Enhanced Extraction of Bioactive Compounds from Red Grape Pomace: Optimizing Ultrasound-Assisted Extraction with Ethanol and NaDES as Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Plant Material
2.3. Ultrasound-Assisted Extraction (UAE)
2.3.1. Hydroalcoholic Solutions
2.3.2. Natural Deep Eutectic Solvents (NaDES)
2.4. Experimental Design
2.5. Total Anthocyanin Content (TAC)
2.6. Total Polyphenol Content (TPC)
2.7. Total Flavonoid Content (TFC)
2.8. Antioxidant Activity
2.9. High-Performance Liquid Chromatography
2.10. In Vitro Digestibility of Grape Pomace Extracts
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization of UAE Process Parameters
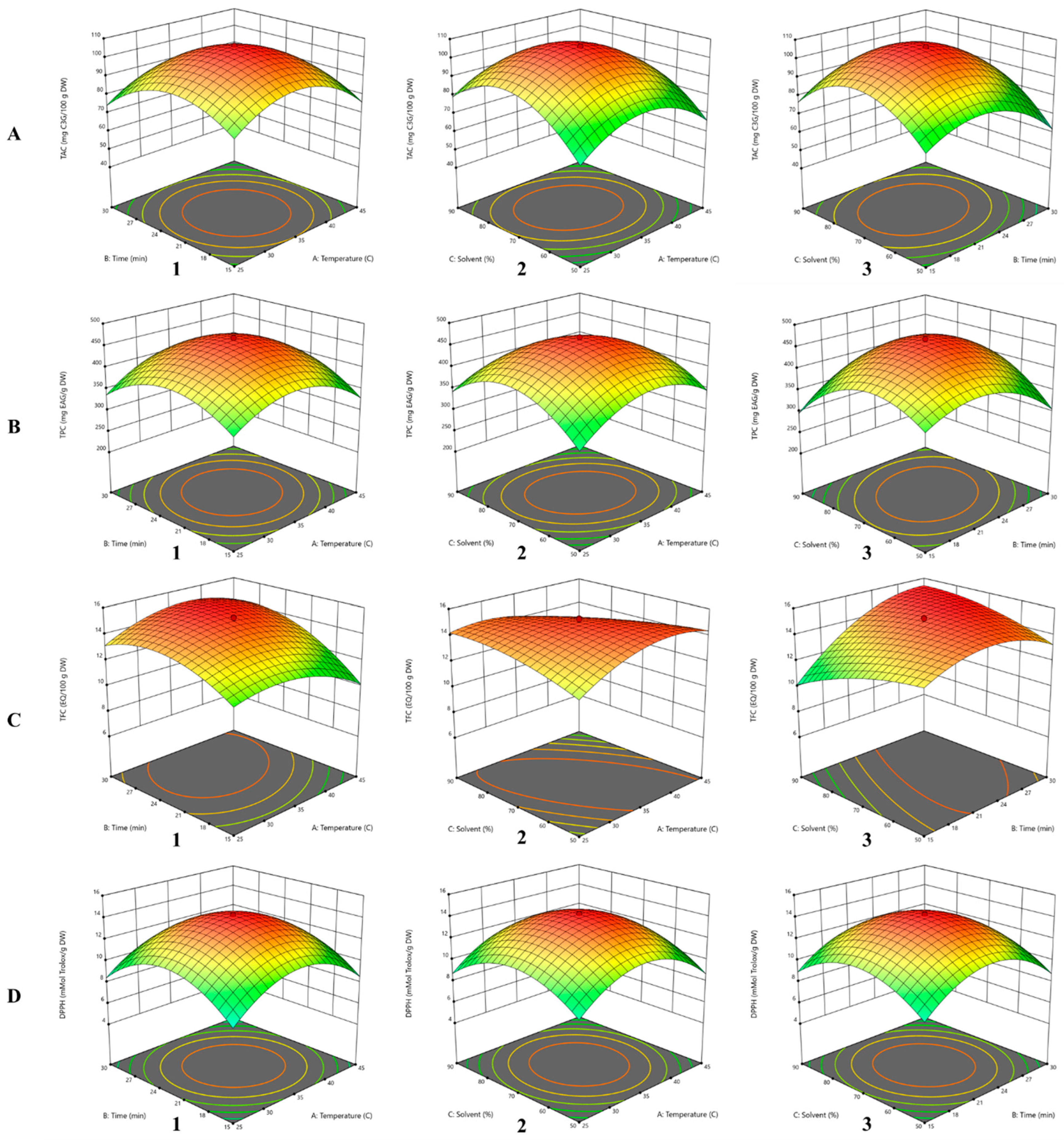
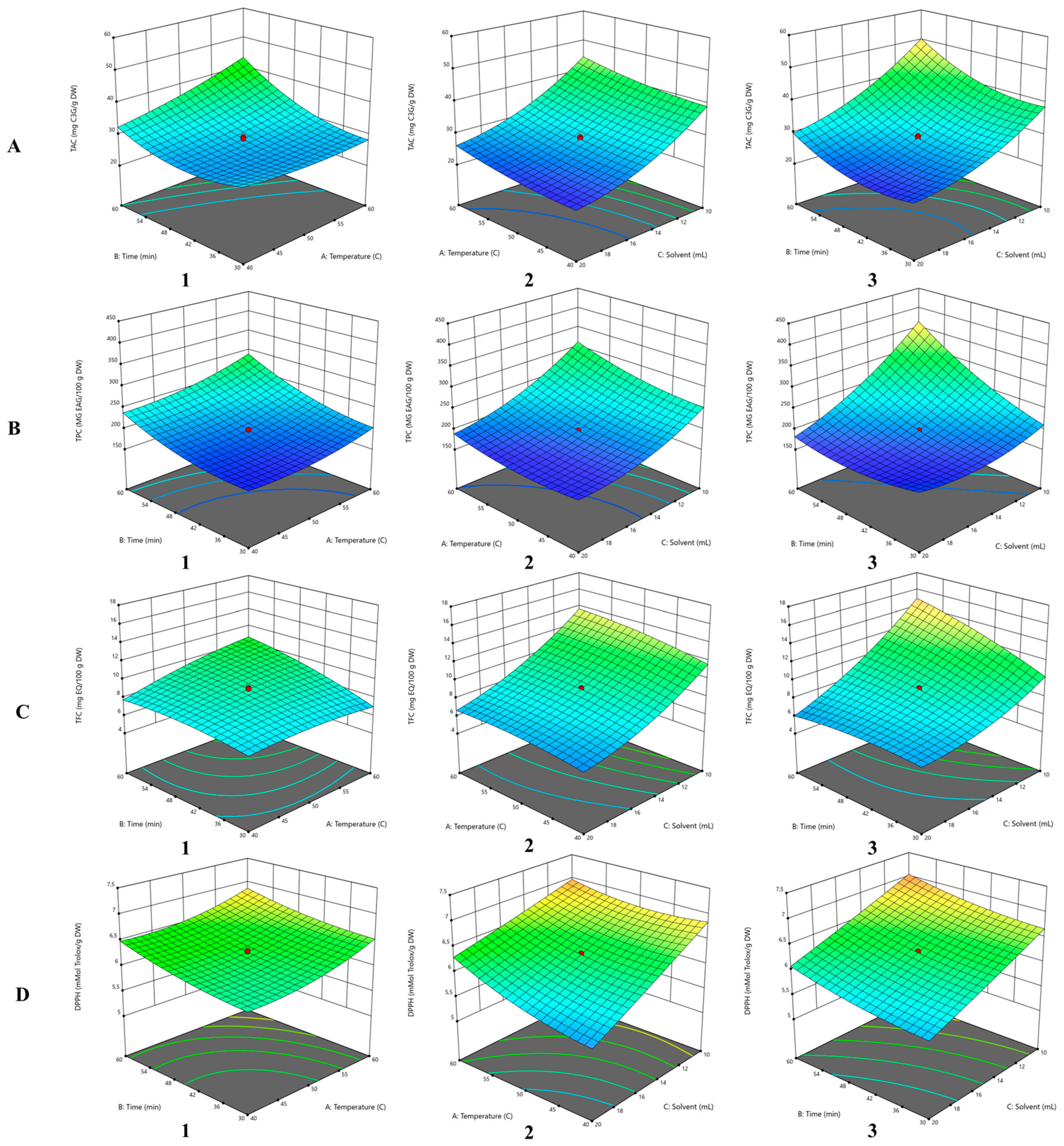
3.2. Influence of Independent Variables on TAC
3.3. Influence of Independent Variables on TPC
3.4. Influence of Independent Variables on TFC
3.5. Influence of Independent Variables on DPPH
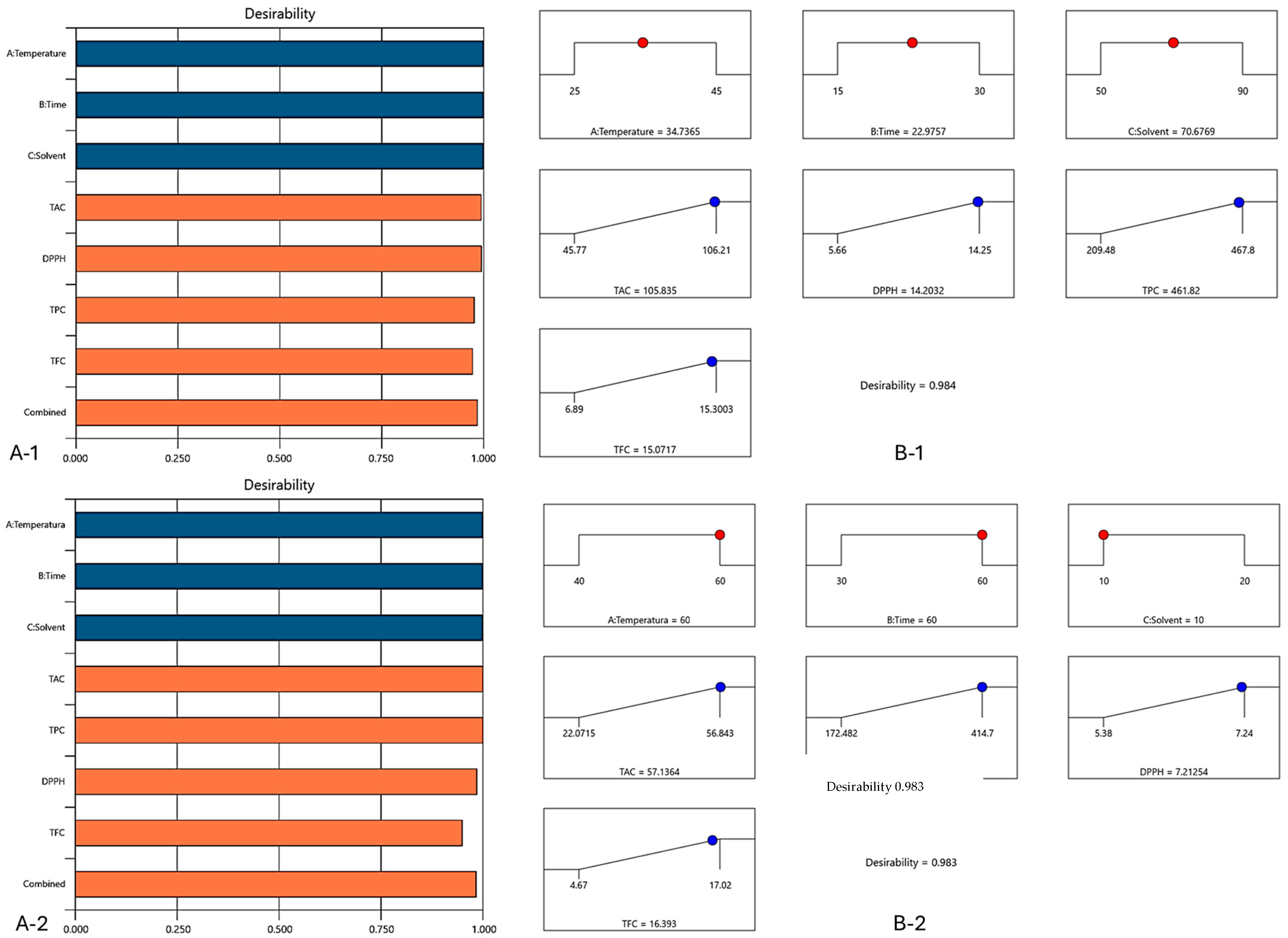
3.6. Optimization and Validation of Extraction Parameters
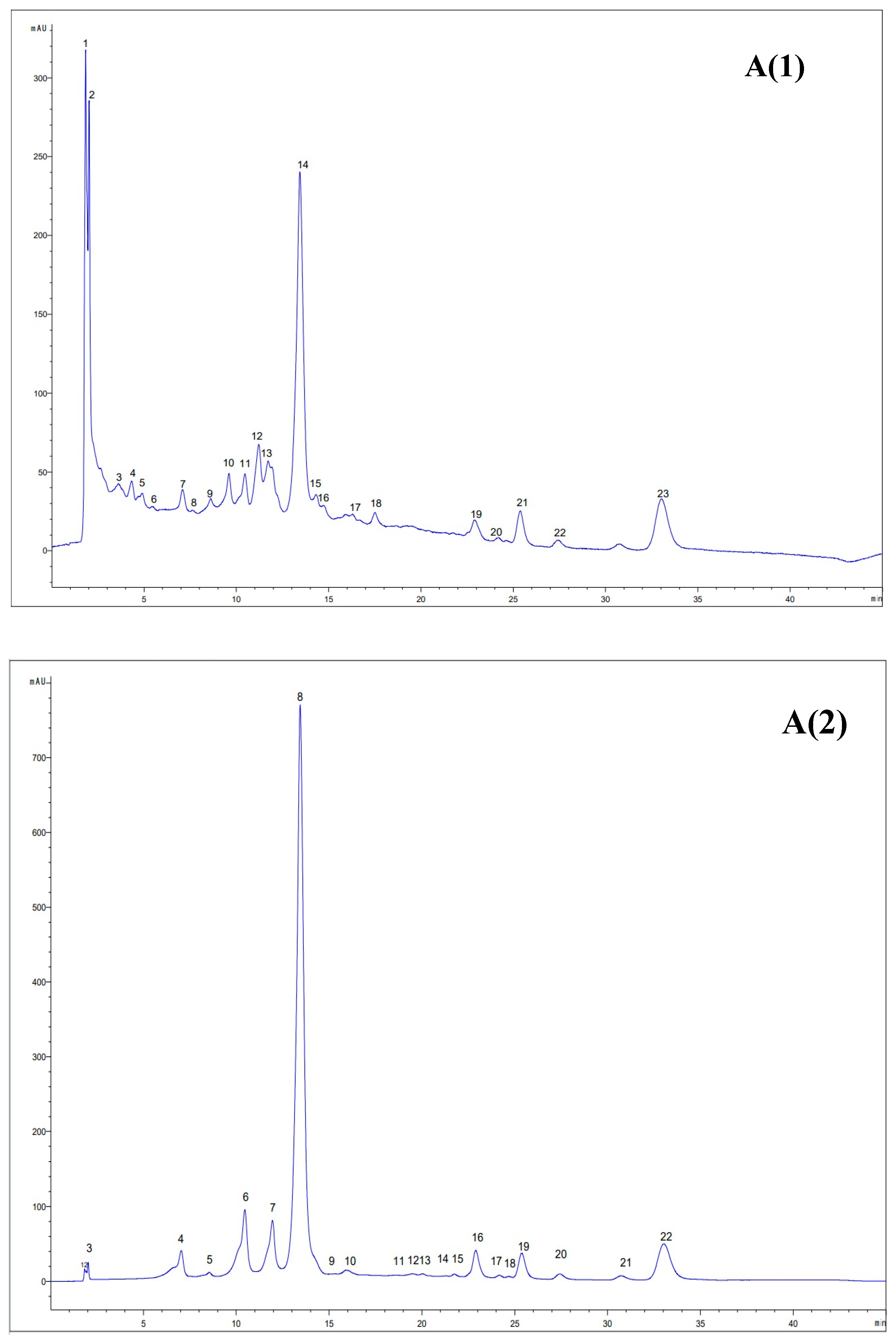
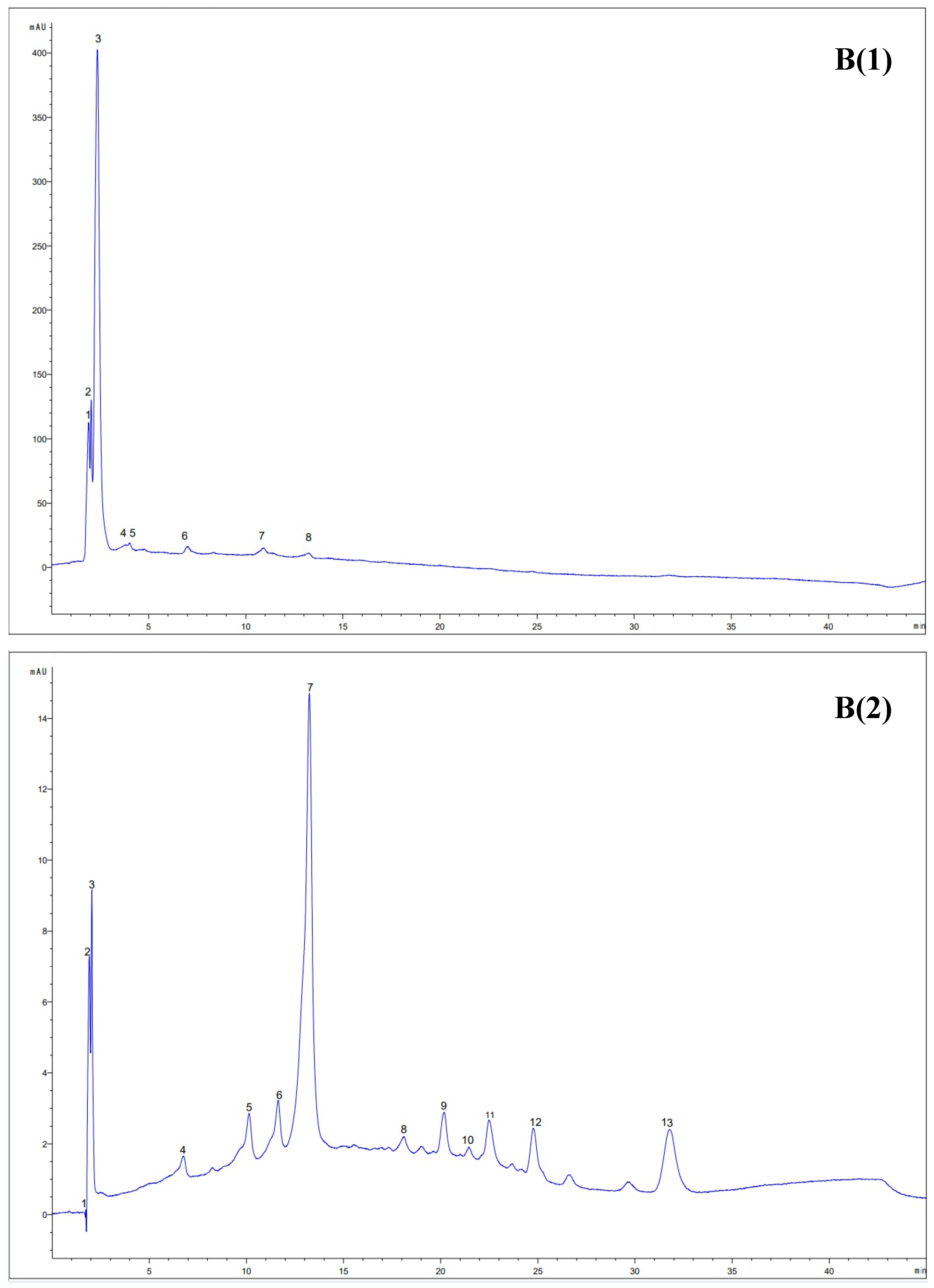
3.7. HPLC Analysis of the Extracts
3.8. In Vitro Digestibility of Grape Pomace Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asadnezhad, B.; Pirmohammadi, R.; Alijoo, Y.; Khalilvandi-Behroozyar, H.; Abdi-Benmar, H.; Tirado-González, D.N.; Hernández Ruiz, P.E.; Ordoñez, V.V.; Lackner, M.; Salem, A.Z.M. The Effects of Dietary Supplementation with Red Grape Pomace Treated with Ozone Gas on Ruminal Fermentation Activities, Nutrient Digestibility, Lactational Performance, and Blood Metabolites in Dairy Ewes. J. Agric. Food Res. 2024, 18, 101452. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ballesteros, M.; Negro, M.J. Biorefineries for the Valorization of Food Processing Waste. In The Interaction of Food Industry and Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 155–190. [Google Scholar] [CrossRef]
- FAO. Production: Crops and Livestock Products; FAOSTAT: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 January 2025).
- Llobera, A.; Cañellas, J. Dietary Fibre Content and Antioxidant Activity of Manto Negro Red Grape (Vitis vinifera): Pomace and Stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Galanakis, C.M. Preface. In Handbook of Grape Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. xv–xvi. [Google Scholar] [CrossRef]
