Engeletin Targets Mitochondrial Dysfunction to Attenuate Oxidative Stress and Experimental Colitis in Intestinal Epithelial Cells Through AMPK/SIRT1/PGC-1α Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Mouse Specimen Collection and Colitis Assessment
2.3. Colonic Organoid Culture and Treatment
2.4. Analysis of Intestinal Barrier Dysfunction
2.5. Assessment of IECs Apoptosis
2.6. Detection of Mitochondrial Dysfunction
2.7. Western Blot Analysis of Signaling Pathways
2.8. Oxidative Stress Detection
2.9. Statistical Analysis
3. Results
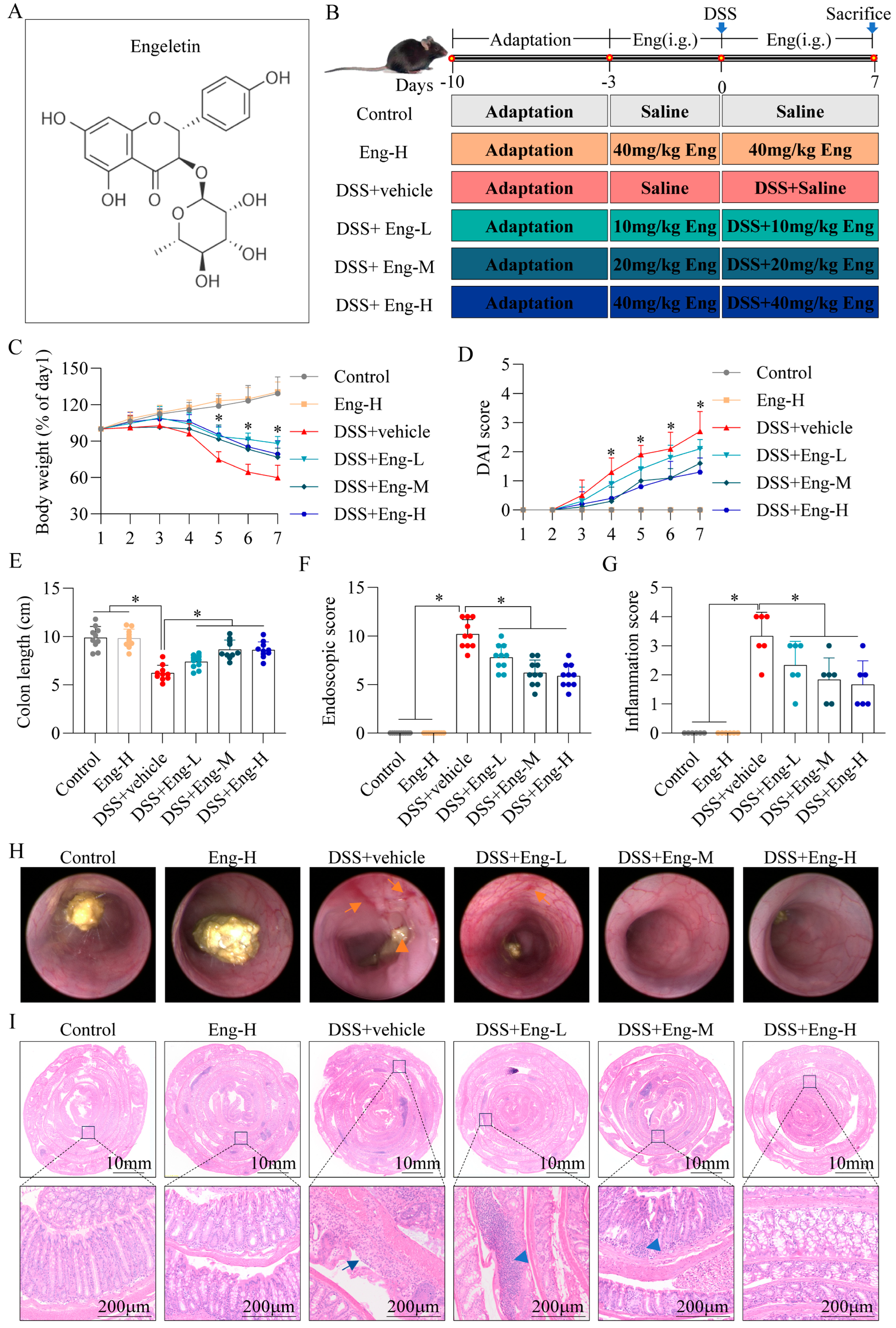
3.1. Engeletin Reduces DSS-Induced Colonic Damage in Mice
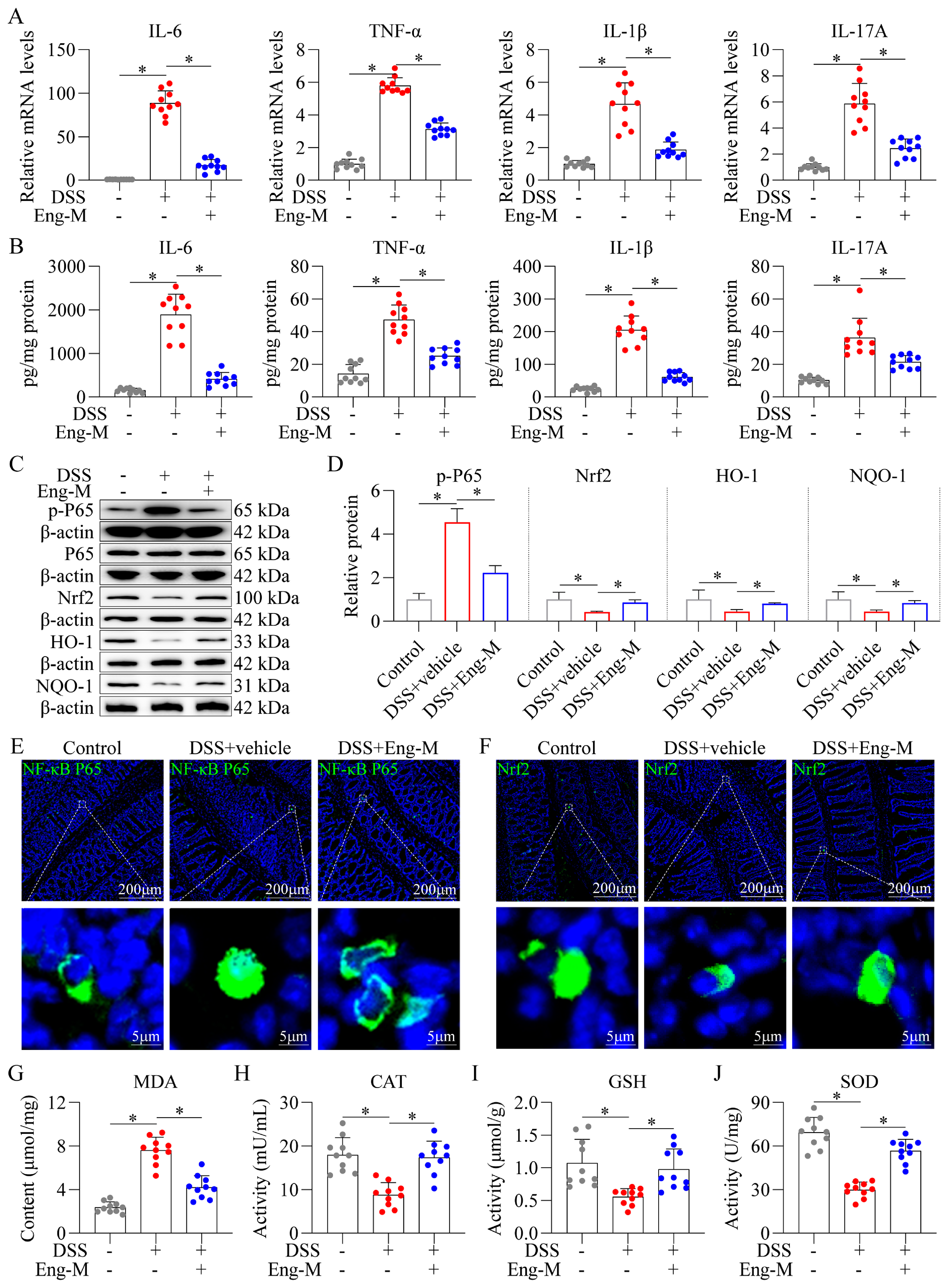
3.2. Engeletin Suppresses NF-κB-Driven Inflammation and Nrf2-Regulated Oxidative Stress in DSS-Induced Colitis Mice
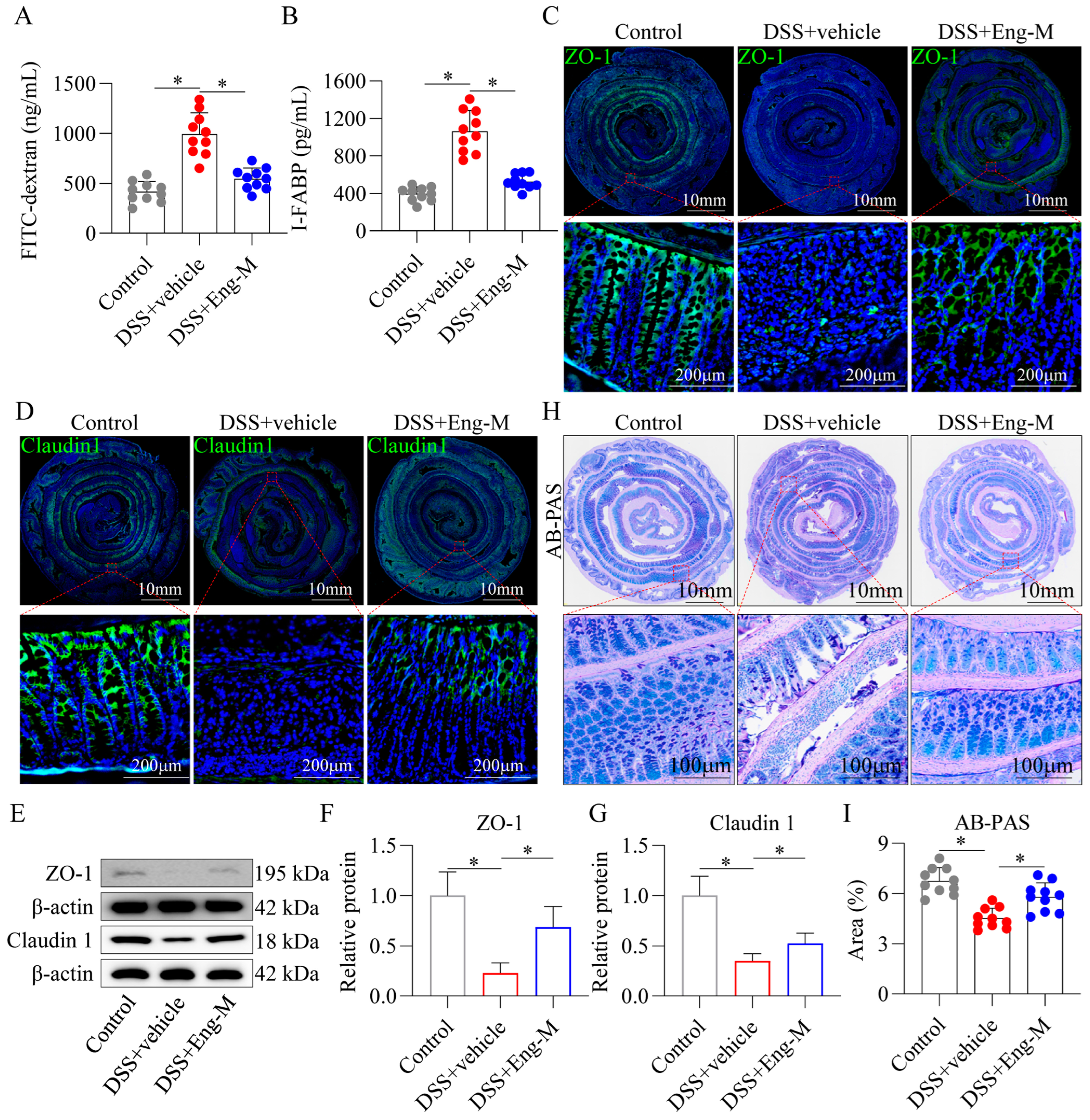
3.3. Engeletin Protects Intestinal Barrier Function In Vivo
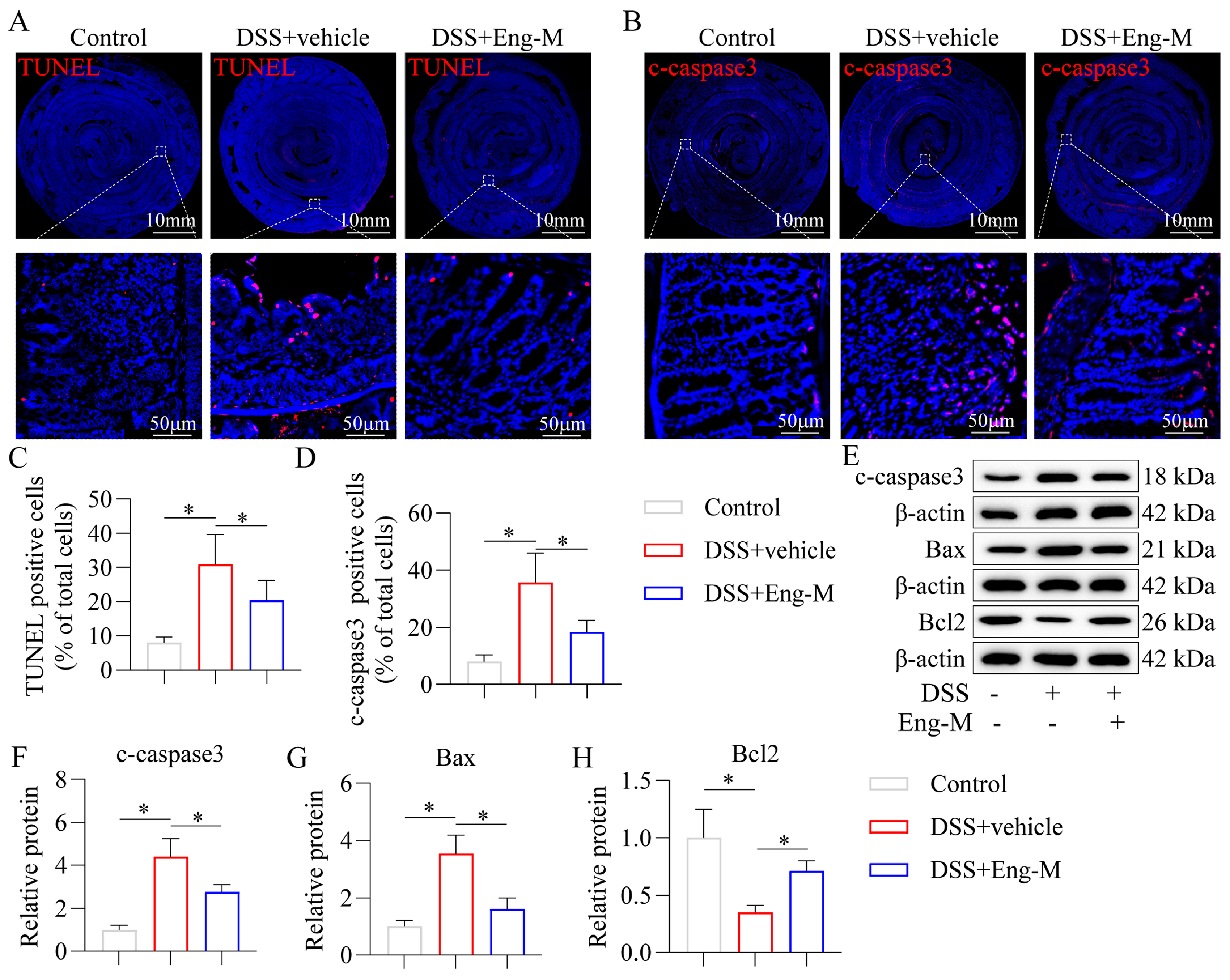
3.4. Engeletin Inhibits Epithelial Cell Apoptosis in DSS-Treated Mice
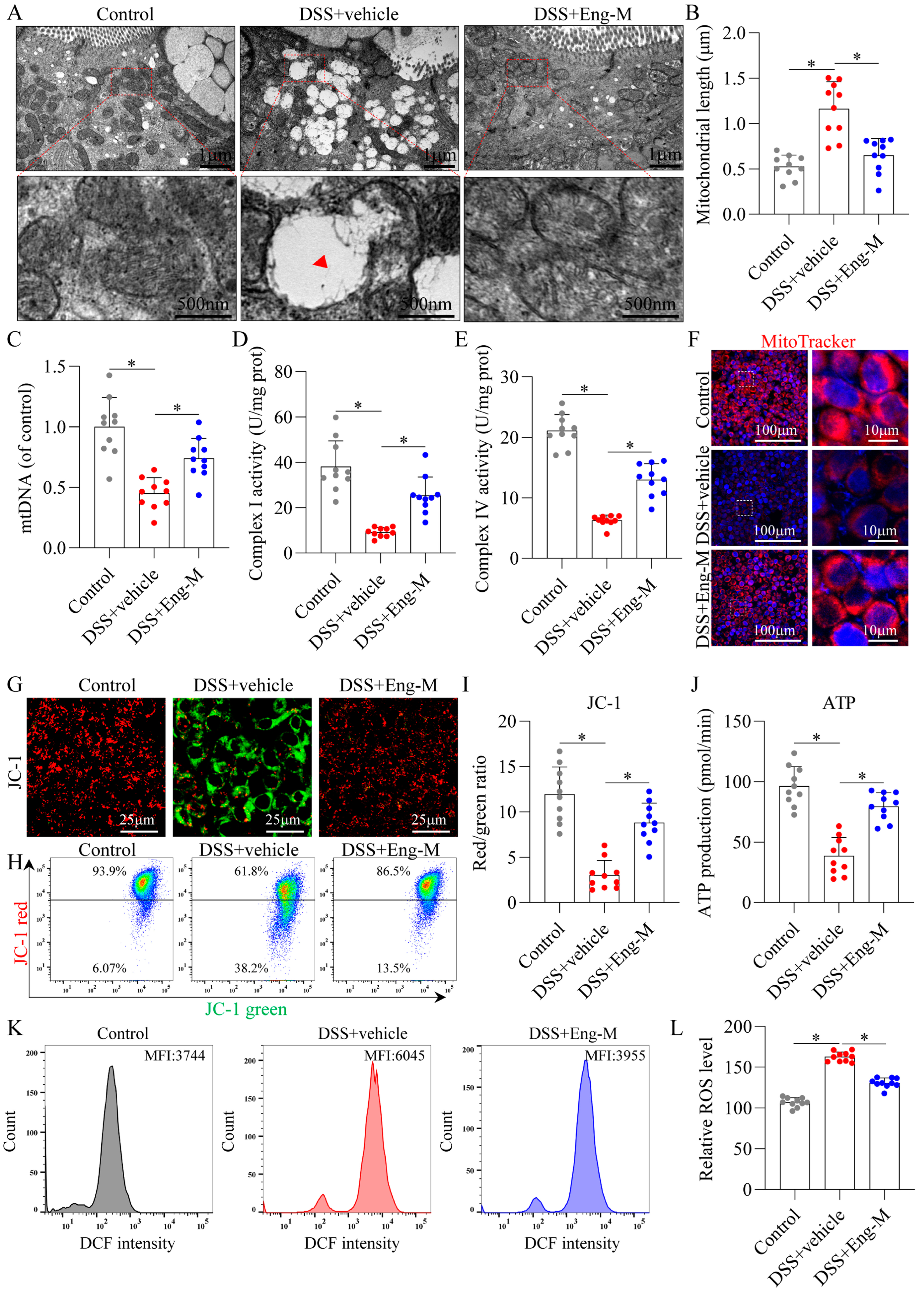
3.5. Engeletin Ameliorates Mitochondrial Dysfunction and Oxidative Stress in Epithelial Cells
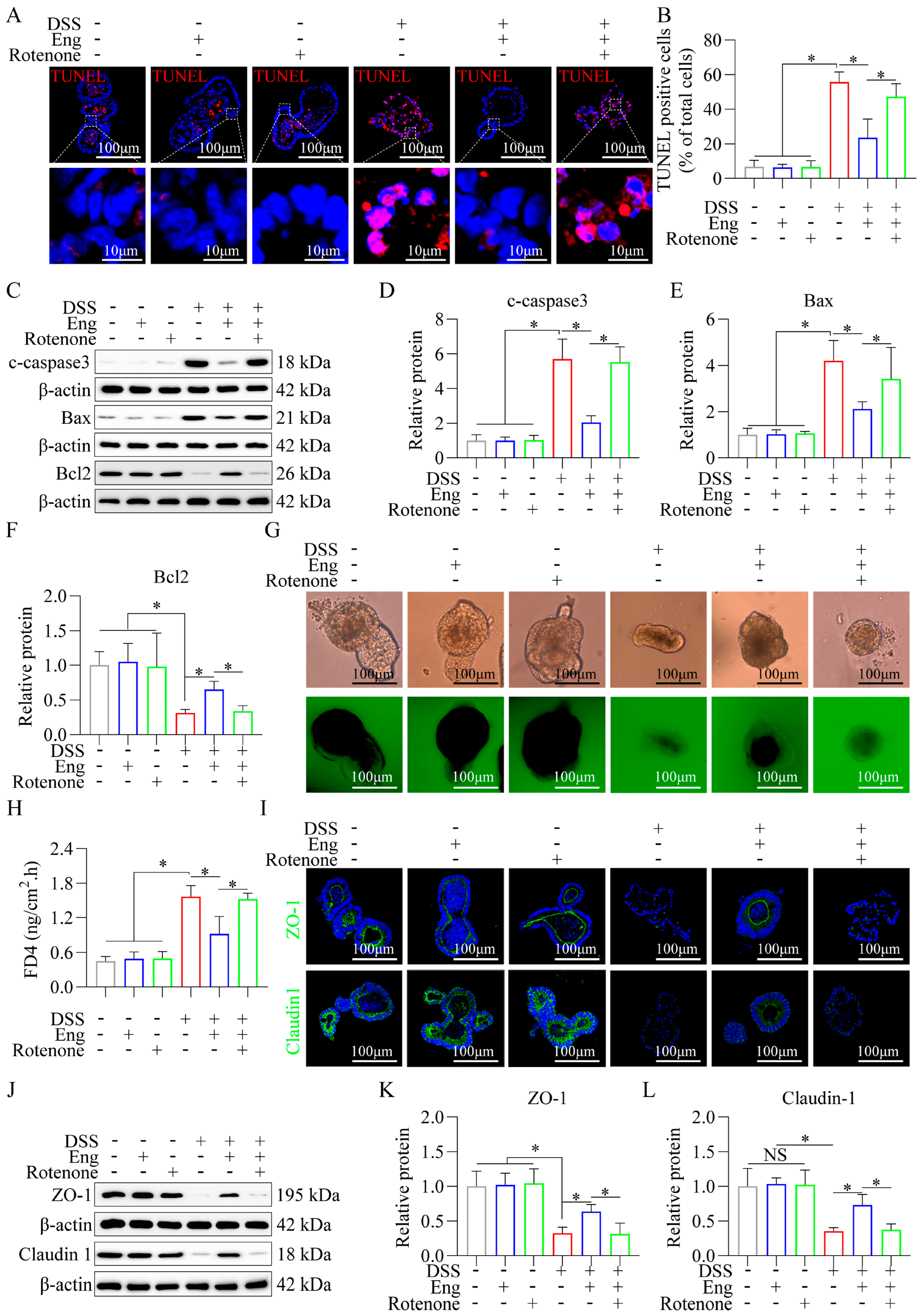
3.6. Engeletin Ameliorates Mitochondrial Dysfunction-Related IECs Apoptosis and Tight Junction Impairment
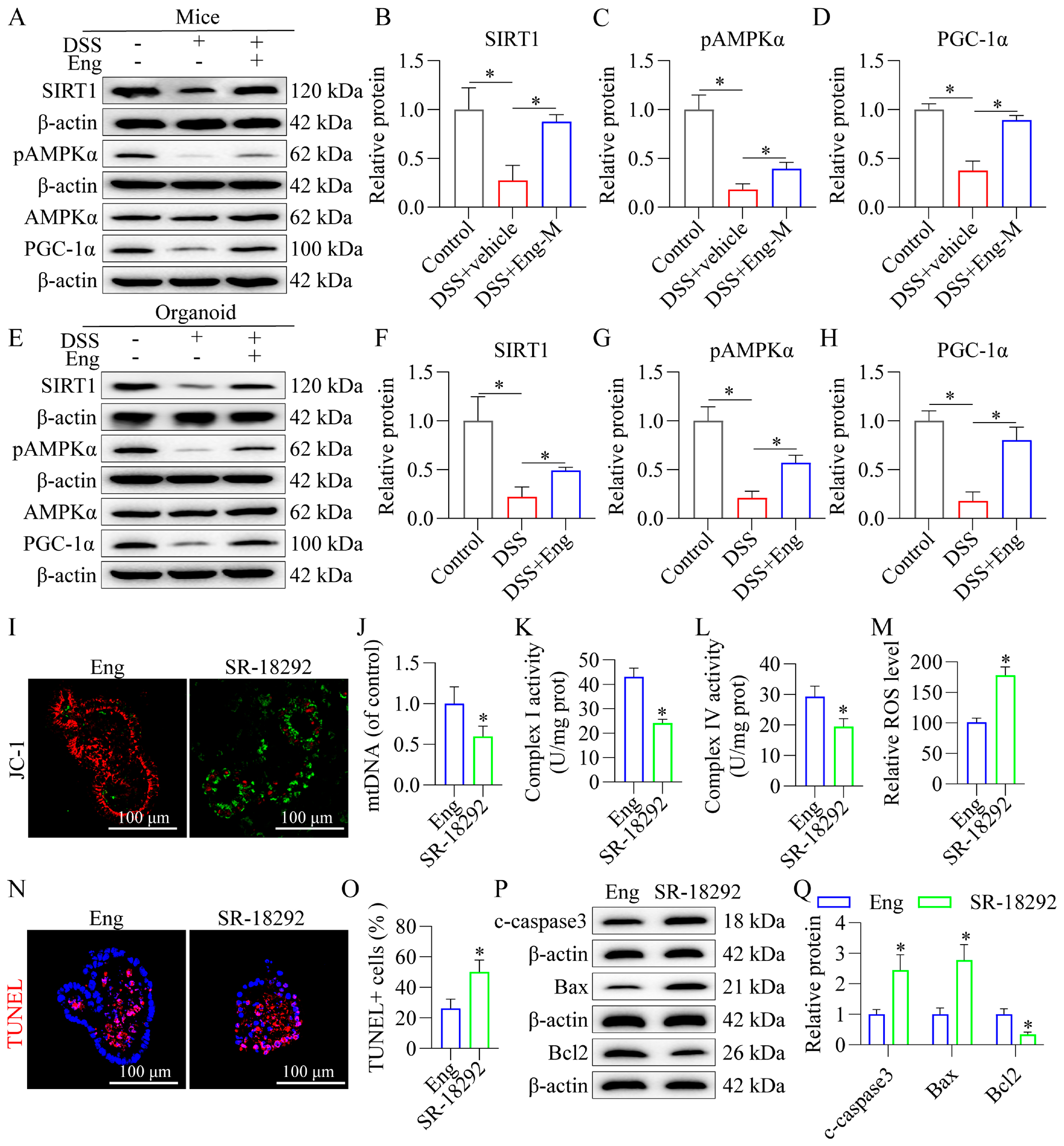
3.7. Engeletin Improves Mitochondrial Dysfunction and Reduces Apoptosis in Epithelial Cells Associated with AMPK/SIRT1 Signaling via PGC-1α-Dependent Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Yan, W.; Xu, L. Macrophage polarization in inflammatory bowel disease. Cell Commun. Signal. 2023, 21, 367. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zheng, C.; Xiang, Y.; Malik, S.; Su, D.; Xu, G.; Zhang, M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 2023, 69, 28–42. [Google Scholar] [CrossRef]
