Abstract
Over the past decade, Urtica dioica L. (U. dioica) has gained prominence in biomedical research, particularly for its potential therapeutic applications in neurodegenerative diseases. This comprehensive review explores its botanical characteristics, toxicological considerations, and extensive traditional medicinal uses. Emphasizing the roles of phytochemical constituents such as flavonoids and overall polyphenolic compounds, this review examines their impact on mitigating critical pathways, such as neuroinflammation, oxidative stress, and mitochondrial dysfunction—all of which are implicated in Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Multiple Sclerosis (MS)—and, overall, in neurodegenerative processes in both humans and animal models. Notably, some phytochemicals are known to modulate crucial pathways for neuronal plasticity, learning, and memory, thereby enhancing cognitive functions. Hence, the potential of U. dioica-based therapies to improve cognitive function and pave the way for future therapeutic developments in neuroprotection is underscored.
1. Introduction
Across the century, neurodegenerative diseases such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Amyotrophic Lateral Sclerosis (ALS) have posed enduring challenges due to their complex etiology and profound impact on neuronal function [1]. These disorders are characterized by the progressive degeneration of neurons, leading to cognitive decline, motor impairment, and ultimately, significant disability [2]. A common hallmark is neuronal disruption driven by metabolic failure, inflammation, and protein misfolding [3]. For example, mitochondria, which are crucial for energy synthesis, metabolite oxidation, and cellular homeostasis, which play pivotal roles in maintaining neuronal health [4]. Moreover, studies indicate that interleukin-6 (IL-6) can promote Amyloid-beta (Aβ) clearance in AD models through reactive gliosis, suggesting complex interactions between inflammation and disease pathology [5]. Despite decades of intensive research, effective treatments for these diseases remain elusive, highlighting the urgent need for innovative therapeutic strategies that can address their multifaceted pathophysiology. In this context, the use of neuroprotective medicinal plants from alternative medicines has gained prominence [6]. Neuroprotective medicinal plants have the potential to repair brain damage associated with neurodegeneration and enhance learning and memory by promoting the development of new synapses [7]. Urtica dioica L. (U. dioica), a perennial plant in the Urticaceae family, has been used in folk medicine for centuries and across continents [8,9]. Its rich phytochemical composition has been shown to be effective in cellular and molecular systems related to neurodegenerative diseases, suggesting promising prospects for disease prognosis [10]. Studies suggest that U. dioica enhances the synthesis of trophic factors in the brain, which is associated with improved learning, memory, and long-term potentiation [11]. Additionally, U. dioica supplementation has been shown to promote neurogenesis, enhance brain plasticity, increase resilience to brain damage, and improve cognitive and memory performance [12]. To date, no comprehensive review has been published regarding the current state of U. dioica-based remedies for neuroprotection. The current review aims to provide an updated overview of existing neurodegenerative diseases and the potential role of U. dioica in combating them. This goal can be achieved by considering animal models and discussing recent advancements in experimental design. In fact, increasing evidence has shown that U. dioica can improve memory and reduce chronic stress-related dysfunctions of the central nervous system (CNS) in animal models [13]. Despite this promising evidence, exploration in this field is relatively recent, making it an appealing option for medicinal chemists to further investigate.
Evidence from studies published between 2015 and 2025 is incorporated into this review. The terms “Urtica dioica,” “neurodegenerative diseases,” “oxidative stress,” “mitochondrial dysfunction,” and “neuroinflammation” were used in a systematic search on PubMed, Scopus, and Web of Science to find relevant publications. Peer-reviewed publications providing experimental and/or in vivo and/or mechanistic findings were the only ones that were accepted. Only when necessary to provide a summary of more general ideas, review articles, and meta-analyses were cited.
2. Urtica dioica
2.1. Botanical Study
U. dioica, commonly known as the “stinging nettle” (Figure 1), is a perennial herb belonging to the Urticaceae family [8]. This herbaceous plant typically grows to a height of 1 to 2 m and possesses rhizomes. It falls under the Rosales order and is characterized by the presence of stinging cells in the genus Urtica [14]. The erect stems of U. dioica are strong, hairy, usually unbranched, and quadrangular in shape [15]. Young plants exhibit green leaves, whereas mature plants develop purple or reddish foliage [16]. The opposite leaves are elongated and egg-shaped, with a distinctly serrated edge and pointed tip [17].

Figure 1.
Parts of the plant Urtica dioica: (A) Urtica dioica whole plant, (B) flowers, (C) leaves, (D) stinging hairs, and (E) roots.
Both the leaves and stems are densely covered in stinging hairs, many of which contain sharp points that release a stinging liquid upon contact [18]. The flowering period of U. dioica typically occurs from July to August in temperate regions, although it may vary depending on geographic location and climate. The species is dioecious, meaning it has separate male and female plants, which is a consistent trait across its range [19]. The small, unisexual flowers are clustered in groups on slender, branching spikes that emerge from the leaf axils. With their greenish coloration, female flowers feature a single ovary that contains one style and a brush-like stigma [20]. Male flowers possess four elongated, flexible stamens that are curved inwards in their bud stage and have a yellowish hue [14]. Following pollination, stinging U. dioica produces oval-shaped achenes, which are one-seeded fruits predominantly black or dark brown in colour. The root system consists of a taproot that branches into fine rootlets, enabling U. dioica to spread and thrive [21].
2.2. Toxicological Studies
Numerous toxicological studies have been undertaken to evaluate the toxicity profile of this plant, and the findings consistently indicate that its utilization is linked to minimal to negligible harm. Dar et al. [22] utilized the well-established Artemia salina (A. salina) technique to evaluate the toxicity of an extract derived from U. dioica. The results of the investigation showed that when utilized against A. salina larvae, both the aqueous extract and the herbal formulation termed Hexane Fraction-2 (HF2) of U. dioica offered exceptionally large margins of safety with a 50% lethal concentration (LC50) > 1000 μg/mL. The two crustacean species A. salina, as a toxicity assay model, presents some practical advantages for use at a preliminary stage, but the interpretation should be verified under some conditions [23]. It’s dependent on salinity, pH, oxygen content, and solvent concentration, and all these parameters need to be precisely controlled to have a reliable and consistent output [24]. The variation in LC50 results makes it clear that we need to be careful when applying data from A. salina to other species [25]. For risk assessments to be meaningful, toxicity estimates have to be both accurate and consistent [26]. By understanding what drives this variability, we can design better tests and make more informed interpretations of ecotoxicological data [27].
Dar et al. [22] also performed an acute toxicity test in Wistar rats, and the findings indicated no mortality after 24-hour of treatment with aqueous and hexane extracts of U. dioica, indicating that this herbal medication has sufficient safety potential.
Tekin et al. [28] conducted an acute toxicity investigation using the fixed oil of U. dioica in rodent models. The oil was administered in doses ranging from 0.2 to 12.8 mL/kg body weight (bw) intraperitoneally (i.p.), consistent with the liquid nature of the extract. No mortality occurred within the 72-hour observation period, even at the maximum dose of 12.8 mL/kg bw i.p., confirming its non-toxic profile within the tested range.
In zebrafish larval models [29], U. dioica ethanolic extract (UDE) demonstrated a safe profile when it was administered at a concentration of 25 mg/L in E3 medium. This concentration, applied in both preventive and curative treatment protocols, caused no observable toxic effects or mortality in the control groups. Additionally, UDE exhibited significant protective effects against chlorpyrifos-induced toxicity, reducing teratogenic effects, preserving telencephalon morphology, and mitigating abnormal locomotor behavior. Overall, these observations reinforce the potential of the extract for therapeutic applications in mitigating environmental toxicity.
Nencu et al. [30] conducted a study using rabbit models to assess the potential hazards associated with this herb by administering 50 mL of a 50% UDE for a duration of 10 days. Occasional instances of diarrhea were observed. However, subcutaneous injections with doses ranging from 5 to 20 mL showed varying tolerability, with chronic injections leading to significant weight loss and mortality in some cases. The authors determined that the intravenous fatal dosage of extract is 1.5 mL at fivefold greater concentrations and that heating the extract reduces its toxicity. The major symptoms displayed by the animals under research were increased breathing and central excitatory behaviour.
2.3. Traditional Uses
U. dioica is widely distributed across South Asia and the Indian subcontinent and has a rich medicinal heritage [31]. Indeed, it is widely recognized as a significant source of bioactive compounds (Table 1) with pharmacological relevance [32]. Ethnopharmacologically, it is esteemed for its therapeutic properties and is known to both prevent and treat various illnesses [33]. Traditionally, it has been utilized to address conditions such as hypertension and hepatic disorders [34], although a comprehensive study of its pharmacological profile and chemical constituents has only been undertaken recently [35]. Medicinally, it is employed for treating ailments such as arthritis, allergies, urinary tract issues, and skin disorders [36]. Additionally, in nutritional contexts, U. dioica has been applied in the food and pharmaceutical industries as a source of chlorophyll, which is utilized as a food coloring ingredient (E140) [37].

Table 1.
Phytochemicals found in Urtica dioica leaves.
2.4. The Roles of Phytochemical Compounds of Urtica dioica in Neurodegenerative Diseases
Several studies have looked into the therapeutic potential of U. dioica in the context of neurodegenerative diseases [43]. Instead of examining isolated compounds on their own, it makes more sense to consider the plant as a whole—as a complete extract where various nutrients and phytochemicals may work together [44]. In practice, the compounds found in U. dioica are probably not acting in isolation [45]. More likely, there’s some level of interaction between them—one affecting how the other is taken up, processed, or even stabilized in the body [46]. That might be part of the reason why it’s difficult to link the observed effects to a single constituent [47]. The response seems to reflect the system as a whole, not any one molecule alone [48]. The cumulative response is more plausibly the result of coordinated actions within the plant’s phytochemical matrix [48]. These interactions—which reflect the full complexity of the plant—are outlined in more detail in Table 2 and Table 3, which summarize some of the key antioxidant, anti-inflammatory, and neuro-supportive effects reported so far.

Table 2.
Protective and Therapeutic Effects in Animal and Human Studies of Compounds Isolated from Urtica dioica.

Table 3.
Summary of the Potential Neuroprotective Effects of Urtica dioica L. Phytochemicals from Different Regions.
3. Neuroinflammation, Oxidative Stress, and Mitochondrial Dysfunction in Neurodegenerative Diseases
Alzheimer’s and Parkinson’s diseases as well as ALS are all characterized by common molecular abnormalities, most notably chronic neuroinflammation, oxidative stress, and mitochondrial dysfunction [5]. These interrelated pathways lead to gradual neuronal death and cognitive decline, and they have been seen in both clinical and experimental research [83]. Microglia are the resident immune cells of the central nervous system and play a major role in neuroinflammation [84]. When triggered by infections or injury, they become active and release pro-inflammatory molecules [85]. Nuclear Factor kappa B (NF-κB) is one of the main pathways involved in this process, regulating genes linked to inflammation [86]. It has a dual role: in neurons, it supports survival and synaptic function, but in microglia, its activation contributes to chronic inflammation and neurodegeneration [87]. Some of the compounds found in U. dioica, like tannins and chlorogenic acid, seem to play a role in how microglia behave during inflammation [88,89]. These plant-derived compounds might help by keeping microglial activation under control, which could, in turn, ease the pressure that chronic inflammation puts on the nervous system [90].