- Lopes, J.D.C.; Madureira, J.; Margaça, F.M.A.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, J.C.; Miljić, U.; Puškaš, V. Extraction of Bioactive Compounds from Grape Processing By-Products. In Handbook of Grape Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–135. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis vinifera, L. Var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol 2020, 11, 990. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Luongo, D.; Naviglio, D.; Vetrani, C.; Ciciola, P.; Tura, A.; Castello, F.; Mena, P.; Del Rio, D.; et al. Grape Pomace Polyphenols Improve Insulin Response to a Standard Meal in Healthy Individuals: A Pilot Study. Clin. Nutr. 2019, 38, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of Polyphenols from Red Grape Pomace and Assessment of Their Antioxidant and Anti-Cholesterol Activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef]
- Osamede Airouyuwa, J.; Sivapragasam, N.; Ali Redha, A.; Maqsood, S. Sustainable Green Extraction of Anthocyanins and Carotenoids Using Deep Eutectic Solvents (DES): A Review of Recent Developments. Food Chem. 2024, 448, 139061. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Sganzerla, W.G.; Silva, A.P.G.; John, O.D.; Barroso, T.L.C.T.; Rostagno, M.A.; Forster-Carneiro, T. Sustainable Extraction Methods for the Recovery of Polyphenolic Compounds from Grape Pomace and Its Biological Properties: A Comprehensive Review. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable Green Processing of Grape Pomace for the Production of Value-Added Products: An Overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A Comparative Study on Different Extraction Techniques to Recover Red Grape Pomace Polyphenols from Vinification Byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Holland, B.; Barrow, C.J.; Suleria, H.A.R. An Optimization of the Extraction of Phenolic Compounds from Grape Marc: A Comparison between Conventional and Ultrasound-Assisted Methods. Chemosensors 2024, 12, 177. [Google Scholar] [CrossRef]
- Vannuchi, N.; Braga, A.R.C.; DeRosso, V.V. High-Performance Extraction Process of Anthocyanins from Jussara (Euterpe edulis) Using Deep Eutectic Solvents. Processes 2022, 10, 615. [Google Scholar] [CrossRef]
- Spaggiari, C.; Hiemstra, I.S.A.; Kazbar, A.; Costantino, G.; Righetti, L. Towards Eco-Metabolomics: NADES-Guided Extraction Enables Semi-Quantitative Metabolomics for Melissa officinalis. Adv. Sample Prep. 2025, 13, 100154. [Google Scholar] [CrossRef]
- Moutinho, J.; Gouvinhas, I.; Domínguez-Perles, R.; Barros, A. Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules 2023, 28, 3885. [Google Scholar] [CrossRef]
- Mangiapelo, L.; Blasi, F.; Ianni, F.; Suvieri, C.; Sardella, R.; Volpi, C.; Cossignani, L. Optimization of a Simple Analytical Workflow to Characterize the Phenolic Fraction from Grape Pomace. Food Bioprocess Technol. 2024, 17, 1942–1957. [Google Scholar] [CrossRef]