- Huang, L.J.; Mao, X.T.; Li, Y.Y.; Liu, D.D.; Fan, K.Q.; Liu, R.B.; Wu, T.T.; Wang, H.L.; Zhang, Y.; Yang, B.; et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity 2021, 54, 1728–1744.e7. [Google Scholar] [CrossRef]
- Rath, E.; Moschetta, A.; Haller, D. Mitochondrial function—Gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 497–516. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Chang, X.; Xie, L.; Song, Y.; Wu, H.; Zhang, H.; Wang, S.; Zhang, X.; Bai, Y. Mitochondrial damage-associated molecular patterns: New perspectives for mitochondria and inflammatory bowel diseases. Mucosal Immunol. 2025, 18, 290–298. [Google Scholar] [CrossRef]
- Arumugam, P.; Saha, K.; Nighot, P. Intestinal Epithelial Tight Junction Barrier Regulation by Novel Pathways. Inflamm. Bowel Dis. 2025, 31, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ruan, X.; Sun, Y.; Lu, S.; Hu, S.; Yuan, S.; Li, X. Multi-omic insight into the molecular networks of mitochondrial dysfunction in the pathogenesis of inflammatory bowel disease. EBioMedicine 2024, 99, 104934. [Google Scholar] [CrossRef]
- Ho, G.T.; Theiss, A.L. Mitochondria and Inflammatory Bowel Diseases: Toward a Stratified Therapeutic Intervention. Annu. Rev. Physiol. 2022, 84, 435–459. [Google Scholar] [CrossRef]
- Özsoy, M.; Stummer, N.; Zimmermann, F.A.; Feichtinger, R.G.; Sperl, W.; Weghuber, D.; Schneider, A.M. Role of Energy Metabolism and Mitochondrial Function in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1443–1450. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, F.; Liu, X.; Shuai, B.; Fan, H. The Role of AMPK Signaling in Ulcerative Colitis. Drug Des. Dev. Ther. 2023, 17, 3855–3875. [Google Scholar] [CrossRef] [PubMed]