As described in Figure 2, microglial activation plays an important role in Alzheimer’s disease, regulating plaque dynamics and immunological signaling. These cells exhibit diverse functions such as phagocytosis, chemotaxis, and activation, which impact plaque dynamics and coverage [91]. The complex interactions among microglia, β-amyloid protein, and neurofibrillary tau tangles in AD underscore the importance of understanding microglial responses to develop effective therapeutic strategies [92]. Signaling cascades, including Nuclear factor erythroid 2-Related Factor 2 (Nrf2)-Antioxidant Response Element (ARE) pathway and Mitogen-Activated Protein Kinase (MAPK)-Extracellular signal-Regulated Kinase (ERK), which are already dysregulated in Alzheimer’s disease, lead to prolonged neuroinflammation and neuronal stress [93].
Neuroinflammation also affects signaling pathways involved in mood, behavior, and stress-related disorders [94]. Elevated levels of inflammatory mediators in the brain have been correlated with impaired neurological function in both clinical studies and animal experiments [95]. This inflammatory state has also been found to reduce neural plasticity, hampering the ability of the brain to adapt and reorganize [96]. Reducing brain inflammation has been shown to improve related behavioral impairments [97].
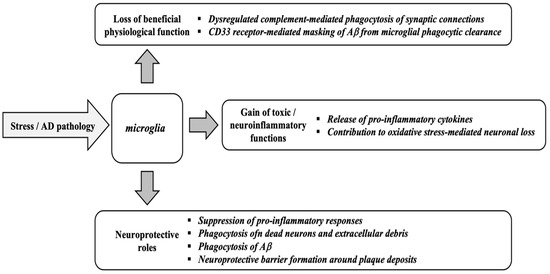
Figure 2.
Microglial functions in response to stress and Alzheimer’s disease (AD) pathology. Adapted from [98].
Figure 2.
Microglial functions in response to stress and Alzheimer’s disease (AD) pathology. Adapted from [98].
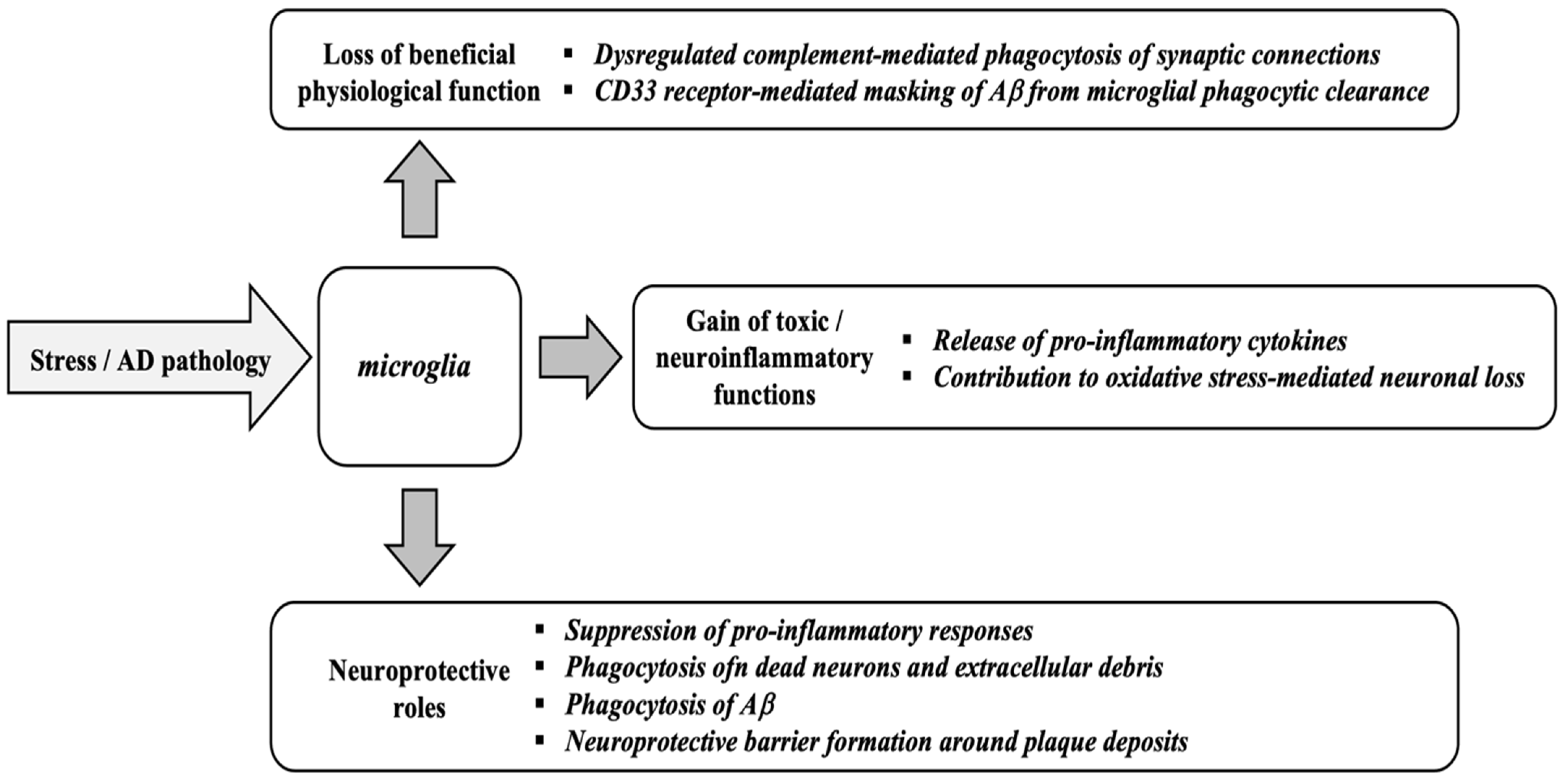
Figure 3 and Figure 4 provides an integrative view of converging mechanisms that drive neurodegeneration, including oxidative stress, protein aggregation, neuroinflammation, excitotoxicity, mitochondrial dysfunction, and apoptosis. These interconnected pathways—each detailed in preceding sections—collectively contribute to progressive neuronal loss in disorders such as Alzheimer’s and Parkinson’s diseases [99].
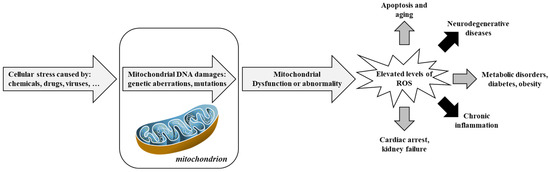
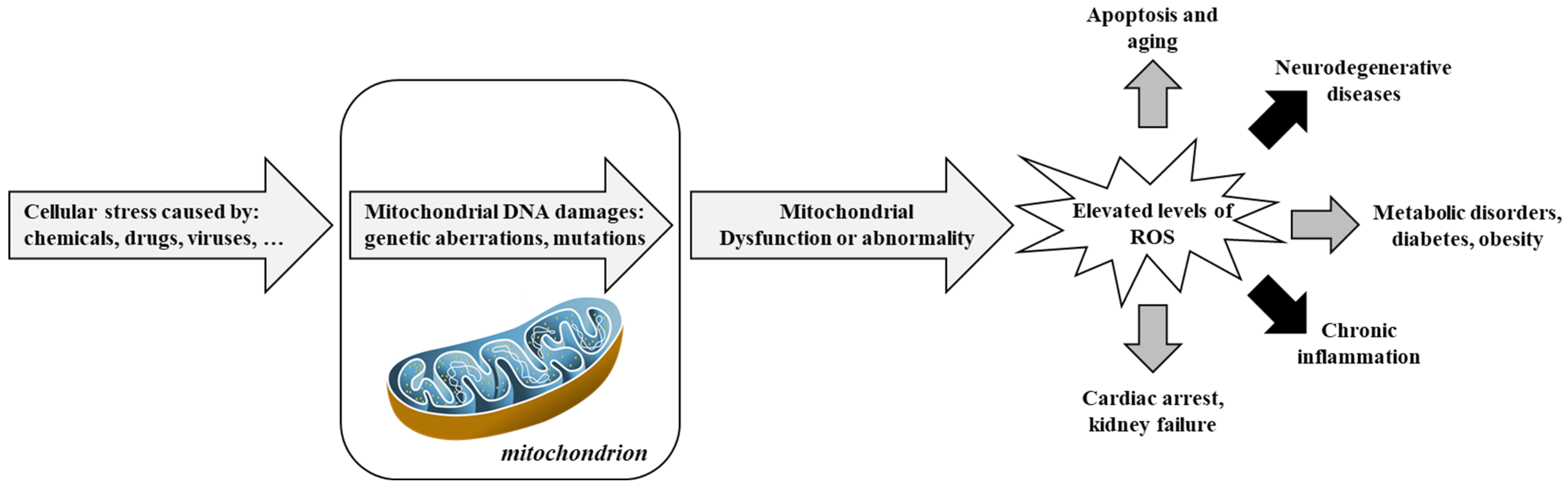
Figure 3.
Involvement of mitochondria in oxidative stress and diseases. Adapted from [100]. Reactive Oxygen Species, ROS.
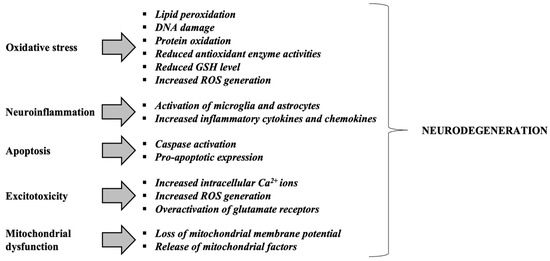
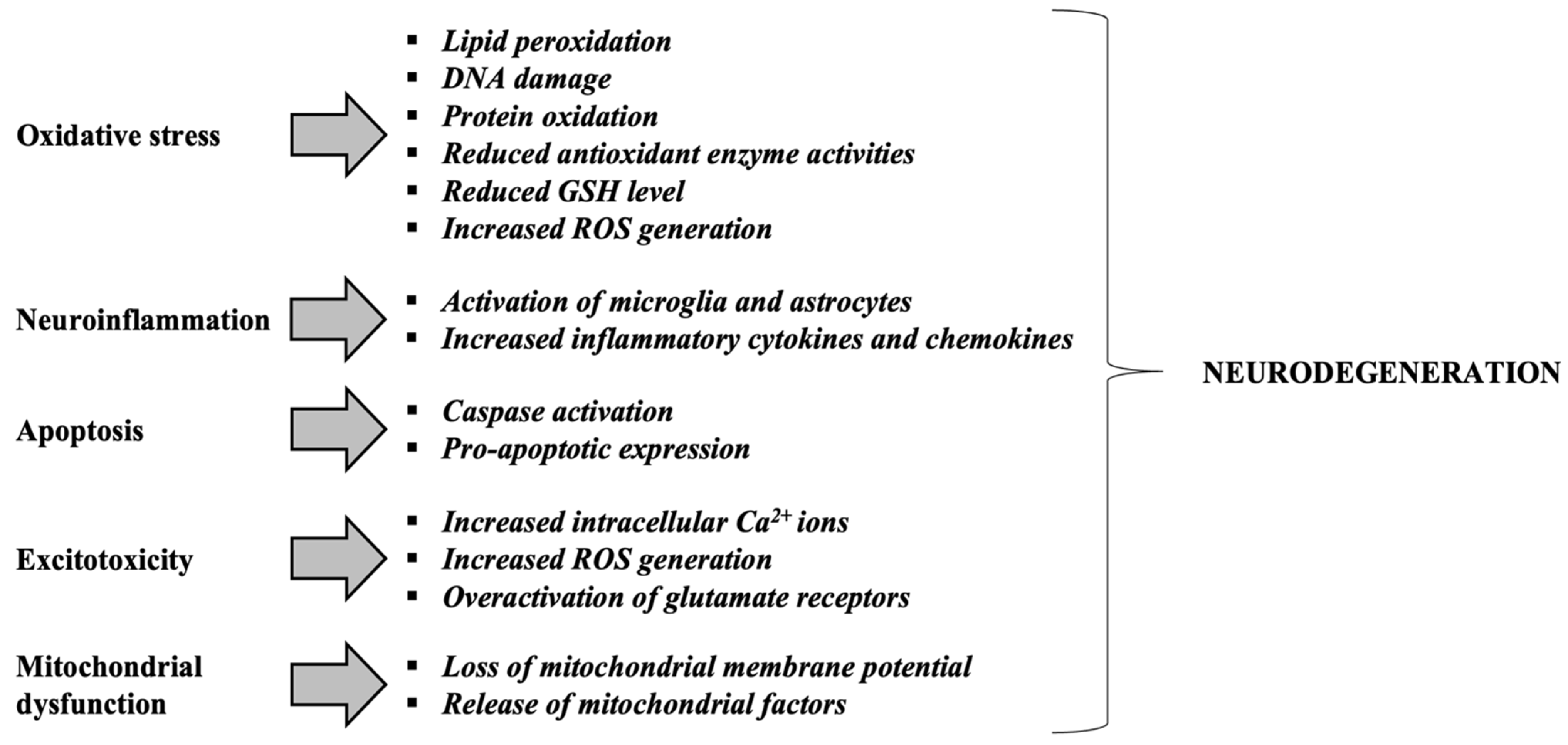
Figure 4.