- Li, Z.; Cui, R.; Liu, W.; Wang, M.; Li, L.; Liu, F.; Du, B.; Song, L. Application of Green Deep Eutectic Solvents for Anthocyanins Extraction from Grape Pomace: Optimization, Stability, Antioxidant Activity, and Molecular Dynamic Simulation. Lebensm. Wiss. Technol. 2024, 211, 116878. [Google Scholar] [CrossRef]
- Gutfinger, T. Polyphenols in Olive Oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Serea, D.; Constantin, O.E.; Horincar, G.; Stănciuc, N.; Aprodu, I.; Bahrim, G.E.; Râpeanu, G. Optimization of Extraction Parameters of Anthocyanin Compounds and Antioxidant Properties from Red Grape (Băbească neagră) Peels. Inventions 2023, 8, 59. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, L.; Qi, Y.; Xie, C.; Zhao, W.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of Processing on Polyphenols in Butternut Pumpkin (Cucurbita moschata). Food Biosci. 2022, 49, 101925. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Z.; Gonzalez Viejo, C.; Ahmadi, F.; Dunshea, F.R.; Suleria, H.A.R. Comparison of Phenolic Composition in Australian-Grown Date Fruit (Phoenix dactylifera L.) Seeds from Different Varieties and Ripening Stages. Food Res. Int. 2024, 181, 114096. [Google Scholar] [CrossRef] [PubMed]
- Mërtiri, I.; Coman, G.; Cotârlet, M.; Turturică, M.; Balan, N.; Râpeanu, G.; Stănciuc, N.; Mihalcea, L. Phytochemical Profile Screening and Selected Bioactivity of Myrtus communis Berries Extracts Obtained from Ultrasound-Assisted and Supercritical Fluid Extraction. Separations 2025, 12, 8. [Google Scholar] [CrossRef]
- Stoica, F.; Condurache, N.N.; Aprodu, I.; Andronoiu, D.G.; Enachi, E.; Stănciuc, N.; Bahrim, G.E.; Croitoru, C.; Râpeanu, G. Value-Added Salad Dressing Enriched with Red Onion Skin Anthocyanins Entrapped in Different Biopolymers. Food Chem. X 2022, 15, 100374. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bai, Q.; Zhou, J.; de Souza, T.S.P.; Suleria, H.A.R. In Vitro Gastrointestinal Bioaccessibility, Bioactivities and Colonic Fermentation of Phenolic Compounds in Different Vigna Beans. Foods 2022, 11, 3884. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Darra, N.E.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of Total Phenolic Compounds, Flavonoids, Anthocyanins and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, D. Economical and Eco-Friendly Isolation of Anthocyanins from Grape Pomace with Higher Efficiency. Food Chem. X 2022, 15, 100419. [Google Scholar] [CrossRef]
- Abouelenein, D.; Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S. Phenolic and Nutritional Profiles, and Antioxidant Activity of Grape Pomaces and Seeds from Lacrima Di Morro d’Alba and Verdicchio varieties. Food Biosci. 2023, 53, 102808. [Google Scholar] [CrossRef]