- Petito, G.; Giacco, A.; Cioffi, F.; Mazzoli, A.; Magnacca, N.; Iossa, S.; Goglia, F.; Senese, R.; Lanni, A. Short-term fructose feeding alters tissue metabolic pathways by modulating microRNAs expression both in young and adult rats. Front. Cell Dev. Biol. 2023, 11, 1101844. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, R.; Neto, A.C.; Rodrigues, A.R.; Gouveia, A.M.; Almeida, H.; Neves, D. Anti-oxidant effect of metformin through AMPK/SIRT1/PGC-1α/SIRT3- independent GPx1 expression in the heart of mice with endometriosis. Horm. Mol. Biol. Clin. Investig. 2022, 43, 405–414. [Google Scholar] [CrossRef]
- Ho, J.H.; Baskaran, R.; Wang, M.F.; Yang, H.S.; Lo, Y.H.; Mohammedsaleh, Z.M.; Lin, W.T. Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats. Pharmaceuticals 2022, 15, 819. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Zhang, B.; Li, Y.L.; Ren, Z.X.; Huang, J.J.; Zhang, Z.Q.; Lin, Z.J.; Zhang, X.M. Smilax glabra Roxb.: A Review of Its Traditional Usages, Phytochemical Constituents, Pharmacological Properties, and Clinical Applications. Drug Des. Dev. Ther. 2022, 16, 3621–3643. [Google Scholar] [CrossRef]
- Abaidullah, M.; La, S.; Liu, M.; Liu, B.; Cui, Y.; Wang, Z.; Sun, H.; Ma, S.; Shi, Y. Polysaccharide from Smilax glabra Roxb Mitigates Intestinal Mucosal Damage by Therapeutically Restoring the Interactions between Gut Microbiota and Innate Immune Functions. Nutrients 2023, 15, 4102. [Google Scholar] [CrossRef]
- Huang, Z.; Ji, H.; Shi, J.; Zhu, X.; Zhi, Z. Engeletin Attenuates Aβ1-42-Induced Oxidative Stress and Neuroinflammation by Keap1/Nrf2 Pathway. Inflammation 2020, 43, 1759–1771. [Google Scholar] [CrossRef]
- Li, B.; Yang, X.; Zhang, P.; Guo, J.; Rong, K.; Wang, X.; Cao, X.; Zhou, T.; Zhao, J. Engeletin Alleviates the Inflammation and Apoptosis in Intervertebral Disc Degeneration via Inhibiting the NF-κB and MAPK Pathways. J. Inflamm. Res. 2022, 15, 5767–5783. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Gao, F.; Cheng, W.; Zhang, Y.; Wei, C.; Zhang, S.; Gao, X. Engeletin alleviates cerebral ischemia reperfusion-induced neuroinflammation via the HMGB1/TLR4/NF-κB network. J. Cell. Mol. Med. 2023, 27, 1653–1663. [Google Scholar] [CrossRef]