Mechanisms contributing to neurodegeneration in neurodegenerative disorders. Adapted from [101]. Glutathione or γ-glutamyl-cysteinyl-glycine, GSH; Reactive Oxygen Species, ROS.
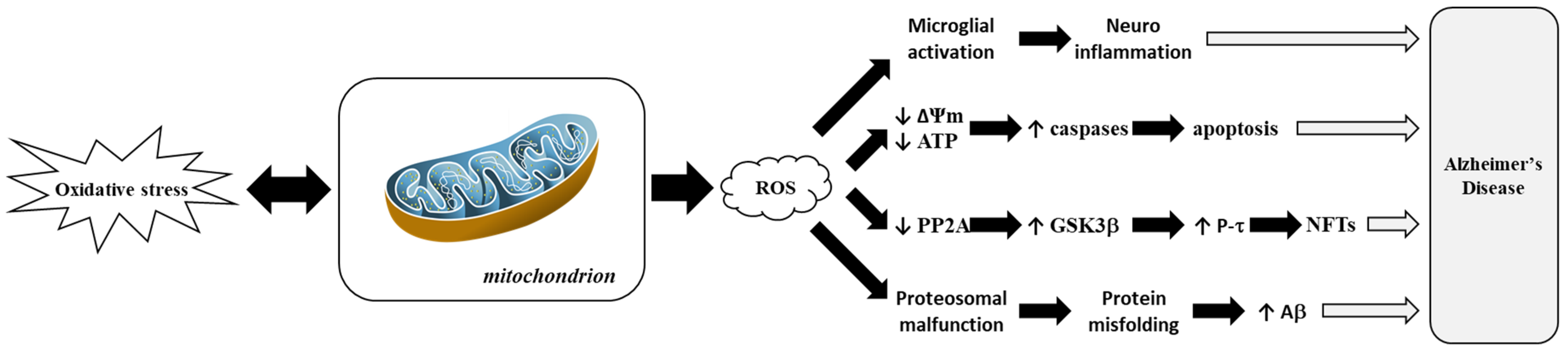
Figure 5 highlights how ROS-driven mitochondrial dysfunction contributes to AD progression. This process is closely linked to hallmark features of AD, such as amyloid-β (Aβ) plaque accumulation and tau hyperphosphorylation, which correlate with cognitive decline [102]. Disturbances in tau and other cytoskeletal proteins also play significant roles in AD. Microtubule-Associated Protein 2 (MAP2), which is crucial for neuronal maintenance and plasticity, shows altered expression levels in AD [103]. Phosphorylation events involving MAPK3, which is pivotal in neurogenesis, and Amyloid Precursor Protein (APP) processing, are dysregulated in AD [104]. In transgenic mouse models, oxidative stress precedes amyloid pathology, while mitochondrial dysfunction amplifies this effect through lipid peroxidation and elevated secretase activity [105,106].
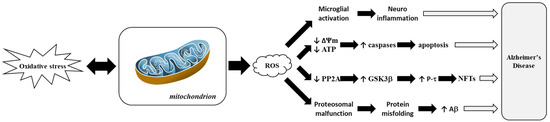
Figure 5.
Representation of mitochondrial dysfunction induced by Reactive Oxygen Species (ROS) in Alzheimer’s disease. Adapted from [103]. Amyloid beta-peptide or amyloid-beta, Aβ; Adenosine TriPhosphate, ATP; Glycogen Synthase Kinase 3 beta, GSK3β; Mitochondrial Membrane Potential or delta psi membrane, ΔΨm; NeuroFibrillary Tangle, NFT; Protein Phosphatase 2A, PP2A; tau Protein, P-τ.
3.1. Alzheimer’s Disease
In Alzheimer’s disease (AD), deregulation of the MAPK/ERK and Nrf2-ARE pathways contributes to characteristic symptoms such as memory loss, synaptic dysfunction, and tau hyperphosphorylation [107]. Chronic activation of the MAPK/ERK pathway, frequently triggered by amyloid-beta (Aβ) peptides binding to α7 nicotinic acetylcholine receptors, leads to negative consequences such as tau protein hyperphosphorylation, which destabilizes microtubules and impairs neuronal transport [108]. Aberrant signaling leads to neuronal death and increased amyloidogenic processing of APP, resulting in increased Aβ buildup and synaptic dysfunction [109]. Concurrently, the Nrf2-ARE pathway, a critical regulator of cellular antioxidant defenses, is impaired in AD due to dysregulation of its inhibitor Kelch-like ECH-Associated Protein 1 (Keap1), resulting in reduced nuclear translocation of Nrf2 and decreased expression of vital detoxifying enzymes such as Heme Oxygenase-1 (HO-1) and NAD(P)H Quinone Oxidoreductase 1 (NQO) [110]. This failure to establish an efficient antioxidant response permits oxidative stress and neuroinflammation to worsen, harming both astrocytes and neurons and inducing ferroptosis, a type of iron-dependent cell death that exacerbates neuronal loss [111]. The interplay between damaged pathways causes a vicious cycle where Aβ accumulation, tau pathology, and oxidative stress feed into one another, gradually reducing neuronal resilience and ultimately leading to the hallmark cognitive deficits of Alzheimer’s disease [112].
3.2. Parkinson’s Disease
Parkinson’s disease (PD) is characterized by progressive neuronal loss in the substantia nigra and the accumulation of Lewy bodies—abnormal protein aggregates within neuronal cytoplasm [113]. Pathologically, this process involves neuronal loss in the substantia nigra and the presence of Lewy bodies, which are abnormal protein deposits in the neuron cytoplasm [114]. Early studies of PD showed that the neurotoxin 1-Methyl-4-PhenylPyridinium (MPP+) inhibits mitochondrial complex I, linking disease onset [115]. Mutations in the parkin gene, which encodes a ubiquitin E3 ligase, disrupt the proteasome system and impair protein clearance [116]. Parkin normally protects neurons by regulating mitochondrial function and inhibiting apoptotic pathways, but its mutation diminishes this protective role [117].
The Parkinsonism-associated deglycase (DJ-1) protein protects neurons against oxidative stress-induced damage in Parkinson’s disease. Mutations in the DJ-1 gene impair this defense, increasing neuronal vulnerability [100,118].
3.3. Multiple Sclerosis
Multiple sclerosis (MS) is characterized by neuroinflammatory demyelinating lesions and neuronal loss, often driven by progressive disruption of the Blood-Brain Barrier (BBB) [119]. This barrier breakdown allows infiltration of natural killer cells, T and B lymphocytes, and dendritic cells into the spinal cord and brain during MS onset [120]. Epigenetic modifications, particularly DNA methylation, have been observed in immune cells and brain tissues of MS patients, although their precise role in this disease remains unclear [121]. Cellular senescence, which is characterized by the accumulation of lipofuscin in neurons and glial cells within demyelinated lesions across white and gray matter, is proposed to be a significant contributor to progressive MS [122].
The etiology of MS involves a complex interplay between genetic susceptibility and environmental factors, although exact mechanisms remain elusive [123]. These risk factors are believed to synergistically trigger autoimmunity in susceptible individuals [124]. The Antigen-Presenting Cells (APCs) present myelin-derived peptides using class II Major Histocompatibility Complex (MHC) receptors within the central nervous system, activating autoreactive CD4+ T lymphocytes [125]. This interaction induces the differentiation of naive CD4+ T cells into proinflammatory Th1 cells, which secrete cytokines such as interferon-gamma (IFN-γ), attracting CD8+ T cells, B cells, and monocytes to the peripheral regions [126]. These proinflammatory cells then migrate to the BBB, attaching to endothelial cells and intensifying the neuroinflammatory response in MS [123].
3.4. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a deadly neurodegenerative disease characterized by the gradual loss of both upper and lower motor neurons, which causes paralysis [127]. Early symptoms often include muscle twitching (fasciculations), cramping, stiffness, and weakness in the limbs or bulbar muscles, causing difficulties with walking, speaking, swallowing, and eventually breathing [128]. As the disease advances, muscle paralysis spreads throughout the body, severely impairing mobility and respiratory function [129]. Despite these physical declines, ALS typically does not affect a person’s intelligence or sensory functions, although some patients may experience cognitive or behavioral changes, including frontotemporal dementia in a subset of cases [130].
Its pathophysiology is complicated, including genetic abnormalities, immunological dysregulation, oxidative stress, and altered protein and RNA metabolism [131]. Mutations in these genes disturb protein homeostasis by disrupting the ubiquitin-proteasome system and autophagy, resulting in toxic protein aggregation and neuronal death [132]. When the RNA-binding proteins TDP-43 and FUS are mislocalized or aggregated, they lead to aberrant RNA metabolism [133]. Recent research has also identified genetic variations in genes such as CNTN4, DPP6, and INPP5B that may influence ALS susceptibility. These coupled circuits form a complicated disease process that gradually compromises motor neuron function.
3.5. Huntington’s Disease
As detailed in Section 3, Huntington’s Disease (HD) involves disrupted cellular processes including mitochondrial dysfunction, protein misfolding, and excitotoxicity, which together compromise neuronal function and survival [128,129]. The huntingtin protein is ubiquitously expressed in human and animal cells, with relatively high levels in the brain and testes, and moderate levels in the liver, heart, and lungs [134]. Orthologues of this protein are found in various species, including zebrafish, fruit flies, and slime molds [135].
