The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits
Abstract
:1. Introduction
1.1. The Vast Array of Secondary Metabolites of Avocado and Their Biological Significance
1.2. Nutritional Composition of P. americana
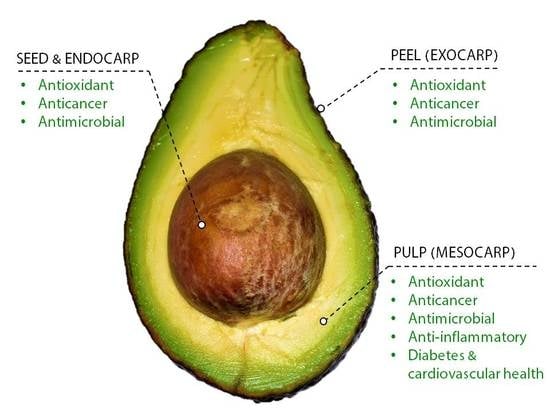
1.3. Antioxidant Properties of P. americana
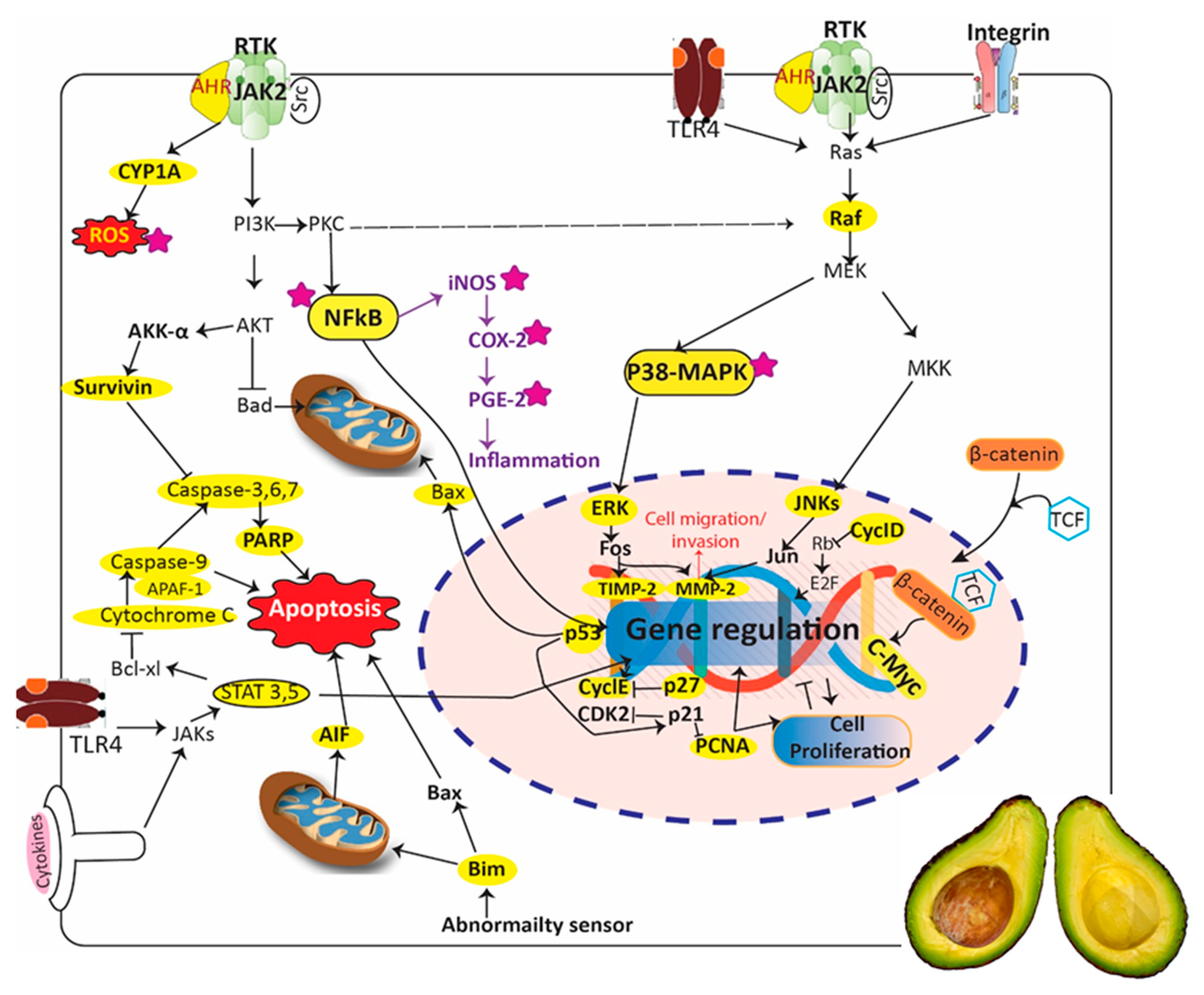
1.4. Anticancer Properties of P. americana
1.5. Antimicrobial Properties of P. americana
1.6. Anti-Inflammatory Properties of P. americana
1.7. Effect of P. americana on Cardiovascular Health and Diabetes
1.8. Bioavailability and Pharmacokinetic of Compounds from P. americana
2. Conclusions and Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Bergh, B.; Ellstrand, N. Taxonomy of the avocado. Calif. Avocado Soc. Yearb. 1986, 70, 135–145. [Google Scholar]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef]
- Cowan, A.K.; Wolstenholme, B.N. Avocado. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 294–300. [Google Scholar]
- Taulavuori, K.; Julkunen-Tiitto, R.; Hyöky, V.; Taulavuori, E. Blue Mood for Superfood. Nat. Prod. Commun. 2013, 8, 1934578X1300800627. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, G.; Martin-Smith, J.; Sullivan, P. The Avocado Hand. Ir. Med. J. 2017, 110, 658. [Google Scholar] [PubMed]
- Agricultural Marketing Resource Center. Avocados; Iowa State University in Ames: Ames, IA, USA, 2018. [Google Scholar]
- Combined Chemical Dictionary 23.1; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Available online: http://ccd.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml;jsessionid=7B7405700267BD91E58E52C6333BF438 (accessed on 1 August 2019).
- The Human Metabolome Database. 2019. Available online: http://www.hmdb.ca/ (accessed on 1 August 2019).