- Muñoz, P.; Pérez, K.; Cassano, A.; Ruby-Figueroa, R. Recovery of Anthocyanins and Monosaccharides from Grape Marc Extract by Nanofiltration Membranes. Molecules 2021, 26, 2003. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J.L. Valorisation Potential of Cabernet Grape Pomace for the Recovery of Polyphenols: Process Intensification, Optimisation and Study of Kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Craciunescu, O.; Seciu-Grama, A.-M.; Mihai, E.; Utoiu, E.; Negreanu-Pirjol, T.; Lupu, C.E.; Artem, V.; Ranca, A.; Negreanu-Pirjol, B.-S. The Chemical Profile, Antioxidant, and Anti-Lipid Droplet Activity of Fluid Extracts from Romanian Cultivars of Haskap Berries, Bitter Cherries, and Red Grape Pomace for the Management of Liver Steatosis. Int. J. Mol. Sci. 2023, 24, 16849. [Google Scholar] [CrossRef]
- Arboleda Mejia, J.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; Parpinello, G.P.; De Pinho, M.N. Recovery of Phenolic Compounds from Red Grape Pomace Extract through Nanofiltration Membranes. Foods 2020, 9, 1649. [Google Scholar] [CrossRef] [PubMed]
- Gerçek, Y.C.; Kutlu, N.; Çelik, S.; Bayram, S.; Kırkıncı, S.; Ecem Bayram, N. Optimized Ultrasonic-NaDES Extraction of Anthocyanins, Polyphenolics, and Organic Acids from Chokeberry Fruit with Blueness and Antimicrobial Evaluation. Microchem. J. 2025, 210, 113061. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Nguyen, V.B.; Kha, T.C. Optimization of Bioactive Compounds Extraction from Red Cardinal Grape Pomace Using Choline Chloride and Lactic Acid-Based Deep Eutectic Solvent. Prep. Biochem. Biotechnol. 2025, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Nguyen, V.B.; Kha, T.C.; Hoang, V.C. Impact of Choline Chloride and Organic Acid-Based Deep Eutectic Solvents on the Extraction of Bioactive Compounds from Grape Pomace. J. Tech. Educ. Sci. 2024, 19, 15–23. [Google Scholar] [CrossRef]
- Frontini, A.; Luvisi, A.; Negro, C.; Apollonio, M.; Accogli, R.; De Pascali, M.; De Bellis, L. Polyphenols Extraction from Different Grape Pomaces Using Natural Deep Eutectic Solvents. Separations 2024, 11, 241. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Gajardo-Parra, N.; Pérez-Correa, J.R.; Canales, R.I.; Martínez-Cifuentes, M.; Contreras-Contreras, G.; Mariotti-Celis, M.S. Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures. Antioxidants 2023, 12, 1446. [Google Scholar] [CrossRef]
- Serea, D.; Râpeanu, G.; Constantin, O.E.; Bahrim, G.E.; Stănciuc, N.; Croitoru, C. Ultrasound and Enzymatic Assisted Extractions of Bioactive Compounds Found in Red Grape Skins Băbească Neagră (Vitis vinifera) Variety. Ann. Univ. Dunarea Jos Galati Fascicle VI-Food Technol. 2021, 45, 9–25. [Google Scholar] [CrossRef]
