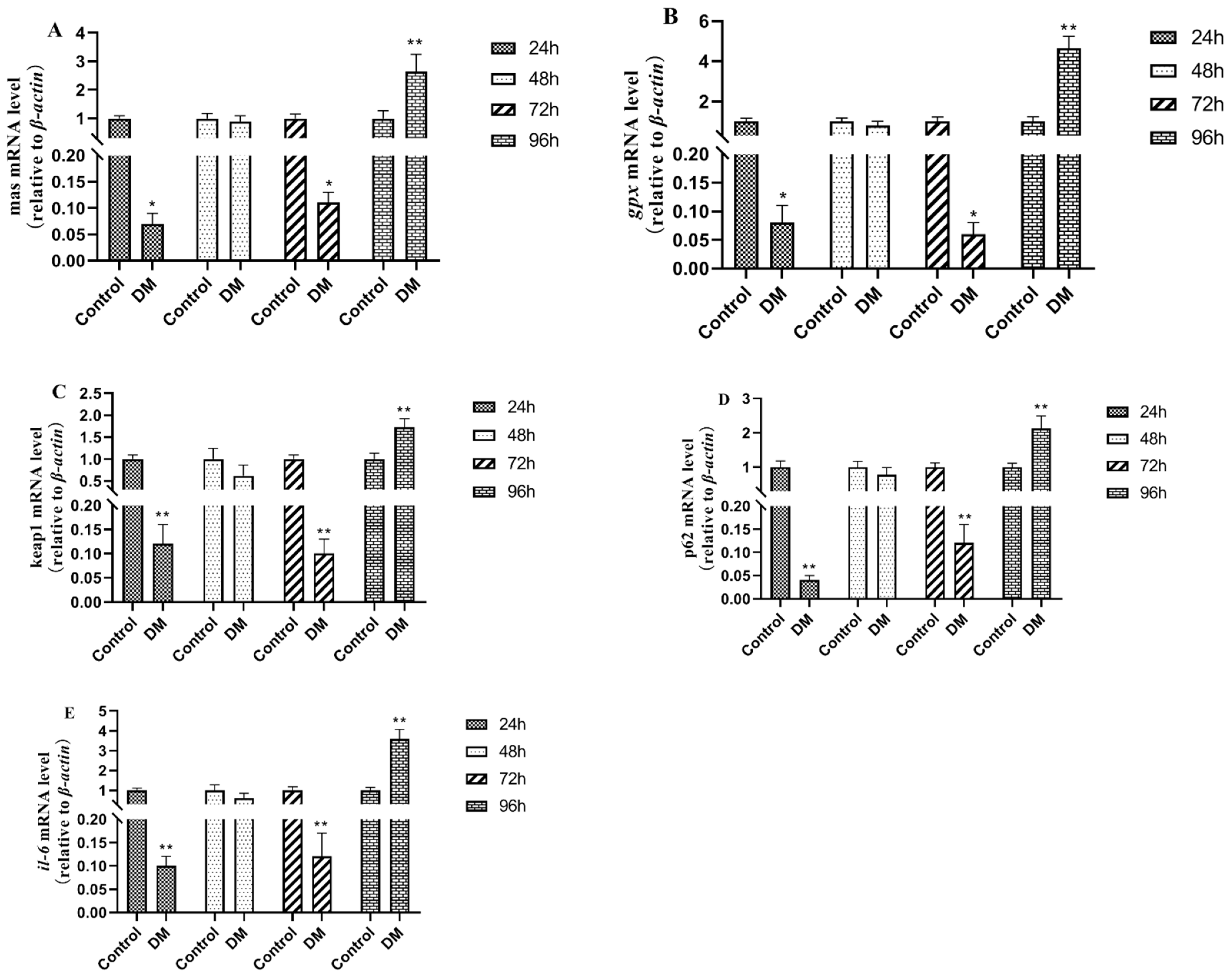
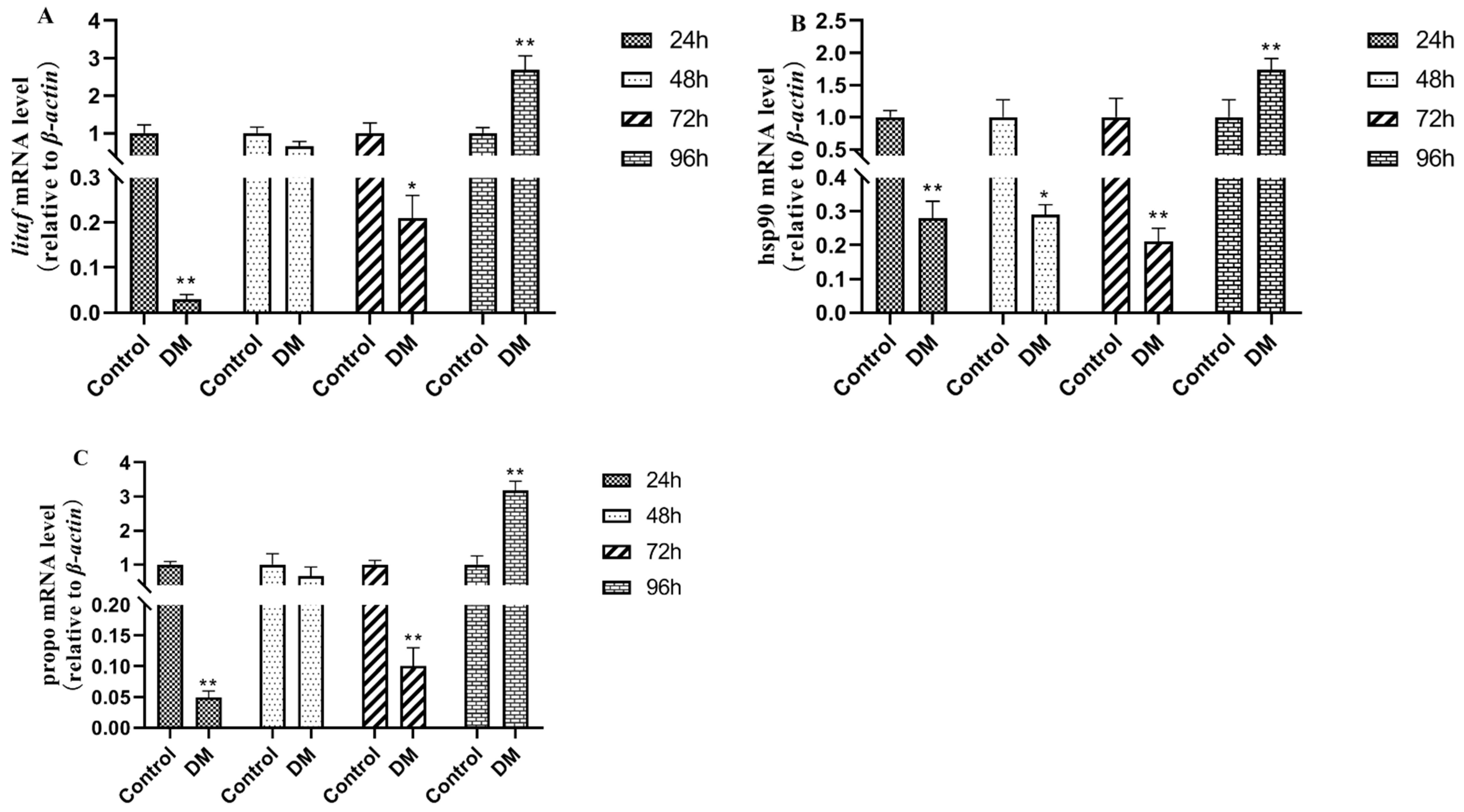
| Type | Gene | Primer Sequence (5′-3′) | GenBank Number/References |
|---|---|---|---|
| Apoptosis autophagy-related genes | bcl-2 | F: AGGACACGCAGTTCTCTTGG | QBA85626.1 |
| R: AACAAGACCCAGGATGCCAG | |||
| jnk | F: TGGTGCGTAACCGACCTAAC | KC900087 | |
| R: ACTGGTCCAATGACTGGCTG | |||
| lc3c | F: CACGTTGCCTATCCTCGACA | XM_050842539.1 | |
| R: GTCATCGTCCCTACACTCGC | |||
| caspase 8 | F: TGGAGCGTCATGGTTCAGAC | AKS36884.1 | |
| R: CAGACAAGCCACCACTGCTA | |||
| beclin1 | F: GCCCATATACTGTGGCGAGG | MH173046.1 | |
| R: CCAGGTCAAAGAGCCCAGTT | |||
| Oxidative stress-related genes | mas | F: GACGATGGTTATGGAGTGTCCT | [26] |
| R: GAGGAGCTCTTTTTGCTGGAC | |||
| gpx | F: GGCTGGACACCCTAGACAAC | FJ617305.1 | |
| R: TAAGGGCCGTCACAAGGAAC | |||
| keap1 | F: AGGCATCTTCATTGTGGGGG | XP_027210665.1 | |
| R: GTTACCAACGACCACCGAGT | |||
| p62 | F: ACAGACGCCAAGTACCAAGG | XM_050829992.1 | |
| R: AGGCTCACTCGTCTCCTGAT | |||
| il-16 | F: AGAGGTTGTTCTTGTGCTGTCC | MG182159.1 | |
| R: ACGAGGGTAATGGTGAATGGAG | |||
| Immune-related genes | litaf | F:TAAAGGCAAGGGAGGCTTCG | KF892539.1 |
| R:GAATGGAGCTTGAGGTGGCA | |||
| hsp90 | F: TCACCAACGACTGGGAGGAT | XM_050873094.1 | |
| R: CAGGAAGAGGAGTGCCCTGA | |||