- Yasir, M.; Das, S.; Kharya, M.D. The phytochemical and pharmacological profile of Persea americana Mill. Pharmacogn. Rev. 2010, 4, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.H.; Tseng, C.K.; Wu, H.C.; Wei, C.K.; Lin, C.K.; Chen, I.S.; Chang, H.S.; Lee, J.C. Avocado (Persea americana) fruit extract (2R,4R)-1,2,4-trihydroxyheptadec-16-yne inhibits dengue virus replication via upregulation of NF-kappaB-dependent induction of antiviral interferon responses. Sci. Rep. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Adikaram, N.K.B.; Ewing, D.F.; Karunaratne, A.M.; Wijeratne, E.M.K. Antifungal Compounds from Immature Avocado Fruit Peel. Phytochemistry 1992, 31, 93–96. [Google Scholar] [CrossRef]
- Abe, F.; Nagafuji, S.; Okawa, M.; Kinjo, J.; Akahane, H.; Ogura, T.; Martinez-Alfaro, M.A.; Reyes-Chilpa, R. Trypanocidal Constituents in Plants 5. Evaluation of some mexican plants for their trypanocidal activity and active constituents in the seeds of Persea americana. Biol. Pharm. Bull. 2005, 28, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Chang, H.S.; Peng, C.F.; Lin, C.H.; Chen, I.S. Secondary metabolites from the unripe pulp of Persea americana and their antimycobacterial activities. Food Chem. 2012, 135, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Domergue, F.; Helms, G.L.; Prusky, D.; Browse, J. Antifungal compounds from idioblast cells isolated from avocado fruits. Phytochemistry 2000, 54, 183–189. [Google Scholar] [CrossRef]
- Lee, T.-H.; Tsai, Y.-F.; Huang, T.-T.; Chen, P.-Y.; Liang, W.-L.; Lee, C.-K. Heptadecanols from the leaves of Persea americana var. americana. Food Chem. 2012, 132, 921–924. [Google Scholar] [CrossRef]
- Bull, S.D.; Carman, R.M. Synthesis of the Avocado Antifungal,(Z, Z)-2-Hydroxy-4-oxohenicosa-12, 15-dien-1-yl Acetate. Aust. J. Chem. 1994, 47, 1661–1672. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Millar, J.G.; Trumble, J.T. Isolation, Identification, and Biological Activity of Isopersin, a New Compound from Avocado Idioblast Oil Cells. J. Nat. Prod. 1998, 61, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Kashman, Y.; Néeman, I.; Lifshitz, A. New compounds from avocado pear. Tetrahedron 1969, 25, 4617–4631. [Google Scholar] [CrossRef]
- Kim, O.K.; Murakami, A.; Nakamura, Y.; Takeda, N.; Yoshizumi, H.; Ohigashi, H. Novel Nitric Oxide and Superoxide Generation Inhibitors, Persenone A and B, from Avocado Fruit. J. Agric. Food Chem. 2000, 48, 1557–1563. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, C.-H.; Wong, C.-H.; Liu, Y.-W.; Lin, Y.-S.; Wang, Y.-D.; Hsui, Y.-R. Cytotoxic Constituents of the Stems of Cinnamomum subavenium. J. Nat. Prod. 2007, 70, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, N.H.; Rogers, L.L.; Martin, J.M.; McLaughlin, J.L. Cytotoxic and insecticidal constituents of the unripe fruit of Persea americana. J. Nat. Prod. 1998, 61, 781–785. [Google Scholar] [CrossRef]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Neuroprotective effects of methanolic extract of avocado Persea americana (var. Colinred) peel on paraquat-induced locomotor impairment, lipid peroxidation and shortage of life span in transgenic knockdown parkin drosophila melanogaster. Neurochem. Res. 2019, 44, 1986–1998. [Google Scholar] [CrossRef]
- Ramos-Jerz Mdel, R.; Villanueva, S.; Jerz, G.; Winterhalter, P.; Deters, A.M. Persea americana Mill. Seed: Fractionation, Characterization, and Effects on Human Keratinocytes and Fibroblasts. Evid. Based Complement. Altern. Med. 2013, 2013, 391247. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Arteaga, J.R.; Zhang, Q.; Huerta, S.; Go, V.L.; Heber, D. Inhibition of prostate cancer cell growth by an avocado extract: Role of lipid-soluble bioactive substances. J. Nutr. Biochem. 2005, 16, 23–30. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.S.; Paul, S.; Dutta, S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Anti-oncogenic potentials of a plant coumarin (7-hydroxy-6-methoxy coumarin) against 7,12-dimethylbenz [a] anthracene-induced skin papilloma in mice: The possible role of several key signal proteins. Chin. J. Integr. Med. 2010, 8, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Kunesch, G.; Martin-Tanguy, J.; Negrel, J.; Paynot, M.; Carre, M. Effect of cinnamoyl putrescines on in vitro cell multiplication and differentiation of tobacco explants. Plant Cell. Rep. 1985, 4, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Santana, I.; Castelo-Branco, V.N.; Guimarães, B.M.; Silva, L.d.O.; Peixoto, V.O.D.S.; Cabral, L.M.C.; Freitas, S.P.; Torres, A.G. Hass avocado (Persea americana Mill.) oil enriched in phenolic compounds and tocopherols by expeller-pressing the unpeeled microwave dried fruit. Food Chem. 2019, 286, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Gabai, M.; Lifshitz, A.; Sklarz, B. Structures of some carotenoids from the pulp of Persea americana. Phytochemistry 1974, 13, 1917–1921. [Google Scholar] [CrossRef]
- Gross, J.; Gabai, M.; Lifshitz, A.; Sklarz, B. Carotenoids in pulp, peel and leaves of Persea americana. Phytochemistry 1973, 12, 2259–2263. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Maynard, D.F.; Phillips, S.; Trumble, J.T. Alkylfurans: Effects of Alkyl Side-Chain Length on Insecticidal Activity. J. Nat. Prod. 1999, 62, 191–193. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Millar, J.G.; Maynard, D.F.; Trumble, J.T. Novel Antifeedant and Insecticidal Compounds from Avocado Idioblast Cell Oil. J. Chem. Ecol. 1998, 24, 867–889. [Google Scholar] [CrossRef]
- Fraga, B.M.; Terrero, D. Alkene-γ-lactones and avocadofurans from Persea indica: A revision of the structure of majorenolide and related lactones. Phytochemistry 1996, 41, 229–232. [Google Scholar] [CrossRef]
- Rosenblat, G.; Kagan, H.M.; Shah, M.A.; Spiteller, G.; Neeman, I. Chemical characterization of lysyl oxidase inhibitor from avocado seed oil. J. Am. Oil Chem. Soc. 1995, 72, 225–229. [Google Scholar] [CrossRef]
- Zaki, A.; Zentmyer, G.; Pettus, J.; Sills, J.; Keen, N.; Sing, V. Borbonol from Persea spp.-chemical properties and antifungal activity against Phytophthora cinnamomi. Physiol. Plant Pathol. 1980, 16, 205–212. [Google Scholar] [CrossRef]
- Falodun, A.; Engel, N.; Kragl, U.; Nebe, B.; Langer, P. Novel anticancer alkene lactone from Persea americana. Pharm. Biol. 2013, 51, 700–706. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, C.-H.; Lo, Y.-C.; Wu, B.-N.; Wang, H.-M.; Lo, W.-L.; Yen, C.-M.; Lin, R.-J. Anticancer Activity of Isoobtusilactone A from Cinnamomum kotoense: Involvement of Apoptosis, Cell-Cycle Dysregulation, Mitochondria Regulation, and Reactive Oxygen Species. J. Nat. Prod. 2008, 71, 933–940. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Maynard, D.F.; Phillips, S.; Trumble, J.T. Avocadofurans and their tetrahydrofuran analogues: Comparison of growth inhibitory and insecticidal activity. J. Agric. Food Chem. 2000, 48, 3642–3645. [Google Scholar] [CrossRef]
- Fraga, B.M.; González-Coloma, A.; Gutiérrez, C.; Terrero, D. Insect Antifeedant Isoryanodane Diterpenes from Persea indica. J. Nat. Prod. 1997, 60, 880–883. [Google Scholar] [CrossRef]