Cognitive decline is another significant feature of HD, affecting memory, executive functions, and decision-making abilities. Psychiatric disturbances such as depression, anxiety, irritability, and personality changes are also commonly observed [136]. Disruptions in protein clearance mechanisms, including autophagy and the ubiquitin-proteasome system, contribute to the buildup of toxic protein aggregates [137]. Excitotoxicity from neurotransmitter imbalance worsens neuronal damage [138].
Dysregulation of intracellular signaling pathways involving calcium, cAMP Response Element-Binding (CREB) protein, and Brain-Derived Neurotrophic Factor (BDNF) also contributes to the pathophysiology of HD [139]. Although haploinsufficiency—a deficiency in protein production due to genetic factors—has been proposed in autosomal-dominant diseases, it is unlikely to explain HD since partial loss of the HD gene does not cause the disease phenotype in humans [139]. In cell culture models, HD is associated with the activation of proapoptotic enzymes such as caspase 1 or 8, contributing to polyglutamine toxicity [140]. Molecular chaperones, such as Hsp 40 and 70, play crucial roles in protein folding and have been shown to mitigate the aggregation and toxicity of polyglutamine-containing proteins [141].
3.6. Creutzfeldt-Jakob Disease
Diagnosis of Creutzfeldt-Jakob Disease (CJD) is often delayed due to its rarity and low clinical suspicion during a patient’s lifetime [142]. The symptoms of CJD typically include rapidly progressive dementia, neurological disturbances, and involuntary movements [143]. As the disease progresses, individuals may experience severe cognitive impairment, muscle stiffness, myoclonus (sudden jerking movements), and difficulties with coordination and balance. Other symptoms can include changes in mood and behavior, hallucinations, and sensory abnormalities [144].
The exact cause of prion diseases, such as CJD, is not fully understood [145]. Genetic factors or environmental exposure are not typically associated with prion diseases. Instead, it is believed that a stochastic misfolding event of the normal prion protein (PrPC) or a somatic mutation in the PRNP gene within a single or clade of cells may trigger the disease [146]. Prion diseases are transmissible, and cases of acquired variants have been documented. One notable example is the outbreak of “kuru”, which affected the Fore ethnic group and neighboring communities in Papua New Guinea [147]. This outbreak, which occurred due to ritual cannibalism, resulted in over 3000 cases [147]. More recently, variant CJD (vCJD) emerged as a zoonotic outbreak with over 230 confirmed cases caused by the dietary transfer of BSE prions [148].
3.7. Perinatal Stroke
Perinatal Stroke (PS) is a complex condition that causes brain damage at a key developmental phase [147]. The growing brains of neonates have amazing flexibility, allowing them to use alternate neural pathways and perhaps achieve unexpected beneficial results despite the harm sustained [148]. A medical history, physical examination, and imaging methods, such as Magnetic Resonance Imaging (MRI) or computed tomography (CT), are usually used to diagnose the brain and discover abnormalities or indicators of damage. PS refers to six unique prenatal stroke syndromes classified by vascular involvement, stroke mechanism, time of damage, and clinical presentation [149].
Acute presentations include Newborn Arterial Ischemic Stroke (NAIS), Neonatal Cerebral Sinovenous Thrombosis (CSVT), and Neonatal Hemorrhagic Stroke (NHS). Delayed presentations include Presumptive Prenatal Hemorrhagic Stroke (PPHS), Periventricular Venous Infarction (PVI), and Arterial Presumed Perinatal Ischemic Stroke (APPIS) [150]. Treatment for PS primarily focuses on managing immediate complications and providing supportive care [151]. This may involve medications to prevent seizures or manage other medical issues, as well as rehabilitation therapies such as physical therapy or occupational therapy to promote optimal development [152].
3.8. Duchenne Muscular Dystrophy
Duchenne Muscular Dystrophy (DMD) frequently begins with delayed motor milestones in early childhood [149]. Children with DMD often exhibit an abnormal, waddling gait and experience frequent falls [153]. As the disease progresses, muscle weakness and wasting become more pronounced, making activities requiring muscle strength, such as climbing stairs or lifting objects, increasingly difficult [154].
The diagnosis of DMD typically involves genetic testing to identify mutations or deletions in the DMD gene [155]. Elevated levels of the enzyme Creatine Kinase (CK) in the blood can also indicate muscle damage, supporting the diagnostic process [156]. Muscle biopsies may be performed to assess dystrophin levels and evaluate muscle pathology.
The pathophysiology of DMD involves the absence or deficiency of dystrophin, which disrupts the structural stability of muscle fibers [157]. Dystrophin is part of the Dystrophin-associated Glycoprotein Complex (DGC), which links the internal cytoskeleton of muscle cells to the extracellular matrix [158]. This linkage is crucial for maintaining muscle cell membrane integrity during contraction and relaxation [158]. Without dystrophin, muscle fibers are more susceptible to damage, leading to progressive muscle degeneration and replacement with fibrotic tissue [159].
Table 4 highlights key mechanistic pathways and reported therapeutic effects of U. dioica across models.

Table 4.
Comparative Summary of Disease Mechanisms and Urtica dioica Effects in Neurodegenerative Models.
4. Effects of Urtica dioica on Neurodegenerative Disorders in Animal Models
New experimental research in a variety of preclinical settings supports U. dioica’s neurotherapeutic potential [176]. Its activities seem to converge on a number of fundamental protective pathways rather than separate outcomes, most notably redox homeostasis, anti-inflammatory signaling, and neurotrophin control —as illustrated in Figure 6 [177]. In zebrafish neurotoxicity studies, ethanolic U. dioica extract showed neuroprotection against chlorpyrifos-induced damage by reducing abnormalities, preserving forebrain shape, and restoring locomotor function [29].
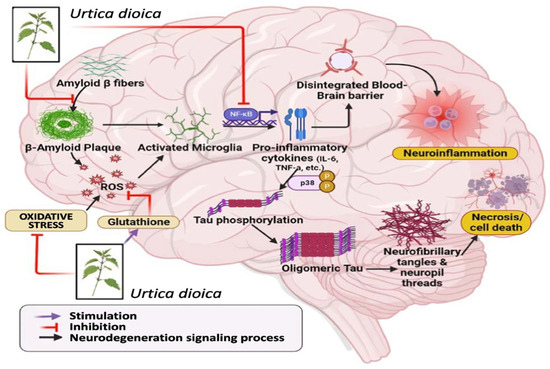
Figure 6.
Mechanisms of neuroprotection associated with Urtica dioica. Reprinted with permission from [34].
Diabetic rat models, frequently employed as a proxy for neurodegenerative progression, improved with U. dioica (50–100 mg/kg), displaying recovered memory performance and endocrine balance, rivaling established treatments like rosiglitazone [178]. Additionally, hydroalcoholic preparations demonstrated histological and molecular advantages in the hippocampus of streptozotocin-induced rats, indicating Nerve Growth Factor (NGF) and BDNF regulation [12]. In cholinergic mice, U. dioica leaf extract not only corrected scopolamine-induced cognitive loss [70], but it also increased the efficiency of co-administered minocycline, indicating synergistic neuroprotection [179]. A separate line of research confirms that therapy with U. dioica reduces acetylcholinesterase activity, lowers oxidative stress indicators, and improves memory learning [7]. These effects correspond to increased transcription of genes associated with sporadic Alzheimer’s disease, providing mechanistic evidence for its functional results.
5. Conclusions
Urtica dioica has been utilized for decades, serving as both a nutritional resource and a traditional medicinal remedy. The neuroprotective effectiveness of medicinal plants can be attributed to their ability to exhibit diverse mechanisms, including antioxidant activity and inflammation inhibition. The promising outcomes of these studies suggest that U. dioica, with its diverse phytochemical composition, has the potential to manage neuroinflammation, oxidative stress, and mitochondrial dysfunction [179,180].
While previous research has provided insights into its anti-neurological properties, more comprehensive studies are needed to pinpoint the specific signaling pathways affected by U. dioica. This identification could potentially facilitate the development of future drugs for treating neurodegenerative disorders. Additionally, integrating Artificial Intelligence (AI) into U. dioica research offers a promising pathway to accelerate discoveries. AI-driven data analysis and predictive modeling can identify key bioactive compounds and their mechanisms, optimize experimental protocols, and enhance precision in biomarker identification. Such innovative approaches could deepen our understanding of U. dioica and pave the way for its personalized and clinical applications in managing neurodegenerative diseases.
Author Contributions
A.C., conceptualization, drafting, and final writing, revision; S.L., drafting and final writing, revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nabi, M.; Tabassum, N. Role of Environmental Toxicants on Neurodegenerative Disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, M.; Elshafae, S.M.; Abdelhiee, E.Y.; Fadl, S.E.; Soliman, A.; Abdelkader, A.; Abdel-Daim, M.M.; Bayoumi, K.A.; Baty, R.S.; Elgendy, E.; et al. Antioxidant and Anti-Inflammatory Potential of Thymoquinone and Lycopene Mitigate the Chlorpyrifos-Induced Toxic Neuropathy. Pharmaceuticals 2021, 14, 940. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Bosch, L.V.D.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell. 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Smit, T.; Deshayes, N.A.C.; Borchelt, D.R.; Kamphuis, W.; Middeldorp, J.; Hol, E.M. Reactive Astrocytes as Treatment Targets in Alzheimer’s Disease—Systematic Review of Studies Using the APPswePS1dE9 Mouse Model. Glia. 2021, 69, 1852–1881. [Google Scholar] [CrossRef]
- Ara, I.; Maqbool, M.; Gani, I. Neuroprotective Activity of Herbal Medicinal Products: A Review. Int. J. Curr. Res. Physiol. Pharmacol. 2022, 6, 1–10. [Google Scholar]
- Tyler, S.E.B.; Tyler, L.D.K. Pathways to Healing: Plants with Therapeutic Potential for Neurodegenerative Diseases. IBRO Neurosci. Rep. 2023, 14, 210–234. [Google Scholar] [CrossRef]
- Chira, A.; Rekik, I.; Rahmouni, F.; Ben Amor, I.; Gargouri, B.; Kallel, C.; Jamoussi, K.; Allouche, N.; El Feki, A.; Kadmi, Y.; et al. Phytochemical Composition of Urtica dioica Essential Oil with Antioxidant and Anti-Inflammatory Properties: In Vitro and in Vivo Studies. Curr. Pharm. Biotechnol. 2022, 23, 728–739. [Google Scholar] [CrossRef]
- Chira, A.; Dridi, I.; Rahmouni, F.; Ben Amor, I.; Gargouri, B.; Kallel, C.; Jamoussi, K.; El Feki, A.; Saoudi, M. Neuroprotective and Antioxidant Effects of Urtica dioica Extract against Chlorpyrifos-Induced Toxicity: An in Vivo Study. 3 Biotech 2025, 15, 86. [Google Scholar] [CrossRef]
- Namazi, F.; Bordbar, E.; Bakhshaei, F.; Nazifi, S. The Effect of Urtica dioica Extract on Oxidative Stress, Heat Shock Proteins, and Brain Histopathology in Multiple Sclerosis Model. Physiol. Rep. 2022, 10, e15404. [Google Scholar] [CrossRef]
- Parente, R.; Paiva-Santos, A.C.; Cabral, C.; Costa, G. Comprehensive Review of Urtica dioica L. (Urticaceae) Phytochemistry and Anti-Inflammatory Properties. Phytochem. Rev. 2024, 24, 1591–1628. [Google Scholar] [CrossRef]
- Rahmati, M.; Keshvari, M.; Xie, W.; Yang, G.; Jin, H.; Li, H.; Chehelcheraghi, F.; Li, Y. Resistance Training and Urtica dioica Increase Neurotrophin Levels and Improve Cognitive Function by Increasing Age in the Hippocampus of Rats. Biomed. Pharmacother. 2022, 153, 113306. [Google Scholar] [CrossRef]
- Rajabian, A.; Sadeghnia, H.; Fanoudi, S.; Hosseini, A. Genus Boswellia as a New Candidate for Neurodegenerative Disorders. Iran. J. Basic Med. Sci. 2020, 23, 277. [Google Scholar] [PubMed]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging Nettle, Urtica dioica L.: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Malik, K.; Ahmad, M.; Öztürk, M.; Altay, V.; Zafar, M.; Sultana, S. Medicinal Plants Used for Musculoskeletal Disorders. In Herbals of Asia; Springer International Publishing: Cham, Switzerland, 2021; pp. 371–432. ISBN 978-3-030-85221-4. [Google Scholar]
- Shonte, T.T. Sensory and Nutritional Properties of Stinging Nettle (Urtica dioica L.) Leaves and Leaf Infusions; University of Pretoria: Pretoria, South Africa, 2017. [Google Scholar]
- Said, A.A.H.; Otmani, I.S.E.; Derfoufi, S.; Benmoussa, A. Highlights on nutritional and therapeutic value of stinging nettle (Urtica dioica). Int. J. Pharm. Pharm. Sci. 2015, 7, 8–14. Available online: https://journals.innovareacademics.in/index.php/ijpps/article/view/8165 (accessed on 20 May 2025).