- Wei, Z.; Ni, X.; Cui, H.; Shu, C.; Peng, Y.; Liu, J.; Li, Y. Engeletin attenuates the inflammatory response via inhibiting TLR4-NFκB signaling pathway in Crohn’s disease-like colitis. J. Ethnopharmacol. 2025, 336, 118733. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, Z.; Tao, B.; Jiang, X. Engeletin mediates antiarrhythmic effects in mice with isoproterenol-induced cardiac remodeling. Biomed. Pharmacother. 2023, 161, 114439. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; La, L.; Feng, H.; Yang, Q.; Wu, F.; Wang, C.; Wu, J.; Hou, L.; Hou, C.; Liu, W. Aldose Reductase Inhibitor Engeletin Suppresses Pelvic Inflammatory Disease by Blocking the Phospholipase C/Protein Kinase C-Dependent/NF-κB and MAPK Cascades. J. Agric. Food Chem. 2020, 68, 11747–11757. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, J.; Zhang, D.; Wang, J.; Tan, Y.; Feng, W.; Peng, C. Ginsenoside Rg1 Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Microbial Tryptophan Metabolism. Front. Immunol. 2022, 13, 817600. [Google Scholar] [CrossRef]
- Spencer, D.M.; Veldman, G.M.; Banerjee, S.; Willis, J.; Levine, A.D. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology 2002, 122, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Fantini, M.C.; Neurath, M.F. High resolution colonoscopy in live mice. Nat. Protoc. 2006, 1, 2900–2904. [Google Scholar] [CrossRef]
- Porras, M.; Martín, M.T.; Yang, P.C.; Jury, J.; Perdue, M.H.; Vergara, P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm. Bowel Dis. 2006, 12, 843–852. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.B.; Zhang, N.; Xiao, L.Y.; Li, W.J.; Wang, W.F.; Xu, M.Z.; Hu, J.G.; Li, J.; Zuo, L.G.; et al. [Application of improved “Swiss roll” method in mouse intestinal tissue section]. Zhonghua Bing Li Xue Za Zhi = Chin. J. Pathol. 2024, 53, 393–397. [Google Scholar] [CrossRef]