- Han, A.; Tao, Y.; Reisman, S.E. 16-Step Synthesis of the Isoryanodane Diterpene (+)-Perseanol. ChemRxiv. Preprint. 2019, in press. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Hernandez, M.G.; Perales, A.; Fraga, B.M. Chemical ecology of canarian laurel forest: Toxic diterpenes from Persea indica (Lauraceae). J. Chem. Ecol. 1990, 16, 2723–2733. [Google Scholar] [CrossRef]
- Fraga, B.M.; Terrero, D.; Gutiérrez, C.; González-Coloma, A. Minor diterpenes from Persea indica: Their antifeedant activity. Phytochemistry 2001, 56, 315–320. [Google Scholar] [CrossRef]
- Gonzĺez-Coloma, A.; Cabrera, R.; Socorro Monzón, A.R.; Frag, B.M. Persea indica as a natural source of the insecticide ryanodol. Phytochemistry 1993, 34, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Hann, R.M.; Hudson, C.S. Proof of the Structure and Configuration of Perseulose (L-Galaheptulose). J. Am. Chem. Soc. 1939, 61, 336–340. [Google Scholar] [CrossRef]
- Sephton, H.H.; Richtmyer, N.K. The isolation of D-erythro-L-galacto-nonulose from the avocado, together with its synthesis and proof of structure through reduction to D-arabino-D-manno-nonitol and D-arabino-D-gluco-nonitol. Carbohyd. Res. 1966, 2, 289–300. [Google Scholar] [CrossRef]
- Sephton, H.H.; Richtmyer, N.K. Isolation of D-erythro-L-gluco-Nonulose from the Avocado1. J. Org. Chem. 1963, 28, 2388–2390. [Google Scholar] [CrossRef]
- Charlson, A.J.; Richtmyer, N.K. The Isolation of an octulose and an octitol from natural sources: D-glycero-D-manno-Octulose and D-erythro-D-galacto-octitol from the avocado and D-glycero-D-manno-octulose from Sedum species1,2. J. Am. Chem. Soc. 1960, 82, 3428–3434. [Google Scholar] [CrossRef]
- Ian-Lih, T.; Chih-Feng, H.; Chang-Yih, D.; Ih-Sheng, C. Cytotoxic neolignans from Persea obovatifolia. Phytochemistry 1996, 43, 1261–1263. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, W. Asymmetric synthesis of machilin C and its analogue. Chem. Pap. 2010, 64, 630–636. [Google Scholar] [CrossRef]
- Ward, R.S. Lignans neolignans, and related compounds. Nat. Prod. Rep. 1993, 10, 1–28. [Google Scholar] [CrossRef]
- Tsai, I.-L.; Hsieh, C.-F.; Duh, C.-Y.; Chen, I.-S. Further study on the chemical constituents and their cytotoxicity from the leaves of Persea obovatifolia. Chin. Pharm. J. 1999, 51, 335–346. [Google Scholar]
- Tsai, I.-L.; Hsieh, C.-F.; Duh, C.-Y. Additional cytotoxic neolignans from Persea obovatifolia. Phytochemistry 1998, 48, 1371–1375. [Google Scholar] [CrossRef]
- Sepulveda-Boza, S.; Delhvi, S.; Cassels, B.K. An aryltetralin lignan from Persea lingue. Phytochemistry 1990, 29, 2357–2358. [Google Scholar] [CrossRef]
- Chang, C.-F.; Isogai, A.; Kamikado, T.; Murakoshi, S.; Sakurai, A.; Tamura, S. Isolation and structure elucidation of growth inhibitors for silkworm larvae from avocado leaves. Agric. Biol. Chem. 1975, 39, 1167–1168. [Google Scholar] [CrossRef]
- El Kharrassi, Y.; Samadi, M.; Lopez, T.; Nury, T.; El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; Vamecq, J.; Moustaid, K.; Latruffe, N.; et al. Biological activities of Schottenol and Spinasterol, two natural phytosterols present in argan oil and in cactus pear seed oil, on murine miroglial BV2 cells. Biochem. Biophys. Res. Commun. 2014, 446, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, A.; Kubota, T.; Asaka, Y. Perseapicroside A, hexanorcucurbitacin-type glucopyranoside from Persea mexicana. Phytochemistry 1990, 29, 1330–1332. [Google Scholar] [CrossRef]
- Cascinu, S.; Catalano, V.; Cordella, L.; Labianca, R.; Giordani, P.; Baldelli, A.M.; Beretta, G.D.; Ubiali, E.; Catalano, G. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: A randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2002, 20, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.F.; Bowman, A.; Perren, T.; Wilkinson, P.; Prescott, R.J.; Quinn, K.J.; Tedeschi, M. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: Results of a double-blind, randomised trial. Ann. Oncol. 1997, 8, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Flagg, E.W.; Coates, R.J.; Jones, D.P.; Byers, T.E.; Greenberg, R.S.; Gridley, G.; McLaughlin, J.K.; Blot, W.J.; Haber, M.; Preston-Martin, S.; et al. Dietary Glutathione Intake and the Risk of Oral and Pharyngeal Cancer. Am. J. Epidemiol. 1994, 139, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Vajczikova, I. Variations in the essential oil composition of Persea bombycina (King ex Hook. f.) Kost and its effect on muga silkworm (Antheraea assama Ww)—A new report. Indian J. Chem. B 2003, 42B, 641–647. [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service. Avocados, Raw, California. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171706/nutrients (accessed on 22 September 2019).
- Duarte, P.F.; Chaves, M.A.; Borges, C.D.; Mendonça, C.R.B. Avocado: Characteristics, health benefits and uses. Cienc. Rural 2016, 46, 747–754. [Google Scholar] [CrossRef]
- Duester, K.C. Avocados a look beyond basic nutrition for one of nature’s whole foods. Nutr. Today 2000, 35, 151–157. [Google Scholar] [CrossRef]
- Bao, J.; Atkinson, F.; Petocz, P.; Willett, W.C.; Brand-Miller, J.C. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: Glycemic load compared with carbohydrate content alone. Am. J. Clin. Nutr. 2011, 93, 984–996. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Landahl, S.; Meyer, M.D.; Terry, L.A. Spatial and temporal analysis of textural and biochemical changes of imported avocado cv. Hass during fruit ripening. J. Agric. Food Chem. 2009, 57, 7039–7047. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bordi, P.L.; Fleming, J.A.; Hill, A.M.; Kris-Etherton, P.M. Effect of a moderate fat diet with and without avocados on lipoprotein particle number, size and subclasses in overweight and obese adults: A randomized, controlled trial. J. Am. Heart Assoc. 2015, 4, e001355. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Thiagarajan, P. A review on Persea americana Mill.(avocado)-its fruits and oil. Int. J. Pharmtech Res. 2015, 8, 72–77. [Google Scholar]
- de Melo, M.F.F.T.; Pereira, D.E.; Moura, R.d.L.; da Silva, E.B.; de Melo, F.A.L.T.; Dias, C.d.C.Q.; Silva, M.d.C.A.; de Oliveira, M.E.G.; Viera, V.B.; Pintado, M.M.E.; et al. Maternal supplementation with avocado (Persea americana Mill.) pulp and oil alters reflex maturation, physical development, and offspring memory in rats. Front. Neurosci. 2019, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.P.; Bernal, E.J.; Velásquez, M.A.; Cartagena, V.J.R. Fatty acid content of avocados (Persea americana Mill. cv. Hass) in relation to orchard altitude and fruit maturity stage. Agron. Colomb. 2015, 33, 220–227. [Google Scholar] [CrossRef]
- Swisher, H.E. Avocado oil. J. Am. Oil Chem. Soc. 1988, 65, 1704–1706. [Google Scholar] [CrossRef]
- Murray, M.T.; Pizzorno, J. The Encyclopedia of Healing Foods; Simon and Schuster: NewYork, NY, USA, 2010. [Google Scholar]
- Lidia, D.-A.; Alicia, O.-M.; Felipe, G.-O. Avocado. In Tropical and Subtropical Fruits; Muhammad, S., Ed.; Wiley: Hoboken, NJ, USA, 2012; Volume 1, pp. 435–454. [Google Scholar]
- Dabas, D.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Avocado (Persea americana) seed as a source of bioactive phytochemicals. Curr. Pharm. Des. 2013, 19, 6133–6140. [Google Scholar] [CrossRef]