- Dabetic, N.; Todorovic, V.; Malenovic, A.; Sobajic, S.; Markovic, B. Optimization of Extraction andHPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents. Antioxidants 2022, 11, 1595. [Google Scholar] [CrossRef]
- Anghel, L.; Milea, A.S.; Constantin, O.E.; Barbu, V.; Chițescu, C.; Enachi, E.; Râpeanu, G.; Mocanu, G.-D.; Stănciuc, N. Dried Grape Pomace with Lactic Acid Bacteria as a Potential Source for Probiotic and Antidiabetic Value-Added Powders. Food Chem. X 2023, 19, 100777. [Google Scholar] [CrossRef]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria Baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef]
- Sik, B.; Székelyhidi, R.; Lakatos, E.; Ajtony, Z. Using Natural Deep Eutectic Solvents for the Extraction of Antioxidant Compounds from Cornelian Cherry (Cornus mas L.) Fruits . Green Anal. Chem. 2024, 11, 100154. [Google Scholar] [CrossRef]
- Constantin, O.E.; Skrt, M.; Poklar Ulrih, N.; Râpeanu, G. Anthocyanins Profile, Total Phenolics and Antioxidant Activity of Two Romanian Red Grape Varieties: Feteascǎ Neagrǎ and Bǎbeascǎ Neagrǎ (Vitis vinifera). Chem. Pap. 2015, 69, 1573–1581. [Google Scholar] [CrossRef]
- Nguyen Thao, T.T.; Nguyen Viet, B.; Le Truc, M.X.; Tran An, H.T.; Kha Tuyen, C. Extraction of Bioactive Compounds from Red Cardinal Grape Pomace by Deep Eutectic Solvents. Chem. Eng. Trans. 2023, 106, 889–894. [Google Scholar] [CrossRef]
- Sazali, A.L.; AlMasoud, N.; Amran, S.K.; Alomar, T.S.; Pa’ee, K.F.; El-Bahy, Z.M.; Yong, T.-L.K.; Dailin, D.J.; Chuah, L.F. Physicochemical and Thermal Characteristics of Choline Chloride-Based Deep Eutectic Solvents. Chemosphere 2023, 338, 139485. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of Phenolic Compounds from Grape Pomace Using Ohmic Heating: Chemical Composition, Bioactivity and Bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Kashyap, P.; Riar, C.S.; Jindal, N. Effect of Extraction Methods and Simulated in Vitro Gastrointestinal Digestion on Phenolic Compound Profile, Bio-Accessibility, and Antioxidant Activity of Meghalayan Cherry (Prunus nepalensis) Pomace Extracts. Lebensm. Wiss. Technol. 2022, 153, 112570. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.M.; Spigno, G. Grape Marc Phenolics: Extraction Kinetics, Quality and Stability of Extracts. J. Food Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules 2023, 28, 5285. [Google Scholar] [CrossRef]
| Run | A: Temperature, °C | B: Time, min | C: Solvent, % | TAC, mg C3G/g DW | TPC, mg GAE/100 g DW | TFC, mg CE/100 g DW | DPPH, mMol Trolox/g DW |
|---|---|---|---|---|---|---|---|