- Schultz, M.; Tonkonogy, S.L.; Sellon, R.K.; Veltkamp, C.; Godfrey, V.L.; Kwon, J.; Grenther, W.B.; Balish, E.; Horak, I.; Sartor, R.B. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am. J. Physiol. 1999, 276, G1461–G1472. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Z.; Pang, Z.; Qi, G.; Hua, B.; Yan, Z.; Yuan, H. Engeletin Protects Against TNF-α-Induced Apoptosis and Reactive Oxygen Species Generation in Chondrocytes and Alleviates Osteoarthritis in vivo. J. Inflamm. Res. 2021, 14, 745–760. [Google Scholar] [CrossRef]
- Sayoc-Becerra, A.; Krishnan, M.; Fan, S.; Jimenez, J.; Hernandez, R.; Gibson, K.; Preciado, R.; Butt, G.; McCole, D.F. The JAK-Inhibitor Tofacitinib Rescues Human Intestinal Epithelial Cells and Colonoids from Cytokine-Induced Barrier Dysfunction. Inflamm. Bowel Dis. 2020, 26, 407–422. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Hu, H.; Zhang, X.; Wang, Z.; Wu, Z.; Wang, L.; Yu, X.; Song, X.; Xiang, P.; et al. ZHX2 emerges as a negative regulator of mitochondrial oxidative phosphorylation during acute liver injury. Nat. Commun. 2023, 14, 7527. [Google Scholar] [CrossRef]
- Liu, C.; Fu, Z.; Wu, S.; Wang, X.; Zhang, S.; Chu, C.; Hong, Y.; Wu, W.; Chen, S.; Jiang, Y.; et al. Mitochondrial HSF1 triggers mitochondrial dysfunction and neurodegeneration in Huntington’s disease. EMBO Mol. Med. 2022, 14, e15851. [Google Scholar] [CrossRef] [PubMed]