- Bauman, H.; Moyer, T. Food as Medicine: Avocado (Persea americana, Lauraceae). In HerbalEGram; American Botanical Council: Austin, TX, USA, 2017; Volume 14. [Google Scholar]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Lichtenstein Alice, H.; Deckelbaum Richard, J. Stanol/Sterol Ester–Containing Foods and Blood Cholesterol Levels. Circulation 2001, 103, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, J.L.; Gardner, J.M. Sterol content of foods of plant origin. J. Am. Diet. Assoc. 1978, 73, 39–47. [Google Scholar] [PubMed]
- Duester, K.C. Avocado fruit is a rich source of beta-sitosterol. J. Am. Diet. Assoc. 2001, 101, 404–405. [Google Scholar] [CrossRef]
- Honarbakhsh, S.; Schachter, M. Vitamins and cardiovascular disease. Br. J. Nutr. 2009, 101, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Investigation of phytochemicals and antioxidant capacity of selected Eucalyptus species using conventional extraction. Chem. Pap. 2016, 70, 567–575. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Qin, X.S.; Gan, R.Y.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Santos, J.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Extraction of antioxidants from eucalyptus (Eucalyptus globulus) bark. Wood Sci. Technol. 2012, 46, 443–457. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Khuong, T.; Lovatt, C.J. Effect of harvest date on the nutritional quality and antioxidant capacity in ‘Hass’ avocado during storage. Food Chem. 2012, 135, 694–698. [Google Scholar] [CrossRef]
- Segovia, F.J.; Corral-Pérez, J.J.; Almajano, M.P. Avocado seed: Modeling extraction of bioactive compounds. Ind. Crop. Prod. 2016, 85, 213–220. [Google Scholar] [CrossRef]
- Boyadzhieva, S.; Georgieva, S.; Angelov, G. Optimization of the extraction of natural antioxidants from avocado seeds. Bulg. Chem. Commun. 2018, 50, 80–84. [Google Scholar]
- Boyadzhieva, S.; Georgieva, S.; Angelov, G. Recovery of antioxidant phenolic compounds from avocado peels by solvent extraction. Bulg. Chem. Commun. 2018, 50, 83–89. [Google Scholar]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Indelicato, S.; Massenti, R.; Lo Bianco, R. Quantitative evaluation of the phenolic profile in fruits of six avocado (Persea americana) cultivars by ultra-high-performance liquid chromatography-heated electrospray-mass spectrometry. Int. J. Food Prop. 2017, 20, 1302–1312. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive identification of bioactive compounds of avocado peel by liquid chromatography coupled to ultra-high-definition accurate-mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Fernandez, E.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A. Profiling LC-DAD-ESI-TOF MS method for the determination of phenolic metabolites from avocado (Persea americana). J. Agric. Food Chem. 2011, 59, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Fernández, E.; Pacchiarotta, T.; Mayboroda, O.A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Quantitative characterization of important metabolites of avocado fruit by gas chromatography coupled to different detectors (APCI-TOF MS and FID). Food Res. Int. 2014, 62, 801–811. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G.A. Phenolic Compound Profiles and Antioxidant Capacity of Persea americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.; Vasconcelos, C.F.; Costa-Silva, J.H.; Maranhao, C.A.; Costa, J.; Batista, T.M.; Carneiro, E.M.; Soares, L.A.; Ferreira, F.; Wanderley, A.G. Anti-diabetic activity of extract from Persea americana Mill. leaf via the activation of protein kinase B (PKB/Akt) in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2012, 141, 517–525. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. Lwt Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; Alencar, S.M.d. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef]
- Alkhalf, M.I.; Alansari, W.S.; Ibrahim, E.A.; Elhalwagy, M.E.A. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. Sci. 2018. [Google Scholar] [CrossRef]
- Amado, D.A.V.; Helmann, G.A.B.; Detoni, A.M.; Carvalho, S.L.C.D.; Aguiar, C.M.D.; Martin, C.A.; Tiuman, T.S.; Cottica, S.M. Antioxidant and antibacterial activity and preliminary toxicity analysis of four varieties of avocado (Persea americana Mill.). Braz. J. Food Technol. 2019, 22. [Google Scholar] [CrossRef]
- Bertling, I.; Tesfay, S.; Bower, J. Antioxidants in ‘Hass’ avocado. South Afr. Avocado Grow. Assoc. Yearb. 2007, 30, 17–19. [Google Scholar]
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Pedroza-Islas, R.; Ponce-Alquicira, E. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. Lwt-Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- Daiuto, É.R.; Tremocoldi, M.A.; Alencar, S.M.D.; Vieites, R.L.; Minarelli, P.H. Composição química e atividade antioxidante da polpa e resíduos de abacate ‘Hass’. Rev. Bras. Frutic. 2014, 36, 417–424. [Google Scholar] [CrossRef]
- Oboh, G.; Adelusi, T.; Akinyemi, A. Inhibitory effect of phenolic extract from leaf and fruit of avocado pear (Persea americana) on Fe2+ induced lipid peroxidation in rats’pancreas in vitro. Futa J. Res. Sci. 2013, 2, 276–286. [Google Scholar]
- Rodriguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estevez, M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Vinha, A.F.; Moreira, J.; Barreira, S.V. Physicochemical parameters, phytochemical composition and antioxidant activity of the algarvian avocado (Persea americana Mill.). J. Agric. Sci. 2013, 5, 100. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Pacchiarotta, T.; Gómez-Romero, M.; Schoenmaker, B.; Derks, R.; Deelder, A.M.; Mayboroda, O.A.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Ultra high performance liquid chromatography-time of flight mass spectrometry for analysis of avocado fruit metabolites: Method evaluation and applicability to the analysis of ripening degrees. J. Chromatogr. A 2011, 1218, 7723–7738. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Segura, N.; Amarillo, M.; Martinez, N.; Grompone, M. Improvement in the extraction of Hass avocado virgin oil by ultrasound application. J. Food Res. 2018, 7, 106–113. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.P.; Gao, K.; Byrns, R.; Heber, D. California Hass avocado: Profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar] [CrossRef] [PubMed]
- Plaza, L.; Sánchez-Moreno, C.; de Pascual-Teresa, S.; de Ancos, B.; Cano, M.P. Fatty Acids, Sterols, and Antioxidant Activity in Minimally Processed Avocados during Refrigerated Storage. J. Agric. Food Chem. 2009, 57, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Souza, D.S.; Marques, L.G.; Gomes, E.d.B.; Narain, N. Lyophilization of Avocado (Persea americana Mill.): Effect of Freezing and Lyophilization Pressure on Antioxidant Activity, Texture, and Browning of Pulp. Dry. Technol. 2015, 33, 194–204. [Google Scholar] [CrossRef]
- Soldera-Silva, A.; Seyfried, M.; Campestrini, L.H.; Zawadzki-Baggio, S.F.; Minho, A.P.; Molento, M.B.; Maurer, J.B.B. Assessment of anthelmintic activity and bio-guided chemical analysis of Persea americana seed extracts. Vet. Parasitol. 2018, 251, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Abaide, E.R.; Zabot, G.L.; Tres, M.V.; Martins, R.F.; Fagundez, J.L.; Nunes, L.F.; Druzian, S.; Soares, J.F.; Dal Prá, V.; Silva, J.R.F.; et al. Yield, composition, and antioxidant activity of avocado pulp oil extracted by pressurized fluids. Food Bioprod. Process. 2017, 102, 289–298. [Google Scholar] [CrossRef]