| 1 | 18.18 | 22.5 | 70 | 65.78 ± 2.77 | 287.02 ± 0.97 | 11.59 ± 0.0 | 6.02 ± 0.005 |
| 2 | 35 | 22.5 | 36.36 | 45.77 ± 1.09 | 257.04 ± 0.87 | 13.50 ± 0.29 | 7.16 ± 0.001 |
| 3 | 35 | 35.11 | 70 | 59.78 ± 0.68 | 301.05 ± 1.46 | 13.45 ± 0.0 | 5.74 ± 0.003 |
| 4 | 25 | 30 | 50 | 46.55 ± 1.35 | 226.94 ± 0.32 | 10.49 ± 0.0 | 6.13 ± 0.003 |
| 5 | 25 | 15 | 90 | 64.13 ± 1.88 | 256.88 ± 0.75 | 10.95 ± 0.41 | 5.81 ± 0.003 |
| 6 | 35 | 22.5 | 103.64 | 61.1 ± 2.10 | 265.91 ± 0.79 | 13.20 ± 0.14 | 7.12 ± 0.015 |
| 7 | 35 | 22.5 | 70 | 105.85 ± 1.04 | 467.8 ± 1.00 | 15.30 ± 0.53 | 14.25 ± 0.067 |
| 8 | 35 | 22.5 | 70 | 105.25 ± 3.86 | 460.48 ± 0.61 | 14.93 ± 0.73 | 14.23 ± 0.042 |
| 9 | 45 | 30 | 90 | 58.34 ± 1.51 | 303.33 ± 1.61 | 13.3 ± 0.00 | 5.66 ± 0.007 |
| 10 | 25 | 15 | 50 | 60.36 ± 1.32 | 300.14 ± 0.65 | 12.09 ± 0.18 | 5.89 ± 0.007 |
| 11 | 51.82 | 22.5 | 70 | 57.73 ± 3.5 | 300.3 ± 2.60 | 11.36 ± 0.33 | 5.92 ± 0.006 |
| 12 | 35 | 22.5 | 70 | 106.12 ± 0.68 | 460.35 ± 1.06 | 14.88 ± 0.22 | 14.24 ± 0.030 |
| 13 | 35 | 22.5 | 70 | 106 ± 2.04 | 459.82 ± 0.46 | 14.92 ± 0.32 | 14.21 ± 0.057 |
| 14 | 45 | 15 | 50 | 58.01 ± 2.77 | 302 ± 2.48 | 12.4 ± 0.05 | 5.93 ± 0.006 |
| 15 | 45 | 15 | 90 | 58.01 ± 2.77 | 209.48 ± 0.09 | 6.89 ± 0.36 | 6.31 ± 0.003 |
| 16 | 35 | 9.887 | 70 | 69.14 ± 1.33 | 293.33 ± 0.56 | 8.60 ± 0.00 | 6.03 ± 0.005 |
| 17 | 35 | 22.5 | 70 | 106.21 ± 2.50 | 461.87 ± 1.41 | 15.23 ± 0.37 | 14.18 ± 0.012 |
| 18 | 35 | 22.5 | 70 | 106 ± 2.04 | 460.92 ± 0.68 | 14.73 ± 0.33 | 14.25 ± 0.012 |
| 19 | 45 | 30 | 50 | 46.45 ± 1.59 | 274.53 ± 1.65 | 13.63 ± 0.34 | 5.7 ± 0.009 |
| 20 | 25 | 30 | 90 | 64.18 ± 1.55 | 312.00 ± 2.10 | 14.63± 0.27 | 5.83 ± 0.003 |
| Run | A: Temperature, °C | B: Time min | C: Solvent, % | TAC, mg C3G/g DW | TPC, mg GAE/100 g DW | TFC, mg CE/100 g DW | DPPH, mMol Trolox/g DW |
|---|---|---|---|---|---|---|---|
| 1 | 50 | 19.77 | 15 | 32.26 ± 0.70 | 184.46 ± 1.69 | 5.77 ± 0.11 | 6.17 ± 0.07 |
| 2 | 40 | 30 | 10 | 39.56 ± 8.69 | 199.80 ± 3.02 | 9.84 ± 0.37 | 6.73 ± 0.06 |
| 3 | 50 | 45 | 23.41 | 22.07 ± 0.89 | 172.48 ± 3.55 | 5.95 ± 0.58 | 5.519 ± 0.01 |
| 4 | 50 | 45 | 6.59 | 53.46 ± 1.56 | 345.90 ± 4.61 | 17.02 ± 0.00 | 7.11 ± 0.02 |
| 5 | 40 | 60 | 10 | 46.20 ± 2.88 | 341.10 ± 9.25 | 12.66 ± 3.09 | 7.15 ± 0.01 |
| 6 | 50 | 70.23 | 15 | 45.98 ± 7.31 | 309.83 ± 1.13 | 9.75 ± 0.27 | 6.81 ± 0.92 |
| 7 | 50 | 45 | 15 | 28.19 ± 0.74 | 197.40 ± 9.98 | 9.03 ± 0.08 | 6.3 ± 0.03 |
| 8 | 50 | 45 | 15 | 28.69 ± 0.27 | 196.30 ± 5.09 | 9.02 ± 0.03 | 6.26 ± 0.06 |
| 9 | 66.82 | 45 | 15 | 36.07 ± 4.53 | 266.20 ± 2.41 | 9.31 ± 0.22 | 7 ± 0.05 |
| 10 | 60 | 60 | 10 | 56.84 ± 1.52 | 414.70 ± 1.40 | 16.23 ± 0.06 | 7.24 ± 0.01 |