- Ensikat, H.J.; Wessely, H.; Engeser, M.; Weigend, M. Distribution, Ecology, Chemistry and Toxicology of Plant Stinging Hairs. Toxins 2021, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Zafar, M.H.; Zhang, J.; Wang, J.; Yu, X.; Liu, W.; Wang, M. Research Progress in Physiological Effects of Resistant Substances of Urtica dioica L. on Animal Performance and Feed Conversion. Front. Plant Sci. 2023, 14, 1164363. [Google Scholar] [CrossRef]
- Dong, J. Morphological Variation and Floral Development of Major Clades in Urticaceae—A Focus on the Female Flowers. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2016. [Google Scholar]
- Toffolatti, S.L.; Davillerd, Y.; D’Isita, I.; Facchinelli, C.; Germinara, G.S.; Ippolito, A.; Khamis, Y.; Kowalska, J.; Maddalena, G.; Marchand, P.; et al. Are Basic Substances a Key to Sustainable Pest and Disease Management in Agriculture? An Open Field Perspective. Plants 2023, 12, 3152. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.-H.; Bhat, T.M.; Sharma, P. Pharmacological and Toxicological Evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Araya, X.; Okumu, M.; Durán, G.; Gómez, A.; Gutiérrez, J.M.; León, G. Assessment of the Artemia Salina Toxicity Assay as a Substitute of the Mouse Lethality Assay in the Determination of Venom-Induced Toxicity and Preclinical Efficacy of Antivenom. Toxicon X 2024, 22, 100195. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological Evaluation of the Plant Products Using Brine Shrimp (Artemia salina L.) Model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Damayanti, M.; Indarjo, A.; Sedjati, S. Phytochemical Content and Toxicity Test of Kappaphycus Alvarezii Hot Water and Methanol Using BSLT Method. J. Mar. Biotechnol. Immunol. 2025, 3, 49–54. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Amaral, S.; Rocha, E.; Rocha, M.J. Determination of 54 Pesticides in Waters of the Iberian Douro River Estuary and Risk Assessment of Environmentally Relevant Mixtures Using Theoretical Approaches and Artemia Salina and Daphnia Magna Bioassays. Ecotoxicol. Environ. Saf. 2017, 145, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Cazzagon, V.; Faraggiana, E.; Bettiol, C.; Picone, M.; Marcomini, A.; Badetti, E. An Overview on Dispersion Procedures and Testing Methods for the Ecotoxicity Testing of Nanomaterials in the Marine Environment. Sci. Total Environ. 2024, 921, 171132. [Google Scholar] [CrossRef] [PubMed]
- Tekin, M.; Him, A. Investigation of Acute Toxicity, Anti-Inflammatory, and Analgesic Effect of Urtica dioica L. Pharmacologyonline 2009, 1, 1210–1215. Pharmacologyonline 2009, 1, 1210–1215. [Google Scholar]
- Mhalhel, K.; Kadmi, Y.; Ben Chira, A.; Levanti, M.; Pansera, L.; Cometa, M.; Sicari, M.; Germanà, A.; Aragona, M.; Montalbano, G. Urtica dioica Extract Abrogates Chlorpyrifos-Induced Toxicity in Zebrafish Larvae. Int. J. Mol. Sci. 2024, 25, 6631. [Google Scholar] [CrossRef]
- Nencu, I.; Vlase, L.; Istudor, V.; Mircea, T. Preliminary Research Regarding Urtica urens L. and Urtica dioica L. Amino Acids 2015, 63, 710–715. [Google Scholar]
- Sundaram, R.L.; Vasanthi, H.R. Spermacoce Hispida Linn: A Critical Review on Pharmacognosy, Phytochemistry, and Pharmacology Based on Traditional Claims. Phytomedicine Plus 2022, 2, 100143. [Google Scholar] [CrossRef]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Prakash Mishra, A.; Sener, B.; et al. Urtica dioica-Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–30. [Google Scholar] [CrossRef]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica dioica L.): A Review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Semwal, P.; Rauf, A.; Olatunde, A.; Singh, P.; Zaky, M.Y.; Islam, M.M.; Khalil, A.A.; Aljohani, A.S.M.; Al Abdulmonem, W.; Ribaudo, G. The Medicinal Chemistry of Urtica dioica L.: From Preliminary Evidence to Clinical Studies Supporting Its Neuroprotective Activity. Nat. Prod. Bioprospect. 2023, 13, 16. [Google Scholar] [CrossRef]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef]
- Chira, A.; Kadmi, Y.; Badraoui, R.; Aouadi, K.; Alhawday, F.; Boudaya, M.; Jamoussi, K.; Kallel, C.; El Feki, A.; Kadri, A.; et al. GC-MS/MS Analysis and Wound Repair Potential of Urtica dioica Essential Oil: In Silico Modeling and In Vivo Study in Rats. Curr. Pharm. Biotechnol. 2024, 26, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.M.; Moser, S. The Advent of Phyllobilins as Bioactive Phytochemicals—Natural Compounds Derived from Chlorophyll in Medicinal Plants and Food with Immunomodulatory Activities. Pteridines 2023, 34, 20220047. [Google Scholar] [CrossRef]
- Durović, S.; Kojić, I.; Radić, D.; Smyatskaya, Y.A.; Bazarnova, J.G.; Filip, S.; Tosti, T. Chemical Constituents of Stinging Nettle (Urtica dioica L.): A Comprehensive Review on Phenolic and Polyphenolic Compounds and Their Bioactivity. Int. J. Mol. Sci. 2024, 25, 3430. [Google Scholar] [CrossRef]
- Koczkodaj, S.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z.; Bączek, K.B. Intraspecific Variability of Stinging Nettle (Urtica dioica L.). Molecules 2023, 28, 1505. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Shabir, S.; Kauts, S.; Minocha, T.; Obaid, A.A.; Khan, A.A.; Mujalli, A.; Jamous, Y.F.; Almaghrabi, S.; Baothman, B.K.; et al. Appraisal of the Antioxidant Activity, Polyphenolic Content, and Characterization of Selected Himalayan Herbs: Anti-Proliferative Potential in HepG2 Cells. Molecules 2022, 27, 8629. [Google Scholar] [CrossRef] [PubMed]
- Brahmi-Chendouh, N.; Piccolella, S.; Nigro, E.; Hamri-Zeghichi, S.; Madani, K.; Daniele, A.; Pacifico, S. Urtica dioica L. Leaf Chemical Composition: A Never-Ending Disclosure by Means of HR-MS/MS Techniques. J. Pharm. Biomed. Anal. 2021, 195, 113892. [Google Scholar] [CrossRef]
- Đurović, S.; Pezo, L.; Gašić, U.; Gorjanović, S.; Pastor, F.; Bazarnova, J.G.; Smyatskaya, Y.A.; Zeković, Z. Recovery of Biologically Active Compounds from Stinging Nettle Leaves Part II: Processing of Exhausted Plant Material after Supercritical Fluid Extraction. Foods 2023, 12, 809. [Google Scholar] [CrossRef]
- Ogaly, H.A.; Abdel-Rahman, R.F.; Mohamed, M.A.E.; Ahmed-Farid, O.A.; Khattab, M.S.; Abd-Elsalam, R.M. Thymol Ameliorated Neurotoxicity and Cognitive Deterioration in a Thioacetamide-Induced Hepatic Encephalopathy Rat Model; Involvement of the BDNF/CREB Signaling Pathway. Food Funct. 2022, 13, 6180–6194. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.-W.; Li, X.-B.; Chen, Y.; Wang, M.-Y. Chemical constituents from Urtica dioica fruits. Zhongguo Zhong Yao Za Zhi 2022, 47, 4972–4977. [Google Scholar] [CrossRef]
- Abi Sleiman, M.; Younes, M.; Hajj, R.; Salameh, T.; Abi Rached, S.; Abi Younes, R.; Daoud, L.; Doumiati, J.L.; Frem, F.; Ishak, R. Urtica dioica: Anticancer Properties and Other Systemic Health Benefits from In Vitro to Clinical Trials. Int. J. Mol. Sci. 2024, 25, 7501. [Google Scholar] [CrossRef]
- Menzikov, S.A.; Zaichenko, D.M.; Moskovtsev, A.A.; Morozov, S.G.; Kubatiev, A.A. Phenols and GABAA Receptors: From Structure and Molecular Mechanisms Action to Neuropsychiatric Sequelae. Front. Pharmacol. 2024, 15, 1272534. [Google Scholar] [CrossRef] [PubMed]
- Eren, A.; Varol, M.; Unal, R.; Altan, F. Exploring Urtica dioica L. as a Promising Alternative Therapy for Obesity-Related Breast Cancer: Insights from Molecular Mechanisms and Bioinformatic Analysis. Plant Foods Hum. Nutr. 2025, 80, 102. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Ray, R.S.; Sharma, A.; Mehta, V.; Katyal, A.; Udayabanu, M. Antidepressant and Anxiolytic like Effects of Urtica dioica Leaves in Streptozotocin Induced Diabetic Mice. Metab. Brain Dis. 2018, 33, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. Bioactivity-Guided Isolation of Flavonoids from Urtica dioica L. and Their Effect on Endometriosis Rat Model. J. Ethnopharmacol. 2019, 243, 112100. [Google Scholar] [CrossRef]
- Rehman, G.; Khan, I.; Rauf, A.; Rashid, U.; Siddique, A.; Shah, S.M.M.; Akram, Z.; AlMasoud, N.; Alomar, T.S.; Shah, Z.A.; et al. Antidiabetic Properties of Caffeoylmalic Acid, a Bioactive Natural Compound Isolated from Urtica dioica. Fitoterapia 2024, 176, 106024. [Google Scholar] [CrossRef]
- Dar, S.; Yousuf, A.; Ahmad, F.; Sharma, D.P.; Kumar, N.; Singh, D.R. Bioassay Guided Isolation and Identification of Anti-Inflammatory and Anti-Microbial Compounds from Urtica dioica L. (Urticaceae) Leaves. Afr. J. Biotechnol. 2012, 11, 12910–12920. [Google Scholar]
- Gorzalczany, S.; Marrassini, C.; Miño, J.; Acevedo, C.; Ferraro, G. Antinociceptive Activity of Ethanolic Extract and Isolated Compounds of Urtica circularis. J. Ethnopharmacol. 2011, 134, 733–738. [Google Scholar] [CrossRef]
- Francišković, M.; Gonzalez-Pérez, R.; Orčić, D.; Sánchez de Medina, F.; Martínez-Augustin, O.; Svirčev, E.; Simin, N.; Mimica-Dukić, N. Chemical Composition and Immuno-Modulatory Effects of L. (Stinging Nettle) Extracts. Phytother. Res. 2017, 31, 1183–1191. [Google Scholar] [CrossRef]