- dos Santos, M.A.Z.; Alicieo, T.V.R.; Pereira, C.M.P.; Ramis-Ramos, G.; Mendonça, C.R.B. Profile of Bioactive Compounds in Avocado Pulp Oil: Influence of the Drying Processes and Extraction Methods. J. Am. Oil Chem. Soc. 2014, 91, 19–27. [Google Scholar] [CrossRef]
- Prabath Pathirana, U.; Sekozawa, Y.; Sugaya, S.; Gemma, H. Changes in lipid oxidation stability and antioxidant properties of avocado in response to 1-MCP and low oxygen treatment under low-temperature storage. Int. Food Res. J. 2013, 20, 1065–1075. [Google Scholar]
- Foudjo, B.U.S.; Kansci, G.; Fokou, E.; Genot, C. Prediction of critical times for water-extracted avocado oil heated at high temperatures. Int. J.Biol. Chem. Sci. 2018, 12, 2053–2064. [Google Scholar] [CrossRef]
- Corrales-García, J.E.; del Rosario García-Mateos, M.; Martínez-López, E.; Barrientos-Priego, A.F.; Ybarra-Moncada, M.C.; Ibarra-Estrada, E.; Méndez-Zúñiga, S.M.; Becerra-Morales, D. Anthocyanin and Oil Contents, Fatty Acids Profiles and Antioxidant Activity of Mexican Landrace Avocado Fruits. Plant Foods Hum. Nutr. 2019, 74, 210–215. [Google Scholar] [CrossRef]
- Corzzini, S.C.S.; Barros, H.D.F.Q.; Grimaldi, R.; Cabral, F.A. Extraction of edible avocado oil using supercritical CO2 and a CO2/ethanol mixture as solvents. J. Food Eng. 2017, 194, 40–45. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonça, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, D.; Silva-Platas, C.; Rojo, R.P.; Garcia, N.; Cisneros-Zevallos, L.; Garcia-Rivas, G.; Hernandez-Brenes, C. Activity-guided identification of acetogenins as novel lipophilic antioxidants present in avocado pulp (Persea americana). J. Chromatogr. B 2013, 942–943, 37–45. [Google Scholar] [CrossRef]
- Gómez, F.S.; Sánchez, S.P.; Iradi, M.G.G.; Azman, N.A.M.; Almajano, M.P. Avocado Seeds: Extraction Optimization and Possible Use as Antioxidant in Food. Antioxidants 2014, 3, 439–454. [Google Scholar] [CrossRef] [Green Version]
- Kingne, F.K.; Tsafack, H.D.; Boungo, G.T.; Mboukap, A.; Azia, A. Phenolic Content and Antioxidant Activity of Young and Mature Mango (Mangifera indica) and Avocado (Persea americana) Leaves Extracts. J. Food. Stab. 2018, 1, 14–27. [Google Scholar] [Green Version]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Characterization of Virgin Avocado Oil Obtained via Advanced Green Techniques. Eur. J. Lipid Sci. Technol. 2018, 120, 1800170. [Google Scholar] [CrossRef]
- Princwill-Ogbonna, I.; Ogbonna, P.; Ogujiofor, I. Proximate Composition, Vitamin, Mineral and biologically Active Compounds Levels in Leaves of Mangifera indica (Mango), Persea americana (Avocado pea), and Annona muricata (Sour sop). J. Appl. Sci.Environ. Manag. 2019, 23, 65–74. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Nabavi, S.M.N.; Setzer, W.; Nabavi, S.A.; Nabavi, S.A.; Ebrahimzadeh, M.A. Antioxidant and antihemolytic activity of lipid-soluble bioactive substances in avocado fruits. Fruits 2013, 68, 185–193. [Google Scholar] [CrossRef]
- Garcia-Alonso, M.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar] [CrossRef]
- Oboh, G.; Odubanjo, V.O.; Bello, F.; Ademosun, A.O.; Oyeleye, S.I.; Nwanna, E.E.; Ademiluyi, A.O. Aqueous extracts of avocado pear (Persea americana Mill.) leaves and seeds exhibit anti-cholinesterases and antioxidant activities in vitro. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, M.; Sandhya, V.; Supriya, G.; Manju, R.; Pranitha, K.; Shivaji, B.; Lalitha, V.; Kiran, B. Antioxidant and antibacterial activity of avocado (Persea gratissima Gaertner.) seed extract. World Appl. Sci. J. 2010, 9, 695–698. [Google Scholar]
- Wang, C.Y.; Bai, X.Y.; Wang, C.H. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am. J. Chin. Med. 2014, 42, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Sakoff, J.; Bond, D.R.; Predebon, M.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. In vitro anticancer properties of selected Eucalyptus species. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, Y.; He, C. Anticancer activities and mechanisms of heat-clearing and detoxicating traditional Chinese herbal medicine. Chin. Med. 2017, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; Bowyer, M.C.; Scarlett, C.J. An array of bioactive compounds from Australian eucalypts and their relevance in pancreatic cancer therapeutics. Pancreas 2018, 47, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Vuong, Q.V.; Bond, D.R.; Chalmers, A.C.; Bowyer, M.C.; Scarlett, C.J. Eucalyptus microcorys leaf extract derived HPLC-fraction reduces the viability of MIA PaCa-2 cells by inducing apoptosis and arresting cell cycle. Biomed. Pharmacother. 2018, 105, 449–460. [Google Scholar] [CrossRef]
- Mooz, E.D.; Gaiano, N.M.; Shimano, M.Y.H.; Amancio, R.D.; Spoto, M.H.F. Physical and chemical characterization of the pulp of different varieties of avocado targeting oil extraction potential. Food Sci. Technol 2012, 32, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Bonilla-Porras, A.R.; Salazar-Ospina, A.; Jimenez-Del-Rio, M.; Pereanez-Jimenez, A.; Velez-Pardo, C. Pro-apoptotic effect of Persea americana var. Hass (avocado) on Jurkat lymphoblastic leukemia cells. Pharm. Biol. 2013. [Google Scholar] [CrossRef]
- Butt, A.J.; Roberts, C.G.; Seawright, A.A.; Oelrichs, P.B.; MacLeod, J.K.; Liaw, T.Y.E.; Kavallaris, M.; Somers-Edgar, T.J.; Lehrbach, G.M.; Watts, C.K.; et al. A novel plant toxin, persin, with in vivo activity in the mammary gland, induces Bim-dependent apoptosis in human breast cancer cells. Mol. Cancer Ther. 2006, 5, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Dabas, D.; Elias, R.J.; Ziegler, G.R.; Lambert, J.D. In Vitro Antioxidant and Cancer Inhibitory Activity of a Colored Avocado Seed Extract. Int. J. Food Sci. 2019, 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chin, Y.-W.; Kinghorn, A.D.; D’Ambrosio, S.M. Chemopreventive characteristics of avocado fruit. Semin. Cancer Biol. 2007, 17, 386–394. [Google Scholar] [CrossRef]
- Ding, H.; Han, C.; Guo, D.; Chin, Y.W.; Ding, Y.; Kinghorn, A.D.; D’Ambrosio, S.M. Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr. Cancer 2009, 61, 348–356. [Google Scholar] [CrossRef]
- Guzman-Rodriguez, J.J.; Lopez-Gomez, R.; Salgado-Garciglia, R.; Ochoa-Zarzosa, A.; Lopez-Meza, J.E. The defensin from avocado (Persea americana var. drymifolia) PaDef induces apoptosis in the human breast cancer cell line MCF-7. Biomed. Pharmacother. 2016, 82, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Yu, M.-H.; Lee, S.-P.; Lee, I.-S. Antioxidant activities and induction of apoptosis by methanol extracts from avocado. J. Korean Soc. Food Sci. Nutr. 2008, 37, 269–275. [Google Scholar] [CrossRef]
- Leon, L.G.; Carballo, R.M.; Vega-Hernandez, M.C.; Miranda, P.O.; Martin, V.S.; Padron, J.I.; Padron, J.M. β’-Hydroxy-alpha,β-unsaturated ketones: A new pharmacophore for the design of anticancer drugs. Part 2. ChemMedChem 2008, 3, 1740–1747. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2017, 25, 104. [Google Scholar] [CrossRef]
- Gondi, C.S.; Dinh, D.H.; Klopfenstein, J.D.; Gujrati, M.; Rao, J.S. MMP-2 downregulation mediates differential regulation of cell death via ErbB-2 in glioma xenografts. Int. J. Oncol. 2009, 35, 257–263. [Google Scholar] [Green Version]
- Valacca, C.; Tassone, E.; Mignatti, P. TIMP-2 Interaction with MT1-MMP Activates the AKT Pathway and Protects Tumor Cells from Apoptosis. PLoS ONE 2015, 10, e0136797. [Google Scholar] [CrossRef]