| 11 | 60 | 30 | 10 | 38.97 ± 0.86 | 245.40 ± 0.23 | 9.99 ± 0.11 | 6.91 ± 0.05 |
| 12 | 50 | 45 | 15 | 29.29 ± 1.58 | 196.70 ± 5.09 | 8.65 ± 0.22 | 6.26 ± 0.02 |
| 13 | 40 | 30 | 20 | 25.58 ± 0.43 | 197.90 ± 6.87 | 6.61 ± 0.04 | 5.38 ± 0.26 |
| 14 | 50 | 45 | 15 | 28.98 ± 2.04 | 197.20 ± 10.19 | 9.02 ± 0.08 | 6.3 ± 0.05 |
| 15 | 50 | 45 | 15 | 28.32 ± 2.76 | 196.20 ± 4.96 | 9.11 ± 0.05 | 6.29 ± 0.06 |
| 16 | 33.18 | 45 | 15 | 26.92 ± 0.81 | 200.70 ± 6.8 | 6.52 ± 0.16 | 6.29 ± 0.03 |
| 17 | 50 | 45 | 15 | 28.00 ± 0.16 | 196.40 ± 4.84 | 8.87 ± 0.11 | 6.25 ± 0.02 |
| 18 | 60 | 60 | 20 | 36.64 ± 0.98 | 210.10 ± 3.79 | 6.63 ± 0.19 | 6.55 ± 0.18 |
| 19 | 40 | 60 | 20 | 26.07 ± 1.64 | 177.94 ± 2.66 | 4.67 ± 1.26 | 5.86 ± 0.07 |
| 20 | 60 | 30 | 20 | 23.35 ± 0.54 | 202.43 ± 10.38 | 5.77 ± 0.03 | 6.19 ± 0.03 |
| Ethanol Extract | NaDES Extract | |||||||
|---|---|---|---|---|---|---|---|---|
| TAC, mg C3G/g DW | TPC, mg EAG/100 g DW | TFC, mg CE/100 g DW | DPPH, mMol Trolox/g DW | TAC, mg C3G/g DW | TPC, mg GAE/100 g DW | TFC, mg CE/100 g DW | DPPH, mMol Trolox/g DW | |
| Mean | 72.54 | 333.06 | 12.80 | 8.53 | 34.07 | 232.46 | 9.02 | 6.43 |
| Std. dev. | 0.5627 | 5.18 | 0.2735 | 0.0451 | 0.7039 | 0.6441 | 0.2353 | 0.0264 |
| C.V.% | 0.7758 | 1.56 | 2.14 | 0.5288 | 2.07 | 0.2771 | 2.61 | 0.4112 |
| R2 | 0.9997 | 0.9983 | 0.9925 | 0.9999 | 0.9974 | 1.0000 | 0.9972 | 0.9986 |
| Adjusted R2 | 0.9994 | 0.9967 | 0.9858 | 0.9999 | 0.9950 | 0.9999 | 0.9947 | 0.9973 |
| Predicted R2 | 0.9979 | 0.9885 | 0.9579 | 0.9995 | 0.9838 | 0.9997 | 0.9827 | 0.9924 |
| Adeq. precision | 150.6316 | 67.5342 | 41.5830 | 269.0211 | 69.2065 | 533.7221 | 74.1646 | 96.8130 |
| Lack of fit (p-values) | 0.0656 | 0.0503 | 0.2140 | 0.0635 | 0.1269 | 0.1855 | 0.1260 | 0.2754 |
| Dependent Variable | Predicted Value | 95% Confidence Intervals | Experimental Value |
|---|---|---|---|
| TAC (mg C3G/g DW) | 105.91 | 104.55–107.26 | 105.32 ± 0.77 |
| TPC (mg GAE/100 g DW) | 461.88 | 449.41–474.35 | 465.81 ± 1.28 |
| TFC (mg CE/100 g DW) | 15.00 | 14.34–15.66 | 15.3 ± 0.30 |
| DPPH (mMol Trolox/g DW) | 14.23 | 14.12–14.33 | 14.15 ± 0.01 |
| Dependent Variable | Predicted Value | 95% Confidence Intervals | Experimental Value |
|---|---|---|---|
| TAC (mg C3G/g DW) | 57.14 | 55.11–59.16 | 57.58 ± 4.73 |
| TPC (mg GAE/100 g DW) | 415.01 | 431.16–416.87 | 414.04 ± 0.80 |
| TFC (mg CE/100 g DW) | 16.39 | 15.72–17.07 | 15.8 ± 2.22 |
| DPPH (mMol Trolox/g DW) | 7.21 | 7.14–7.29 | 7.28 ± 0.02 |
| No. | Identified Compounds | Concentration (μg/mL) | |
|---|---|---|---|
| Ethanol Extract | NaDES Extract | ||
| 1. | Cafestol | n.d. | Traces |
| 2. | Gallic acid | n.d. | 479.78 ± 84.76 |