| propo | F: CCATCCCTTCCTGCTTACCA | EF493829.1 | |
| R: CTCCATCACAAACCCTAACGACTT | |||
| Internal reference | β-actin | F: AGCGCAAGTACTCCGTCTGGAT | SRR9599542 |
| R: AATGGCAGGGCCAGACTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, C.; Du, J.; Zhu, H.; Gao, J.; Xu, G.; Xu, P. Intestinal Microbiota and Gene Expression Alterations in Chinese Mitten Crab (Eriocheir sinensis) Under Deltamethrin Exposure. Antioxidants 2025, 14, 510. https://doi.org/10.3390/antiox14050510
Zhong C, Du J, Zhu H, Gao J, Xu G, Xu P. Intestinal Microbiota and Gene Expression Alterations in Chinese Mitten Crab (Eriocheir sinensis) Under Deltamethrin Exposure. Antioxidants. 2025; 14(5):510. https://doi.org/10.3390/antiox14050510
Chicago/Turabian StyleZhong, Chunyi, Jinliang Du, Haojun Zhu, Jiancao Gao, Gangchun Xu, and Pao Xu. 2025. "Intestinal Microbiota and Gene Expression Alterations in Chinese Mitten Crab (Eriocheir sinensis) Under Deltamethrin Exposure" Antioxidants 14, no. 5: 510. https://doi.org/10.3390/antiox14050510
APA StyleZhong, C., Du, J., Zhu, H., Gao, J., Xu, G., & Xu, P. (2025). Intestinal Microbiota and Gene Expression Alterations in Chinese Mitten Crab (Eriocheir sinensis) Under Deltamethrin Exposure. Antioxidants, 14(5), 510. https://doi.org/10.3390/antiox14050510