- Roberts, C.G.; Gurisik, E.; Biden, T.J.; Sutherland, R.L.; Butt, A.J. Synergistic cytotoxicity between tamoxifen and the plant toxin persin in human breast cancer cells is dependent on Bim expression and mediated by modulation of ceramide metabolism. Mol. Cancer Ther. 2007, 6, 2777–2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Strasser, A.; O’Reilly, L.A.; Hausmann, G.; Adams, J.M.; Cory, S.; Huang, D.C. Bim: A novel member of the Bcl-2 family that promotes apoptosis. Embo J. 1998, 17, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Moudgil, T.; Ross, H.J.; Hu, H.-M. The BH3-only proapoptotic protein Bim directly links the microtubule poison Paclitaxel to mitochondrial damage and apoptosis. Cancer Res. 2004, 64, 1296. [Google Scholar]
- Flores-Alvarez, L.J.; Guzman-Rodriguez, J.J.; Lopez-Gomez, R.; Salgado-Garciglia, R.; Ochoa-Zarzosa, A.; Lopez-Meza, J.E. PaDef defensin from avocado (Persea americana var. drymifolia) is cytotoxic to K562 chronic myeloid leukemia cells through extrinsic apoptosis. Int. J. Biochem. Cell Biol. 2018, 99, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Brooke, D.G.; Shelley, E.J.; Roberts, C.G.; Denny, W.A.; Sutherland, R.L.; Butt, A.J. Synthesis and in vitro evaluation of analogues of avocado-produced toxin (+)-(R)-persin in human breast cancer cells. Bioorg. Med. Chem. 2011, 19, 7033–7043. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Rodríguez, J.J.; López-Gómez, R.; Suárez-Rodríguez, L.M.; Salgado-Garciglia, R.; Rodríguez-Zapata, L.C.; Ochoa-Zarzosa, A.; López-Meza, J.E. Antibacterial activity of defensin PaDef from avocado fruit (Persea americana var. drymifolia) expressed in endothelial cells against Escherichia coli and Staphylococcus aureus. Biomed Res. Int. 2013, 2013, 986273. [Google Scholar] [CrossRef]
- Meneguetti, B.T.; Machado, L.d.S.; Oshiro, K.G.N.; Nogueira, M.L.; Carvalho, C.M.E.; Franco, O.L. Antimicrobial Peptides from Fruits and Their Potential Use as Biotechnological Tools—A Review and Outlook. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Kulkarni, P.; Paul, R.; Ganesh, N. In Vitro evaluation of genotoxicity of avocado (Persea americana) fruit and leaf extracts in human peripheral lymphocytes. J. Environ. Sci. Health C 2010, 28, 172–187. [Google Scholar] [CrossRef]
- Paul, R.; Kulkarni, P.; Ganesh, N. Avocado fruit (Persea americana Mill) exhibits chemo-protective potentiality against cyclophosphamide induced genotoxicity in human lymphocyte culture. J. Exp. Ther. Oncol. 2011, 9, 221–230. [Google Scholar]
- Engel, N.; Oppermann, C.; Falodun, A.; Kragl, U. Proliferative effects of five traditional Nigerian medicinal plant extracts on human breast and bone cancer cell lines. J. Ethnopharmacol. 2011, 137, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.D.; Walker, S.P.; Simpson-Smith, C.M.; Lindsay, C.M.; Smith, G.; McFarlane-Anderson, N.; Bennett, F.I.; Coard, K.C.M.; Aiken, W.D.; Tulloch, T.; et al. Associations of whole-blood fatty acids and dietary intakes with prostate cancer in Jamaica. Cancer Causes Control 2012, 23, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.J.; Mayne, S.T.; Blumberg, J.B.; Ribaya-Mercado, J.D.; Johnson, E.J.; Cartmel, B. Plasma Carotenoids and Biomarkers of Oxidative Stress in Patients with prior Head and Neck Cancer. Biomark. Insights 2009, 4, 17–26. [Google Scholar] [CrossRef]
- Tamimi, R.M.; Colditz, G.A.; Hankinson, S.E. Circulating carotenoids, mammographic density, and subsequent risk of breast cancer. Cancer Res. 2009, 69, 9323–9329. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Stendell-Hollis, N.R.; Rock, C.L.; Cussler, E.C.; Flatt, S.W.; Pierce, J.P. Plasma and dietary carotenoids are associated with reduced oxidative stress in women previously treated for breast cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Coates, R.J.; Flagg, E.W.; Eley, J.W.; Block, G.; Greenberg, R.S.; Gunter, E.W.; Jackson, B. Glutathione in foods listed in the National Cancer Institute’s Health Habits and History Food Frequency Questionnaire. Nutr. Cancer 1992, 17, 57–75. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, S.M.; Han, C.; Pan, L.; Kinghorn, A.D.; Ding, H. Aliphatic acetogenin constituents of avocado fruits inhibit human oral cancer cell proliferation by targeting the EGFR/RAS/RAF/MEK/ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2011, 409, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.S.; Le, P.U. Free radical scavenging and anti-proliferative activities of avocado (Persea americana Mill.) seed extract. Asian Pac. J. Trop. Biomed. 2019, 9, 91. [Google Scholar]
- Widiyastuti, Y.; Pratiwi, R.; Riyanto, S.; Wahyuono, S. Cytotoxic Activity and Apoptosis Induction of Avocado (Perseaamericana) Seed Extract on MCF-7 Cancer Cell Line. Indones. J. Biotechnol. 2018, 23, 61–67. [Google Scholar] [CrossRef]
- Salazar, L.; López, M.J.V.; Grijalva, M.; Castillo, L.; Maldonado, A. Biological Effect of Organically Coated Grias neuberthii and Persea americana Silver Nanoparticles on HeLa and MCF-7 Cancer Cell Lines. J. Nanotechnol. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Ant, A.; Avcý, A.; Genç, M.; Ýnal, E.; Tunçel, Ü.; Þencan, Z. Avocado leaf extract activates Adenosine Deaminase (ADA) in Larynx cancer tissues. Acta Oncol. Tur. 2018, 51, 199–204. [Google Scholar] [CrossRef]
- Abubakar, A.N.F.; Achmadi, S.S.; Suparto, I.H. Triterpenoid of avocado (Persea americana) seed and its cytotoxic activity toward breast MCF-7 and liver HepG2 cancer cells. Asian Pac. J. Trop. Biomed. 2017, 7, 397–400. [Google Scholar] [CrossRef]
- Vahedi Larijani, L.; Ghasemi, M.; AbedianKenari, S.; Naghshvar, F. Evaluating the effect of four extracts of avocado fruit on esophageal squamous carcinoma and colon adenocarcinoma cell lines in comparison with peripheral blood mononuclear cells. Acta Med. Iran. 2014, 52, 201–205. [Google Scholar] [PubMed]
- Khalifa, N.S.; Barakat, H.S.; Elhallouty, S.; Salem, D. Effect of the Water Extracts of Avocado Fruit and Cherimoya Leaf on Four Human Cancer Cell Lines and Vicia Faba Root Tip Cells. J. Agric. Sci. 2013, 5, 245. [Google Scholar] [CrossRef]
- Kim, O.K.; Murakami, A.; Takahashi, D.; Nakamura, Y.; Torikai, K.; Kim, H.W.; Ohigashi, H. An avocado constituent, persenone A, suppresses expression of inducible forms of nitric oxide synthase and cyclooxygenase in macrophages, and hydrogen peroxide generation in mouse skin. Biosci. Biotechnol. Biochem. 2000, 64, 2504–2507. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Athaydes, B.R.; Alves, G.M.; Assis, A.L.E.M.D.; Gomes, J.V.D.; Rodrigues, R.P.; Campagnaro, B.P.; Nogueira, B.V.; Silveira, D.; Kuster, R.M.; Pereira, T.M.C.; et al. Avocado seeds (Persea americana Mill.) prevents indomethacin-induced gastric ulcer in mice. Food Res. Int. 2019, 119, 751–760. [Google Scholar] [CrossRef]
- Raymond Chia, T.W.; Dykes, G.A. Antimicrobial activity of crude epicarp and seed extracts from mature avocado fruit (Persea americana) of three cultivars. Pharm. Biol. 2010, 48, 753–756. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Hernández-Brenes, C.; Rodríguez-Sánchez, D.G.; Castillo, E.C.; Navarro-Silva, J.M.; Pacheco, A. Inhibitory Activity of Avocado Seed Fatty Acid Derivatives (Acetogenins) Against Listeria Monocytogenes. J. Food Sci. 2017, 82, 134–144. [Google Scholar] [CrossRef]