| 3. | Epigallocatechin | 20,576.01 ± 2067.62 | 4514.44 ± 11.78 |
| 4. | Catechin | n.d. | 9177.5 ± 21.34 |
| 5. | Protocatechuic acid | n.d. | 867.88 ± 15.98 |
| 6. | Chlorogenic acid | n.d. | 227.0797 ± 9.18 |
| 7. | Caffeic acid | n.d. | 40.80 ± 2.35 |
| 8. | Vanillic acid | 18.53 ± 0.31 | n.d. |
| 9. | Epicatechin | 740.51 ± 30.60 | n.d. |
| 10. | Syringic acid | 5.69 ± 0.08 | n.d. |
| 11. | Keracyanin | 143.02 ± 4.75 | n.d. |
| 12. | Quercetin 3-diglucoside | 95.09 ± 1.42 | n.d. |
| 13. | Rutin trihydrate (quercetin 3-rutinoside trihydrate) | 1204.66 ± 46.34 | 34.15 ± 1.97 |
| 14. | Hesperidin | 302.17 ± 19.88 | n.d. |
| 15. | Cinnamic acid | 6.36 ± 0.22 | n.d. |
| 16. | Quercetin | 16.80 ± 1.18 | n.d. |
| 17. | Quercetin dihydrate | 19.59 ± 1.53 | n.d. |
| 18. | Luteolin | 14.28 ± 0.23 | n.d. |
| 19. | Kaempferol | 28.70 ± 0.41 | n.d. |
| 20. | Apigenin | 27.36 ± 0.25 | n.d. |
| 21. | Isorhamnetin | 11.92 ± 0.11 | n.d. |
| 22. | Ellagic acid | n.d. | 142.55 ± 8.23 |
| Extract | Phenolic Content | Before Digestion | After Gastric Simulation | After Intestinal Simulation |
|---|---|---|---|---|
| Ethanol 70% | TAC, mg C3G/g DW | 242.29 | 50.77 | 7.49 |
| TPC, mg GAE/100 g DW | 456.6 | 393.8 | 129.6 | |
| TFC, mg CE/100 g DW | 25.55 | 22.65 | 3.77 | |
| DPPH, mMol Trolox/g DW | 22.63 | 15.21 | Bdl | |
| NaDES | TAC, mg C3G/g DW | 108.77 | 98.81 | 7.51 |
| TPC, mg GAE/100 g DW | 473.8 | 460 | 115.7 | |
| TFC, mg CE/100 g DW | 19.88 | 10.05 | 0.54 | |
| DPPH, mMol Trolox/g DW | 23.33 | 15.69 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, N.; Măntăilă, S.; Râpeanu, G.; Stănciuc, N. Enhanced Extraction of Bioactive Compounds from Red Grape Pomace: Optimizing Ultrasound-Assisted Extraction with Ethanol and NaDES as Solvents. Antioxidants 2025, 14, 526. https://doi.org/10.3390/antiox14050526
Balan N, Măntăilă S, Râpeanu G, Stănciuc N. Enhanced Extraction of Bioactive Compounds from Red Grape Pomace: Optimizing Ultrasound-Assisted Extraction with Ethanol and NaDES as Solvents. Antioxidants. 2025; 14(5):526. https://doi.org/10.3390/antiox14050526
Chicago/Turabian StyleBalan, Nicoleta, Silviu Măntăilă, Gabriela Râpeanu, and Nicoleta Stănciuc. 2025. "Enhanced Extraction of Bioactive Compounds from Red Grape Pomace: Optimizing Ultrasound-Assisted Extraction with Ethanol and NaDES as Solvents" Antioxidants 14, no. 5: 526. https://doi.org/10.3390/antiox14050526
APA StyleBalan, N., Măntăilă, S., Râpeanu, G., & Stănciuc, N. (2025). Enhanced Extraction of Bioactive Compounds from Red Grape Pomace: Optimizing Ultrasound-Assisted Extraction with Ethanol and NaDES as Solvents. Antioxidants, 14(5), 526. https://doi.org/10.3390/antiox14050526