- Afzal, H.R.; Khan, N.u.H.; Sultana, K.; Mobashar, A.; Lareb, A.; Khan, A.; Gull, A.; Afzaal, H.; Khan, M.T.; Rizwan, M.; et al. Schiff Bases of Pioglitazone Provide Better Antidiabetic and Potent Antioxidant Effect in a Streptozotocin–Nicotinamide-Induced Diabetic Rodent Model. ACS Omega 2021, 6, 4470–4479. [Google Scholar] [CrossRef]
- Haidara, M.; Mikhailidis, D.; Rateb, M.; Ahmed, Z.; Yassin, H.; Ibrahim, I.; Rashed, A. Evaluation of the Effect of Oxidative Stress and Vitamin E Supplementation on Renal Function in Rats with Streptozotocin-Induced Type 1 Diabetes. J. Diabetes Its Complicat. 2008, 23, 130–136. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of Quercetin in Neurological Disorders: From Nutrition to Nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.C.; Prakash, A.; Kalia, A.N. Hepatoprotective Potential of Antioxidant Potent Fraction from Urtica dioica Linn.(Whole Plant) in CCl4 Challenged Rats. Toxicol. Rep. 2015, 2, 1101–1110. [Google Scholar] [CrossRef]
- Nahata, A.; Dixit, V.K. Ameliorative Effects of Stinging Nettle (Urtica dioica) on Testosterone-Induced Prostatic Hyperplasia in Rats: Urtica dioica Attenuates Prostatic Hyperplasia. Andrologia 2012, 44, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Rono, C.K.; Maiyoh, G.K.; Muhanji, C.I. Antihyperglycemic Potential of Urticol from Urtica dioica Leaves Using Freshly Isolated Rat Hepatocytes. Int. J. Sci. Eng. Res. 2015, 6, 277–282. [Google Scholar]
- Mahmood, D.; Khanam, R.; Pillai, K.K.; Akhtar, M. Reversal of Oxidative Stress by Histamine H3 Receptor-Ligands in Experimental Models of Schizophrenia. Arzneimittelforschung 2012, 62, 222–229. [Google Scholar] [CrossRef]
- Khan, A.; Park, J.S.; Kang, M.H.; Lee, H.J.; Ali, J.; Tahir, M.; Choe, K.; Kim, M.O. Caffeic Acid, a Polyphenolic Micronutrient Rescues Mice Brains against Aβ-Induced Neurodegeneration and Memory Impairment. Antioxidants 2023, 12, 1284. [Google Scholar] [CrossRef]
- de Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.V.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased Oxidative Stress in the Prefrontal Cortex as a Shared Feature of Depressive- and PTSD-Like Syndromes: Effects of a Standardized Herbal Antioxidant. Front. Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Kasprzak-Drozd, K.; Oniszczuk, A.; Borowicz-Reutt, K. Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies—A Non-Systematic Review. Int. J. Mol. Sci. 2021, 22, 7366. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Rudkowska, M.; Klimek, K.; Mołdoch, J.; Agacka-Mołdoch, M.; Budzyńska, B.; Oniszczuk, A. S-(+)-Carvone, a Monoterpene with Potential Anti-Neurodegenerative Activity—In Vitro, In Vivo and Ex Vivo Studies. Molecules 2024, 29, 4365. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.-R.; Seigneuret, J.-M.; Auguin, D.; Pichon, C.; Lainé, É. Nettle (Urtica dioica L.) as a Source of Antioxidant and Anti-Aging Phytochemicals for Cosmetic Applications. Comptes Rendus Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Wu, T.; Zhang, W.; Shu, J.; He, Y.; Tang, S.-J. Quercetin Attenuates AZT-Induced Neuroinflammation in the CNS. Sci. Rep. 2018, 8, 6194. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. Protection from Interferon-β-induced Neuronal Apoptosis through Stimulation of Muscarinic Acetylcholine Receptors Coupled to ERK1/2 Activation. Br. J Pharmacol. 2016, 173, 2910–2928. [Google Scholar] [CrossRef]
- Yuan, A.; Gao, M.; Wang, B.; Zeng, N. Cholinergic Anti-Inflammatory Pathway: An Insight into Inflammatory Diseases Treatment with Chinese Herbal Medicine. Preprint on Authorea. Available online: https://www.authorea.com/users/369229/articles/488156-cholinergic-anti-inflammatory-pathway-an-insight-into-inflammatory-diseases-treatment-with-chinese-herbal-medicine (accessed on 7 July 2025).
- Yao, Y.; Baronio, D.; Chen, Y.-C.; Jin, C.; Panula, P. The Roles of Histamine Receptor 1 (Hrh1) in Neurotransmitter System Regulation, Behavior, and Neurogenesis in Zebrafish. Mol. Neurobiol. 2023, 60, 6660–6675. [Google Scholar] [CrossRef] [PubMed]
- Abu Almaaty, A.H.; Mosaad, R.M.; Hassan, M.K.; Ali, E.H.; Mahmoud, G.A.; Ahmed, H.; Anber, N.; Alkahtani, S.; Abdel-Daim, M.M.; Aleya, L. Urtica dioica Extracts Abolish Scopolamine-Induced Neuropathies in Rats. Environ. Sci. Pollut. Res. 2021, 28, 18134–18145. [Google Scholar] [CrossRef]
- Yoo, J.-M.; Lee, B.D.; Sok, D.-E.; Ma, J.Y.; Kim, M.R. Neuroprotective Action of N-Acetyl Serotonin in Oxidative Stress-Induced Apoptosis through the Activation of Both TrkB/CREB/BDNF Pathway and Akt/Nrf2/Antioxidant Enzyme in Neuronal Cells. Redox Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef]
- Karg, C.A.; Parráková, L.; Fuchs, D.; Schennach, H.; Kräutler, B.; Moser, S.; Gostner, J.M. A Chlorophyll-Derived Phylloxanthobilin Is a Potent Antioxidant That Modulates Immunometabolism in Human PBMC. Antioxidants 2022, 11, 2056. [Google Scholar] [CrossRef]
- Gardón, D.P.; Cervantes-Llanos, M.; Matamoros, B.P.; Rodríguez, H.C.; Tan, C.; Marín–Prida, J.; Falcón-Cama, V.; Pavón-Fuentes, N.; Lemus, J.G.; Ruiz, L.d.l.C.B. Positive Effects of Phycocyanobilin on Gene Expression in Glutamate-Induced Excitotoxicity in SH-SY5Y Cells and Animal Models of Multiple Sclerosis and Cerebral Ischemia. Heliyon 2022, 8, e09769. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Marchetti, N.; Bonetti, G.; Brandolini, V.; Cavazzini, A.; Maietti, A.; Meca, G.; Mañes, J. Stinging Nettle (Urtica dioica L.) as a Functional Food Additive in Egg Pasta: Enrichment and Bioaccessibility of Lutein and β-Carotene. J. Funct. Foods 2018, 47, 547–553. [Google Scholar] [CrossRef]
- Roy, A.; Das, S.; Chatterjee, I.; Roy, S.; Chakraborty, R. Anti-Inflammatory Effects of Different Dietary Antioxidants. In Plant Antioxidants and Health; Springer: Cham, Switzerland, 2022; pp. 1–25. ISBN 978-3-030-45299-5. [Google Scholar]
- Dhouafli, Z.; Cuanalo-Contreras, K.; Hayouni, E.A.; Mays, C.E.; Soto, C.; Moreno-Gonzalez, I. Inhibition of protein misfolding and aggregation by natural phenolic compounds. Cell Mol. Life Sci. 2018, 75, 3521–3538. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-κB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better Than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.; Martins, V.; Silva, A.; Rocha, H.; Rachetti, V.; Scortecci, K. Phenolic Acids as Antidepressant Agents. Nutrients 2022, 14, 4309. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Hosseini, Z.; Khatami, A.; Abbasi-kolli, M.; Sadri Nahand, J.; Kouchaki, E.; Mirzaei, H. Neuroprotective Effects of Glycosides. In Phytonutrients and Neurological Disorders; Academic Press: Cambridge, MA, USA, 2023; pp. 201–226. ISBN 978-0-12-824467-8. [Google Scholar]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The What and Who of Dietary Lignans in Human Health: Special Focus on Prooxidant and Antioxidant Effects. Trends Food Sci. Technol. 2020, 106, 382–390. [Google Scholar] [CrossRef]
- Giuliano, C.; Siani, F.; Mus, L.; Ghezzi, C.; Cerri, S.; Pacchetti, B.; Bigogno, C.; Blandini, F. Neuroprotective Effects of Lignan 7-Hydroxymatairesinol (HMR/Lignan) in a Rodent Model of Parkinson’s Disease. Nutrition 2020, 69, 110494. [Google Scholar] [CrossRef]
- Goel, P.; Chakrabarti, S.; Goel, K.; Bhutani, K.; Chopra, T.; Bali, S. Neuronal Cell Death Mechanisms in Alzheimer’s Disease: An Insight. Front. Mol. Neurosci. 2022, 15, 937133. [Google Scholar] [CrossRef]
- Gogoleva, V.S.; Drutskaya, M.S.; Atretkhany, K.S.-N. The Role of Microglia in the Homeostasis of the Central Nervous System and Neuroinflammation. Mol. Biol. 2019, 53, 696–703. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Dakhli, N.; López-Jiménez, A.; Cárdenas, C.; Hraoui, M.; Dhaouafi, J.; Bernal, M.; Sebai, H.; Medina, M.Á. Urtica dioica Aqueous Leaf Extract: Chemical Composition and In Vitro Evaluation of Biological Activities. Int. J. Mol. Sci. 2025, 26, 1220. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, A.D.; Wu, L.-J. How Microglia Sense and Regulate Neuronal Activity. Glia 2021, 69, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mathew, S.; Gamage, R.; Bodkin, F.; Doyle, K.; Rossetti, I.; Wagnon, I.; Zhou, X.; Raju, R.; Gyengesi, E.; et al. From the Bush to the Brain: Preclinical Stages of Ethnobotanical Anti-Inflammatory and Neuroprotective Drug Discovery—An Australian Example. Int. J. Mol. Sci. 2023, 24, 11086. [Google Scholar] [CrossRef]
- Pons, V.; Rivest, S. Targeting Systemic Innate Immune Cells as a Therapeutic Avenue for Alzheimer Disease. Pharmacol. Rev. 2022, 74, 1–17. [Google Scholar] [CrossRef]
- McFarland, K.N.; Chakrabarty, P. Microglia in Alzheimer’s Disease: A Key Player in the Transition between Homeostasis and Pathogenesis. Neurotherapeutics 2023, 19, 186–208. [Google Scholar] [CrossRef]
- Kang, Y.J.; Hyeon, S.J.; McQuade, A.; Lim, J.; Baek, S.H.; Diep, Y.N.; Do, K.V.; Jeon, Y.; Jo, D.; Lee, C.J.; et al. Neurotoxic Microglial Activation via IFNγ-Induced Nrf2 Reduction Exacerbating Alzheimer’s Disease. Adv. Sci. 2024, 11, 2304357. [Google Scholar] [CrossRef]