- Cardoso, P.F.; Scarpassa, J.A.; Pretto-Giordano, L.G.; Otaguiri, E.S.; Yamada-Ogatta, S.F.; Nakazato, G.; Perugini, M.R.E.; Moreira, I.C.; Vilas-Boâs, G.T. Antibacterial activity of avocado extracts (Persea americana Mill.) against streptococcus agalactiae. Phyton 2016, 85, 218–224. [Google Scholar]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Guayabo, G.T. Evaluation of a colorant and oil extracted from avocado waste as functional components of a liquid soap formulation. Rev. Fac. Nac. Agron. Medellin 2019, 72, 8855–8862. [Google Scholar] [CrossRef]
- Bamoniri, A.; Ebrahimabadi, A.H.; Mazoochi, A.; Behpour, M.; Kashi, F.J.; Batooli, H. Antioxidant and antimicrobial activity evaluation and essential oil analysis of Semenovia tragioides Boiss. from Iran. Food Chem. 2010, 122, 553–558. [Google Scholar] [CrossRef]
- Bouic, P.J.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; Van Jaarsveld, P.P. beta-Sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. Immunopharmacol. 1996, 18, 693–700. [Google Scholar] [CrossRef]
- Simpson, D.; Amos, S. Chapter 12—Other Plant Metabolites. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 267–280. [Google Scholar]
- Pacheco, A.; Rodríguez-Sánchez, D.G.; Villarreal-Lara, R.; Navarro-Silva, J.M.; Senés-Guerrero, C.; Hernández-Brenes, C. Stability of the antimicrobial activity of acetogenins from avocado seed, under common food processing conditions, against Clostridium sporogenes vegetative cell growth and endospore germination. Int. J. Food Sci. Technol. 2017, 52, 2311–2323. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019, 54, 959–965. [Google Scholar] [CrossRef]
- Adeyemi, O.; Okpo, S.; O Ogunti, O. Analgesic and anti-inflammatory effects of Persea americana Mill (Lauraceae). Fitoterapia 2002, 73, 375–380. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Franco, E.d.S.; Rodrigues Barreto, R.; Cordeiro, D.P.; de Melo, R.G.; de Aquino, C.M.F.; e Silva, A.A.R.; de Medeiros, P.L.; et al. Effect of Semisolid Formulation of Persea Americana Mill (Avocado) Oil on Wound Healing in Rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, H.-C.; Park, J.W.; Kim, I.-S.; Kim, J.-Y.; Kim, K.-C.; Chae, D.-S.; Jo, W.-L.; Song, J.-H. Diagnosis and Treatment of Inflammatory Joint Disease. Hip Pelvis 2017, 29, 211–222. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Ownby, S.L.; Fortuno, L.V.; Au, A.Y.; Grzanna, M.W.; Rashmir-Raven, A.M.; Frondoza, C.G. Expression of pro-inflammatory mediators is inhibited by an avocado/soybean unsaponifiables and epigallocatechin gallate combination. J. Inflamm. 2014, 11, 8. [Google Scholar] [CrossRef]
- Gabay, O.; Gosset, M.; Levy, A.; Salvat, C.; Sanchez, C.; Pigenet, A.; Sautet, A.; Jacques, C.; Berenbaum, F. Stress-induced signaling pathways in hyalin chondrocytes: Inhibition by Avocado–Soybean Unsaponifiables (ASU). Osteoarthr. Cartil. 2008, 16, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Angermann, P. Avocado/soybean unsaponifiables in the treatment of knee and hip osteoarthritis. Ugeskr. Laeger 2005, 167, 3023–3025. [Google Scholar] [PubMed]
- Henrotin, Y.E.; Labasse, A.H.; Jaspar, J.M.; De Groote, D.D.; Zheng, S.X.; Guillou, G.B.; Reginster, J.Y. Effects of three avocado/soybean unsaponifiable mixtures on metalloproteinases, cytokines and prostaglandin E2 production by human articular chondrocytes. Clin. Rheumatol. 1998, 17, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; el-Ghazaly, M.A. The possible “chondroprotective” effect of the unsaponifiable constituents of avocado and soya in vivo. Drugs Exp. Clin. Res. 1998, 24, 41–50. [Google Scholar] [PubMed]
- Christiansen, B.A.; Bhatti, S.; Goudarzi, R.; Emami, S. Management of Osteoarthritis with Avocado/Soybean Unsaponifiables. Cartilage 2015, 6, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, R.; Taylor, J.F.; Yazdi, P.G.; Pedersen, B.A. Effects of Arthrocen, an avocado/soy unsaponifiables agent, on inflammatory mediators and gene expression in human chondrocytes. FEBS Open Bio 2017, 7, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au, R.Y.; Al-Talib, T.K.; Au, A.Y.; Phan, P.V.; Frondoza, C.G. Avocado soybean unsaponifiables (ASU) suppress TNF-α, IL-1β, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr. Cartil. 2007, 15, 1249–1255. [Google Scholar] [CrossRef]
- Oliveira, G.J.P.L.; Paula, L.G.F.; Souza, J.A.C.; Spin-Neto, R.; Stavropoulos, A.; Marcantonio, R.A.C. Effect of avocado/soybean unsaponifiables on ligature-induced bone loss and bone repair after ligature removal in rats. J. Periodontal Res. 2016, 51, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Lippiello, L.; Nardo, J.V.; Harlan, R.; Chiou, T. Metabolic Effects of Avocado/Soy Unsaponifiables on Articular Chondrocytes. Evid. Based Complement. Alternat. Med. 2008, 5, 191–197. [Google Scholar] [CrossRef]
- Kawcak, C.E.; Frisbie, D.D.; McIlwraith, C.W.; Werpy, N.M.; Park, R.D. Evaluation of avocado and soybean unsaponifiable extracts for treatment of horses with experimentally induced osteoarthritis. Am. J. Vet. Res. 2007, 68, 598–604. [Google Scholar] [CrossRef]
- Blotman, F.; Maheu, E.; Wulwik, A.; Caspard, H.; Lopez, A. Efficacy and safety of avocado/soybean unsaponifiables in the treatment of symptomatic osteoarthritis of the knee and hip. A prospective, multicenter, three-month, randomized, double-blind, placebo-controlled trial. Rev. Rhum. Engl. Ed. 1997, 64, 825–834. [Google Scholar] [PubMed]
- Lequesne, M.; Maheu, E.; Cadet, C.; Dreiser, R.-L. Structural effect of avocado/soybean unsaponifiables on joint space loss in osteoarthritis of the hip. Arthritis Care Res. 2002, 47, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Maheu, E.; Cadet, C.; Marty, M.; Moyse, D.; Kerloch, I.; Coste, P.; Dougados, M.; Mazieres, B.; Spector, T.D.; Halhol, H.; et al. Randomised, controlled trial of avocado-soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: The ERADIAS study. Ann. Rheum. Dis. 2014, 73, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Catunda, I.S.; Vasconcelos, B.C.d.E.; Andrade, E.S.d.S.; Costa, D.F.N. Clinical effects of an avocado–soybean unsaponifiable extract on arthralgia and osteoarthritis of the temporomandibular joint: Preliminary study. Int. J. Oral Maxillofac. Surg. 2016, 45, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, L.F.; Grzanna, M.W.; Au, A.Y.; Mochal, C.A.; Rashmir-Raven, A.; Frondoza, C.G. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthr. Cartil. 2010, 18, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frondoza, C.G.; Fortuno, L.V.; Grzanna, M.W.; Ownby, S.L.; Au, A.Y.; Rashmir-Raven, A.M. α-Lipoic Acid Potentiates the Anti-Inflammatory Activity of Avocado/Soybean Unsaponifiables in Chondrocyte Cultures. Cartilage 2017, 9, 304–312. [Google Scholar] [CrossRef]
- Andriamanalijaona, R.; Benateau, H.; Barre, P.E.; Boumediene, K.; Labbe, D.; Compere, J.F.; Pujol, J.P. Effect of Interleukin-1β on Transforming Growth Factor-Beta and Bone Morphogenetic Protein-2 Expression in Human Periodontal Ligament and Alveolar Bone Cells in Culture: Modulation by Avocado and Soybean Unsaponifiables. J. Periodontol. 2006, 77, 1156–1166. [Google Scholar] [CrossRef]