- Leibovitzh, H.; Lee, S.H.; Raygoza Garay, J.A.; Espin-Garcia, O.; Xue, M.; Neustaeter, A.; Goethel, A.; Huynh, H.Q.; Griffiths, A.M.; Turner, D.; et al. Immune response and barrier dysfunction-related proteomic signatures in preclinical phase of Crohn’s disease highlight earliest events of pathogenesis. Gut 2023, 72, 1462–1471. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Q.; Liu, M.; Zhao, T.; Song, X.; Chen, Q.; Yang, Y.; Nan, Y.; Liu, Z.; Zhang, Y.; et al. Precisely Inhibiting Excessive Intestinal Epithelial Cell Apoptosis to Efficiently Treat Inflammatory Bowel Disease with Oral Pifithrin-α Embedded Nanomedicine (OPEN). Adv. Mater. 2023, 35, e2309370. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, D.; Zou, G.; Solitano, V.; Syal, G.; Lee, H.H.; Ma, C.; Jairath, V.; Singh, S. No Impact of Concomitant Medications on Efficacy and Safety of Biologics and Small Molecules for Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Q.; Li, J.; Xue, J.C.; Li, Y.; Meng, H.; Hou, X.T.; Nan, J.X.; Zhang, Q.G. Inflammatory bowel disease: An overview of Chinese herbal medicine formula-based treatment. Chin. Med. 2022, 17, 74. [Google Scholar] [CrossRef]
- Smriti; Singla, M.; Gupta, S.; Porwal, O.; Nasser Binjawhar, D.; Sayed, A.A.; Mittal, P.; El-Demerdash, F.M.; Algahtani, M.; Singh, S.K.; et al. Theoretical design for covering Engeletin with functionalized nanostructure-lipid carriers as neuroprotective agents against Huntington’s disease via the nasal-brain route. Front. Pharmacol. 2023, 14, 1218625. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Xie, R.; Zhong, W.; Wang, H.; Farag, M.A.; Hussain, H.; Arroo, R.; Chen, X.; Xiao, J. Thermal degradation of (2R, 3R)-dihydromyricetin in neutral aqueous solution at 100 °C. Food Chem. 2024, 435, 137560. [Google Scholar] [CrossRef]