- Slavich, G.M.; Sacher, J. Stress, Sex Hormones, Inflammation, and Major Depressive Disorder: Extending Social Signal Transduction Theory of Depression to Account for Sex Differences in Mood Disorders. Psychopharmacology 2019, 236, 3063–3079. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of Gut-Brain Axis, Gut Microbial Composition, and Probiotic Intervention in Alzheimer’s Disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Talamonti, E.; Sasso, V.; To, H.; Haslam, R.P.; Napier, J.A.; Ulfhake, B.; Pernold, K.; Asadi, A.; Hessa, T.; Jacobsson, A. Impairment of DHA Synthesis Alters the Expression of Neuronal Plasticity Markers and the Brain Inflammatory Status in Mice. FASEB J. 2020, 34, 2024. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, X.; Wu, Y.; Hu, J.; Li, Y.; Jin, S.; Wu, X. Activation of GPR55 Attenuates Cognitive Impairment, Oxidative Stress, Neuroinflammation, and Synaptic Dysfunction in a Streptozotocin-Induced Alzheimer’s Mouse Model. Pharmacol. Biochem. Behav. 2022, 214, 173340. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Sharma, K.; Tremblay, M.-È. Chronic Stress as a Risk Factor for Alzheimer’s Disease: Roles of Microglia-Mediated Synaptic Remodeling, Inflammation, and Oxidative Stress. Neurobiol. Stress. 2018, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Duran-Prado, M.; Fj, S.-B.; Fj, A.; Jr, P.; Llanos, E.; Henares Chavarino, Á.A.; Pedrero-Prieto, C.; Garcia, S.; Frontiñán-Rubio, J.; Sancho-Bielsa, F.; et al. Interplay Between Mitochondrial Oxidative Disorders and Proteostasis in Alzheimer’s Disease. Front. Neurosci. 2020, 13, 1444. [Google Scholar]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen Synthase Kinase-3 Signaling in Alzheimer’s Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Yao, Y.; Chinnici, C.; Tang, H.; Trojanowski, J.Q.; Lee, V.M.; Praticò, D. Brain Inflammation and Oxidative Stress in a Transgenic Mouse Model of Alzheimer-like Brain Amyloidosis. J. Neuroinflamm. 2004, 1, 21. [Google Scholar] [CrossRef]
- Belkacemi, A.; Ramassamy, C. Time Sequence of Oxidative Stress in the Brain from Transgenic Mouse Models of Alzheimer’s Disease Related to the Amyloid-β Cascade. Free Radic. Biol. Med. 2012, 52, 593–600. [Google Scholar] [CrossRef]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative Stress in Alzheimer’s Disease: Current Knowledge of Signaling Pathways and Therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-W.; Zong, H.-F.; Ma, K.-G.; Zhai, W.-Y.; Yang, W.-N.; Hu, X.-D.; Xu, J.-H.; Chen, X.-L.; Ji, S.-F.; Qian, Y.-H. Activation of A7 Nicotinic Acetylcholine Receptor Alleviates Aβ1-42-Induced Neurotoxicity via Downregulation of P38 and JNK MAPK Signaling Pathways. Neurochem. Int. 2018, 120, 238–250. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Shah, S.Z.A.; Zhao, D.; Hussain, T.; Sabir, N.; Mangi, M.H.; Yang, L. P62-Keap1-NRF2-ARE Pathway: A Contentious Player for Selective Targeting of Autophagy, Oxidative Stress and Mitochondrial Dysfunction in Prion Diseases. Front. Mol. Neurosci. 2018, 11, 310. [Google Scholar] [CrossRef]
- Kaur, K.; Narang, R.K.; Singh, S. Role of Nrf2 in Oxidative Stress, Neuroinflammation and Autophagy in Alzheimer’s Disease: Regulation of Nrf2 by Different Signaling Pathways. Curr. Mol. Med. 2025, 25, 372–387. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Goyal, L.; Singh, S. Tremor and Rigidity in Patients with Parkinson’s Disease: Emphasis on Epidemiology, Pathophysiology and Contributing Factors. CNS Neurol. Disord.-Drug Targets. 2022, 21, 596–609. [Google Scholar] [CrossRef]
- Váradi, C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Rawat, C.S.; Pandey, S. Parkinson’s Disease–An Introduction. In Techniques for Assessment of Parkinsonism for Diagnosis and Rehabilitation; Arjunan, S.P., Kumar, D.K., Eds.; Series in BioEngineering; Springer Singapore: Singapore, 2022; pp. 1–24. ISBN 9789811630552. [Google Scholar]
- Tröster, A.I. Parkinson’s Disease and Parkinsonism. In APA Handbook of Neuropsychology: Neurobehavioral Disorders and Conditions: Accepted Science and Open Questions; American Psychological Association: Washington, DC, USA, 2023. [Google Scholar]
- Jankovic, J.; Lang, A.E. Diagnosis and Assessment of Parkinson Disease and Other Movement Disorders. In Bradley and Daroff’s Neurology in Clinical Practice, 8th ed.; Daroff, R.B., Jankovic, J., Mazziotta, J.C., Pomeroy, S.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 310–337. [Google Scholar]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in Multiple Sclerosis. WIREs Mech. Dis. 2023, 15, e1583. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. [Google Scholar] [CrossRef]
- Celarain, N.; Tomas-Roig, J. Aberrant DNA Methylation Profile Exacerbates Inflammation and Neurodegeneration in Multiple Sclerosis Patients. J. Neuroinflamm. 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Santos, C.L.; De Souza Almeida, R.R.; Da Silva, A.; Thomaz, N.K.; Costa, N.L.F.; Weber, F.B.; Schmitz, I.; Medeiros, L.S.; Medeiros, L.; et al. Gliotoxicity and Glioprotection: The Dual Role of Glial Cells. Mol. Neurobiol. 2021, 58, 6577–6592. [Google Scholar] [CrossRef]
- Barrie, W.; Yang, Y.; Irving-Pease, E.K.; Attfield, K.E.; Scorrano, G.; Jensen, L.T.; Armen, A.P.; Dimopoulos, E.A.; Stern, A.; Refoyo-Martinez, A.; et al. Elevated Genetic Risk for Multiple Sclerosis Emerged in Steppe Pastoralist Populations. Nature 2024, 625, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Läderach, F.; Münz, C. Epstein Barr Virus Exploits Genetic Susceptibility to Increase Multiple Sclerosis Risk. Microorganisms 2021, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Ishina, I.A.; Zakharova, M.Y.; Kurbatskaia, I.N.; Mamedov, A.E.; Belogurov, A.A., Jr.; Gabibov, A.G. MHC class II presentation in autoimmunity. Cells 2023, 12, 314. [Google Scholar] [CrossRef]
- Accogli, T.; Bruchard, M.; Végran, F. Modulation of CD4 T Cell Response According to Tumor Cytokine Microenvironment. Cancers 2021, 13, 373. [Google Scholar] [CrossRef]
- Rizea, R.E.; Corlatescu, A.-D.; Costin, H.P.; Dumitru, A.; Ciurea, A.V. Understanding Amyotrophic Lateral Sclerosis: Pathophysiology, Diagnosis, and Therapeutic Advances. Int. J. Mol. Sci. 2024, 25, 9966. [Google Scholar] [CrossRef]
- Ashok Verma, M.D. Clinical Manifestation and Management of Amyotrophic Lateral Sclerosis. Exon Publ. 2021, 1–14. [Google Scholar] [CrossRef]
- Shoesmith, C. Chapter 9—Palliative Care Principles in ALS. In Handbook of Clinical Neurology; Miyasaki, J.M., Kluger, B.M., Eds.; Neuropalliative Care, Part II; Elsevier: Amsterdam, The Netherlands, 2023; Volume 191, pp. 139–155. [Google Scholar]
- Rusina, R.; Vandenberghe, R.; Bruffaerts, R. Cognitive and Behavioral Manifestations in ALS: Beyond Motor System Involvement. Diagnostics 2021, 11, 624. [Google Scholar] [CrossRef]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Emerging Insights into the Complex Genetics and Pathophysiology of Amyotrophic Lateral Sclerosis. Lancet Neurol. 2022, 21, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Miao, Z.; Liu, Y.; Chen, X.; Wang, H.; Su, J.; Chen, J. The Brain–Gut–Bone Axis in Neurodegenerative Diseases: Insights, Challenges, and Future Prospects. Adv. Sci. 2024, 11, 2307971. [Google Scholar] [CrossRef]
- Rummens, J.; Da Cruz, S. RNA-Binding Proteins in ALS and FTD: From Pathogenic Mechanisms to Therapeutic Insights. Mol. Neurodegener. 2025, 20, 64. [Google Scholar] [CrossRef]
- Bartl, S.; Xie, Y.; Potluri, N.; Kesineni, R.; Hencak, K.; Cengio, L.D.; Balazs, K.; Oueslati, A.; Parth, M.; Salhat, N.; et al. Reducing Huntingtin by Immunotherapy Delays Disease Progression in a Mouse Model of Huntington Disease. Neurobiol. Dis. 2024, 190, 106376. [Google Scholar] [CrossRef] [PubMed]
- Holen, M.M. Characterization of Genes and Proteins Related to Chitin Metabolism in Atlantic Salmon. Ph.D. Thesis, Norwegian University of Life Sciences, Ås, Norway, 2022. [Google Scholar]
- Migliaccio, R.; Tanguy, D.; Bouzigues, A.; Sezer, I.; Dubois, B.; Le Ber, I.; Batrancourt, B.; Godefroy, V.; Levy, R. Cognitive and Behavioural Inhibition Deficits in Neurodegenerative Dementias. Cortex 2020, 131, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.E.; Samant, R.S. Alternative Systems for Misfolded Protein Clearance: Life beyond the Proteasome. FEBS J. 2021, 288, 4464–4487. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-Induced Excitotoxicity in Parkinson’s Disease: The Role of Glial Cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Gatto, E.M.; Rojas, N.G.; Persi, G.; Etcheverry, J.L.; Cesarini, M.E.; Perandones, C. Huntington Disease: Advances in the Understanding of Its Mechanisms. Clin. Park. Relat. Disord. 2020, 3, 100056. [Google Scholar] [CrossRef]
- Tunalı, N.E. Neurodegenerative Diseases: Molecular Mechanisms and Current Therapeutic Approaches; BoD—Books on Demand: Norderstedt, Germany, 2021; ISBN 978-1-83880-149-6. [Google Scholar]
- Watson, N.; Brandel, J.-P.; Green, A.; Hermann, P.; Ladogana, A.; Lindsay, T.; Mackenzie, J.; Pocchiari, M.; Smith, C.; Zerr, I. The Importance of Ongoing International Surveillance for Creutzfeldt–Jakob Disease. Nat. Rev. Neurol. 2021, 17, 362–379. [Google Scholar] [CrossRef]