- Huang, W.; Xu, S.; Lin, R.; Xiong, X.; Song, J.; Liu, Y.; Li, J. Enzymatic Synthesis of Biflavonoid Glycosides with Enhanced Antitumor Activity Using Glycosyltransferase and Sucrose Synthase. J. Agric. Food Chem. 2025, 73, 4807–4819. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Geertsema, S.; Bourgonje, A.R.; Fagundes, R.R.; Gacesa, R.; Weersma, R.K.; van Goor, H.; Mann, G.E.; Dijkstra, G.; Faber, K.N. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol. Med. 2023, 29, 830–842. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal 2024, 17, eadh1641. [Google Scholar] [CrossRef]
- Wen, X.; Tang, L.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Role of Mitophagy in Regulating Intestinal Oxidative Damage. Antioxidants 2023, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Teshima, C.W.; Dieleman, L.A.; Meddings, J.B. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann. N. Y. Acad. Sci. 2012, 1258, 159–165. [Google Scholar] [CrossRef]
- Danne, C.; Michaudel, C.; Skerniskyte, J.; Planchais, J.; Magniez, A.; Agus, A.; Michel, M.L.; Lamas, B.; Da Costa, G.; Spatz, M.; et al. CARD9 in neutrophils protects from colitis and controls mitochondrial metabolism and cell survival. Gut 2023, 72, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Wang, H.; Chen, C.; Hu, W.; Ai, C.; Chen, L.; Teng, H. Flavonoids and gastrointestinal health: Single molecule for multiple roles. Crit. Rev. Food Sci. Nutr. 2024, 64, 10987–11005. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef]
- Ning, Z.; Li, Y.; Liu, D.; Owoicho Orgah, J.; Zhu, J.; Wang, Y.; Zhu, Y. Tetrahydroxystilbene Glucoside Delayed Senile Symptoms in Old Mice via Regulation of the AMPK/SIRT1/PGC-1α Signaling Cascade. Gerontology 2018, 64, 457–465. [Google Scholar] [CrossRef]
- Xu, W.; Yan, J.; Ocak, U.; Lenahan, C.; Shao, A.; Tang, J.; Zhang, J.; Zhang, J.H. Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats. Theranostics 2021, 11, 522–539. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Xu, K.; Guo, H.; Li, C.; Sun, X. Protective effect of Xinmai’an tablets via mediation of the AMPK/SIRT1/PGC-1α signaling pathway on myocardial ischemia-reperfusion injury in rats. Phytomedicine 2023, 120, 155034. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Sun, X.; Gheinani, P.T.; Guan, X.; Sharma, S.; Zhou, Y.; Jin, C.; Yang, Z.; Naren, A.P.; Yin, J.; et al. Epithelial SMYD5 Exaggerates IBD by Down-regulating Mitochondrial Functions via Post-Translational Control of PGC-1α Stability. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 375–403. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Geng, Z.; Yin, L.; Huang, J.; Niu, M.; Zhang, K.; Song, X.; Wang, Y.; Zuo, L.; Hu, J. Engeletin Targets Mitochondrial Dysfunction to Attenuate Oxidative Stress and Experimental Colitis in Intestinal Epithelial Cells Through AMPK/SIRT1/PGC-1α Signaling. Antioxidants 2025, 14, 524. https://doi.org/10.3390/antiox14050524
Li J, Geng Z, Yin L, Huang J, Niu M, Zhang K, Song X, Wang Y, Zuo L, Hu J. Engeletin Targets Mitochondrial Dysfunction to Attenuate Oxidative Stress and Experimental Colitis in Intestinal Epithelial Cells Through AMPK/SIRT1/PGC-1α Signaling. Antioxidants. 2025; 14(5):524. https://doi.org/10.3390/antiox14050524
Chicago/Turabian StyleLi, Jing, Zhijun Geng, Lixia Yin, Ju Huang, Minzhu Niu, Keni Zhang, Xue Song, Yueyue Wang, Lugen Zuo, and Jianguo Hu. 2025. "Engeletin Targets Mitochondrial Dysfunction to Attenuate Oxidative Stress and Experimental Colitis in Intestinal Epithelial Cells Through AMPK/SIRT1/PGC-1α Signaling" Antioxidants 14, no. 5: 524. https://doi.org/10.3390/antiox14050524
APA StyleLi, J., Geng, Z., Yin, L., Huang, J., Niu, M., Zhang, K., Song, X., Wang, Y., Zuo, L., & Hu, J. (2025). Engeletin Targets Mitochondrial Dysfunction to Attenuate Oxidative Stress and Experimental Colitis in Intestinal Epithelial Cells Through AMPK/SIRT1/PGC-1α Signaling. Antioxidants, 14(5), 524. https://doi.org/10.3390/antiox14050524