- Ritchie, D.L.; Barria, M.A. Prion Diseases: A Unique Transmissible Agent or a Model for Neurodegenerative Diseases? Biomolecules 2021, 11, 207. [Google Scholar] [CrossRef]
- Salehi, P.; Clark, M.; Pinzon, J.; Patil, A. Sporadic Creutzfeldt-Jakob Disease. Am. J. Emerg. Med. 2022, 52, e1–e267. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F. A Literature Review of Movement Disorder Associated with Medications and Systemic Diseases. Preprints 2024, 2024020070. [Google Scholar] [CrossRef]
- Saitoh, Y.; Mizusawa, H. Prion Diseases, Always a Threat? J. Neurol. Sci. 2024, 463, 123119. [Google Scholar] [CrossRef] [PubMed]
- Jaunmuktane, Z.; Brandner, S. Invited Review: The Role of Prion-like Mechanisms in Neurodegenerative Diseases. Neuropathol. Appl. Neurobiol. 2020, 46, 522–545. [Google Scholar] [CrossRef]
- Tiffin, H. Foot in Mouth: Animals, Disease, and the Cannibal Complex. Mosaic Interdiscip. Crit. J. 2021, 54, 131–146. [Google Scholar] [CrossRef]
- Beasley, A.N. Doktor Bilong Kuru; Xlibris Corporation: Bloomington, IN, USA, 2024; ISBN 978-1-66988-097-4. [Google Scholar]
- Srivastava, N.K.; Yadav, R.; Mukherjee, S. Chapter 5—Interconnectivity of Gene, Immune System, and Metabolism in the Muscle Pathology of Duchenne Muscular Dystrophy (DMD). In The Molecular Immunology of Neurological Diseases; Kumar, S., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 51–74. ISBN 978-0-12-821974-4. [Google Scholar]
- Felling, R.J.; Sun, L.R. Stroke in Neonates. In Principles of Neonatology; Maheshwari, A., Ed.; Elsevier: New Delhi, India, 2024; pp. 438–443. ISBN 978-0-323-69415-5. [Google Scholar]
- Whitaker, E.E.; Cipolla, M.J. Chapter 16—Perinatal Stroke. In Handbook of Clinical Neurology; Steegers, E.A.P., Cipolla, M.J., Miller, E.C., Eds.; Neurology and Pregnancy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 171, pp. 313–326. [Google Scholar]
- Elgendy, M.M.; Puthuraya, S.; LoPiccolo, C.; Liu, W.; Aly, H.; Karnati, S. Neonatal Stroke: Clinical Characteristics and Neurodevelopmental Outcomes. Pediatr. Neonatol. 2022, 63, 41–47. [Google Scholar] [CrossRef]
- Thomas, S.; Conway, K.M.; Fapo, O.; Street, N.; Mathews, K.D.; Mann, J.R.; Romitti, P.A.; Soim, A.; Westfield, C.; Fox, D.J.; et al. Time to Diagnosis of Duchenne Muscular Dystrophy Remains Unchanged: Findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000–2015. Muscle Nerve 2022, 66, 193–197. [Google Scholar] [CrossRef]
- Darmahkasih, A.J.; Rybalsky, I.; Tian, C.; Shellenbarger, K.C.; Horn, P.S.; Lambert, J.T.; Wong, B.L. Neurodevelopmental, Behavioral, and Emotional Symptoms Common in Duchenne Muscular Dystrophy. Muscle Nerve 2020, 61, 466–474. [Google Scholar] [CrossRef]
- Limback, K.; Jacobus, W.; Wiggins-McDaniel, A.; Newman, R.; White, R. A Comprehensive Review of Duchenne Muscular Dystrophy: Genetics, Clinical Presentation, Diagnosis, and Treatment. Biotechnol. J. Int. 2022, 26, 1–31. [Google Scholar] [CrossRef]
- Kumar, S.H.; Athimoolam, K.; Suraj, M.; Das, M.S.D.C.; Muralidharan, A.; Jeyam, D.; Ashokan, J.; Karthikeyan, P.; Krishna, R.; Khanna-Gupta, A.; et al. Comprehensive Genetic Analysis of 961 Unrelated Duchenne Muscular Dystrophy Patients: Focus on Diagnosis, Prevention and Therapeutic Possibilities. PLoS ONE 2020, 15, e0232654. [Google Scholar] [CrossRef]
- Zabłocka, B.; Górecki, D.C.; Zabłocki, K. Disrupted Calcium Homeostasis in Duchenne Muscular Dystrophy: A Common Mechanism behind Diverse Consequences. Int. J. Mol. Sci. 2021, 22, 11040. [Google Scholar] [CrossRef]
- Elasbali, A.M.; Al-Soud, W.A.; Anwar, S.; Alhassan, H.H.; Adnan, M.; Hassan, M.I. A Review on Mechanistic Insights into Structure and Function of Dystrophin Protein in Pathophysiology and Therapeutic Targeting of Duchenne Muscular Dystrophy. Int. J. Biol. Macromol. 2024, 264, 130544. [Google Scholar] [CrossRef]
- Dowling, P.; Swandulla, D.; Ohlendieck, K. Cellular Pathogenesis of Duchenne Muscular Dystrophy: Progressive Myofibre Degeneration, Chronic Inflammation, Reactive Myofibrosis and Satellite Cell Dysfunction. Eur. J. Transl. Myol. 2023, 33, 11856. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, A.E.; Alkhalifa, O.; Durdanovic, I.; Ibrahim, D.R.; Maragkou, S. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease: Insights into Pathophysiology and Treatment. J. Dement. Alzheimer’s Dis. 2025, 2, 17. [Google Scholar] [CrossRef]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef]
- Wang, P.-F.; Jiang, F.; Zeng, Q.-M.; Yin, W.-F.; Hu, Y.-Z.; Li, Q.; Hu, Z.-L. Mitochondrial and Metabolic Dysfunction of Peripheral Immune Cells in Multiple Sclerosis. J. Neuroinflamm. 2024, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xu, R. Current Insights in the Molecular Genetic Pathogenesis of Amyotrophic Lateral Sclerosis. Front. Neurosci. 2023, 17, 1189470. [Google Scholar] [CrossRef]
- Du, X.; Dong, Q.; Zhu, J.; Li, L.; Yu, X.; Liu, R. Rutin Ameliorates ALS Pathology by Reducing SOD1 Aggregation and Neuroinflammation in an SOD1-G93A Mouse Model. Int. J. Mol. Sci. 2024, 25, 10392. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Vitkauskaitė, M.; Paulauskienė, A.; Černiauskienė, J. Wild Stinging Nettle (Urtica dioica L.) Leaves and Roots Chemical Composition and Phenols Extraction. Plants 2023, 12, 309. [Google Scholar] [CrossRef]
- Jurcau, A. Molecular Pathophysiological Mechanisms in Huntington’s Disease. Biomedicines 2022, 10, 1432. [Google Scholar] [CrossRef]
- Franco-Iborra, S.; Plaza-Zabala, A.; Montpeyo, M.; David, S.; Vila, M.; Martinez-Vicente, M. Mutant HTT (Huntingtin) Impairs Mitophagy in a Cellular Model of Huntington Disease. Autophagy 2021, 17, 672–689. [Google Scholar] [CrossRef] [PubMed]
- Taran, A.S.; Shuvalova, L.D.; Lagarkova, M.A.; Alieva, I.B. Huntington’s Disease—An Outlook on the Interplay of the HTT Protein, Microtubules and Actin Cytoskeletal Components. Cells 2020, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Mailo, J.; Dunbar, M. Perinatal Stroke in Fetuses, Preterm and Term Infants. In Seminars in Pediatric Neurology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 43, p. 100988. [Google Scholar]
- Alimoddin, M.; Jayakumari, S.; Fatima, B.; Hasan, N.; Ali, S.; Sami, F.; Ali, S.; Nair, R.S.; Ansari, M.T. Pharmacological Applications of Urtica dioica: A Comprehensive Review of Its Traditional Use and Modern Scientific Evidence. J. Herb. Med. 2024, 48, 100935. [Google Scholar] [CrossRef]
- Hedayati Ch, M.; Abedinzade, M.; Khanaki, K.; Khakpour Tleghani, B.; Golshekan, M.; Mohammadi, E. Comparative Protective Effects of Viola Spathulata, Urtica dioica, and Lamium Album on Endoplasmic Reticulum (ER) Stress in Rat Stroke Model. Casp. J. Neurol. Sci. 2021, 7, 172–179. [Google Scholar] [CrossRef]
- Moda, F.; Ciullini, A.; Dellarole, I.; Lombardo, A.; Campanella, N.; Bufano, G.; Giaccone, G. Secondary protein aggregates in neurodegenerative diseases: Almost the rule rather than the exception. Front. Biosci. 2023, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tahir, W.; Zafar, S.; Llorens, F.; Arora, A.S.; Thüne, K.; Schmitz, M.; Gotzmann, N.; Kruse, N.; Mollenhauer, B.; Torres, J.M. Molecular Alterations in the Cerebellum of Sporadic Creutzfeldt–Jakob Disease Subtypes with DJ-1 as a Key Regulator of Oxidative Stress. Mol. Neurobiol. 2018, 55, 517–537. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Charfi, S.; Marekchi, R.; Hammami, S.; Zeghal, K.; Ksouda, K. Protective Effect of Urtica dioica in Induced Neurobehavioral Changes, Nephrotoxicity and Hepatotoxicity after Chronic Exposure to Potassium Bromate in Rats. Environ. Pollut. 2021, 287, 117657. [Google Scholar] [CrossRef]
- Mareedu, S.; Million, E.D.; Duan, D.; Babu, G.J. Abnormal Calcium Handling in Duchenne Muscular Dystrophy: Mechanisms and Potential Therapies. Front. Physiol. 2021, 12, 647010. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Martorell, M.; Sharopov, F.; Tumer, T.B.; Kurt, B.; Lankatillake, C.; Docea, A.O.; Moreira, A.C.; et al. A Pharmacological Perspective on Plant-Derived Bioactive Molecules for Epilepsy. Neurochem. Res. 2021, 46, 2205–2225. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rapposelli, S.; Sestito, S.; Herrera-Bravo, J.; Arancibia-Diaz, A.; Salazar, L.A.; Yeskaliyeva, B.; Beyatli, A.; Leyva-Gómez, G.; González-Contreras, C.; et al. Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. J. Pers. Med. 2022, 12, 1515. [Google Scholar] [CrossRef]
- Patel, S.S.; Udayabanu, M. Urtica dioica Extract Attenuates Depressive like Behavior and Associative Memory Dysfunction in Dexamethasone Induced Diabetic Mice. Metab. Brain Dis. 2014, 29, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Vinod, Y.; Sarkar, D.; Wal, A.; Verma, V.; Wal, P. Urtica dioica (Stinging Nettle): A Comprehensive & Concise Review on Its Nutritional Profile and Therapeutic Applications. Curr. Tradit. Med. 2023, 10, e120923220975. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).