- Oliveira, G.J.P.L.D.; Paula, L.G.F.D.; Souza, J.A.C.D.; Spin-Neto, R.; Stavropoulos, A.; Marcantonio, R.A.C. Effects of avocado/soybean unsaponifiables (ASU) on the treatment of ligature-induced periodontitis in rats. Braz. Oral Res. 2017, 31. [Google Scholar] [CrossRef]
- Noorul, H.; Nesar, A.; Zafar, K.; Khalid, M.; Zeeshan, A.; Vartika, S. Health benefits and pharmacology of Persea americana mill. (Avocado). Int. J. Res. Pharmacol. Pharmacother. 2016, 5, 132–141. [Google Scholar]
- Grant, W.C. Influence of avocados on serum cholesterol. Proc. Soc. Exp. Biol. Med. 1960, 104, 45–47. [Google Scholar] [CrossRef]
- Pieterse, Z.; Jerling, J.C.; Oosthuizen, W.; Kruger, H.S.; Hanekom, S.M.; Smuts, C.M.; Schutte, A.E. Substitution of high monounsaturated fatty acid avocado for mixed dietary fats during an energy-restricted diet: Effects on weight loss, serum lipids, fibrinogen, and vascular function. Nutrition 2005, 21, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L., 3rd; Dreher, M.; Davenport, A.J. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: Results from the National Health and Nutrition Examination Survey (NHANES) 2001-2008. Nutr. J. 2013, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Haddad, E.; Oda, K.; Sabate, J. A randomized 3x3 crossover study to evaluate the effect of Hass avocado intake on post-ingestive satiety, glucose and insulin levels, and subsequent energy intake in overweight adults. Nutr. J. 2013, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Silva Caldas, A.P.; Chaves, L.O.; Linhares Da Silva, L.; De Castro Morais, D.; Gonçalves Alfenas, R.d.C. Mechanisms involved in the cardioprotective effect of avocado consumption: A systematic review. Int. J. Food Prop. 2017, 20, 1675–1685. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Nolasco-Hipolito, C.; Aguilar-Uscanga, M.G.; Melo-Santiesteban, G.; Hayward-Jones, P.M.; Barradas-Dermitz, D.M. Avocado oil supplementation modifies cardiovascular risk profile markers in a rat model of sucrose-induced metabolic changes. Dis. Markers 2014, 2014, 386425. [Google Scholar] [CrossRef] [PubMed]
- Heskey, C.; Oda, K.; Sabate, J. Avocado Intake, and Longitudinal Weight and Body Mass Index Changes in an Adult Cohort. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Mahmassani, H.A.; Avendano, E.E.; Raman, G.; Johnson, E.J. Avocado consumption and risk factors for heart disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Pahua-Ramos, M.E.; Ortiz-Moreno, A.; Chamorro-Cevallos, G.; Hernandez-Navarro, M.D.; Garduno-Siciliano, L.; Necoechea-Mondragon, H.; Hernandez-Ortega, M. Hypolipidemic effect of avocado (Persea americana Mill) seed in a hypercholesterolemic mouse model. Plant Foods Hum. Nutr. 2012, 67, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezejiofor, A.N.; Okorie, A.; Orisakwe, O.E. Hypoglycaemic and tissue-protective effects of the aqueous extract of persea americana seeds on alloxan-induced albino rats. Malays. J. Med. Sci. 2013, 20, 31–39. [Google Scholar]
- Pahua-Ramos, M.E.; Garduno-Siciliano, L.; Dorantes-Alvarez, L.; Chamorro-Cevallos, G.; Herrera-Martinez, J.; Osorio-Esquivel, O.; Ortiz-Moreno, A. Reduced-calorie avocado paste attenuates metabolic factors associated with a hypercholesterolemic-high fructose diet in rats. Plant Foods Hum. Nutr. 2014, 69, 18–24. [Google Scholar] [CrossRef]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014, 10, 208–216. [Google Scholar] [PubMed]
- Park, E.; Edirisinghe, I.; Burton-Freeman, B. Avocado Fruit on Postprandial Markers of Cardio-Metabolic Risk: A Randomized Controlled Dose Response Trial in Overweight and Obese Men and Women. Nutrients 2018, 10, 1287. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Basic Report: 09038, Avocados, raw, California. In USDA Natl. Nutr. Database Stand. Ref.; 2018. Available online: https://ndb.nal.usda.gov/ndb/foods/show/09038?fgcd=&manu=&format=&count=&max=25&offset=&sort=default&order=asc&qlookup=avocado&ds=&qt=&qp=&qa=&qn=&q=&ing= (accessed on 15 August 2019).
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef]
- Tang, G.W.; Qin, J.; Dolnikowski, G.G.; Russell, R.M.; Grusak, M.A. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. Am. J. Clin. Nutr. 2005, 82, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; van den Berg, H.; Arthur, J.; Bast, A.; Dainty, J.; Faulks, R.M.; Gartner, C.; Haenen, G.; Hollman, P.; Holst, B.; et al. Bioavailability and metabolism. Mol. Asp. Med. 2002, 23, 39–100. [Google Scholar] [CrossRef]
- White, W.S.; Zhou, Y.; Crane, A.; Dixon, P.; Quadt, F.; Flendrig, L.M. Modeling the dose effects of soybean oil in salad dressing on carotenoid and fat-soluble vitamin bioavailability in salad vegetables. Am. J. Clin. Nutr. 2017, 106, 1041–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reboul, E. Absorption of vitamin A and carotenoids by the enterocyte: Focus on transport proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef]
- Kopec, R.E.; Cooperstone, J.L.; Schweiggert, R.M.; Young, G.S.; Harrison, E.H.; Francis, D.M.; Clinton, S.K.; Schwartz, S.J. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high-beta-carotene tomato sauce and from carrots. J. Nutr. 2014, 144, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Alam, D.S.; Yunus, M.; Aziz, K.M.A.; Wahed, M.A.; van Raaij, J.M.A.; Hautvast, J.G.A.G.; Fuchs, G.J. Effects of dietary fat supplementation during pregnancy/lactation on maternal blood and breastmilk vitamin A in rural Bangladesh. FASEB J. 1999, 13, A895. [Google Scholar]
- Brown, M.J.; Ferruzzi, M.G.; Nguyen, M.L.; Cooper, D.A.; Eldridge, A.L.; Schwartz, S.J.; White, W.S. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am. J. Clin. Nutr. 2004, 80, 396–403. [Google Scholar] [CrossRef]
- Ostlund, R.E.; McGill, J.B.; Zeng, C.M.; Covey, D.F.; Stearns, J.; Stenson, W.F.; Spilburg, C.A. Gastrointestinal absorption and plasma kinetics of soy Delta(5)-phytosterols and phytostanols in humans. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E911–E916. [Google Scholar] [CrossRef]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G.; Olaniru, O.B. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R.; Participants, S.W. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar] [CrossRef]








| Compound Name and Synonyms | Source | Extracts of Different Parts Used | Biological Significance | Reference |
|---|---|---|---|---|
| Fatty alcohols | ||||
| (2R,4R)-1,2,4-trihydroxyheptadec-16-yne [Avocadyne] 1,2,4-trihydroxyheptadec-16-ene 2,4-methylene-dioxyheptadec-16-ene-1-ol 1-acetoxy-2,4-dihydroxyheptadec-16-yne (2R,4R)1,2,4-Nonadecanetriol. (2R,4R,6E)-6-Nonadecene-1,2,4-triol (2R,4R,16E)-16-Nonadecene-1,2,4-triol [Avocadenol D] | P. americana | Pulp and seeds | Inhibition of the dengue virus replication. Cytotoxic, insecticidal, antimycobacterial, and trypanocidal activity. | [10,11,12,13,21] |
| (Z,Z)-1-Acetoxy-2-hydroxy-4-oxo-heneicosa-12,15-triene (Z,Z,E)-1-Acetoxy-2-hydroxy-4-oxo-heneicosa-5,12,15-triene 1,2,4-trihydroxyheptadec-16-ene | P. americana | Idioblast cells of pulp | Antifungal activity | [14] |
| (2R,4R)16-Heptadecene-1,2,4-triol and the following derivatives: 1,2, or 4 acetate (1,2), (1,4) or (2,4) di acetate 1-hexadecanolyl derivative (Avocadoin) | P. americana | Peel, idioblast cell, and leaves | Antifungal, cytotoxic, and insecticidal activity. | [11,14,15] |
| 2-(isopropyl)-(2E,4E)-16-Heptadecene-1,2,4-triol 2-(isopropyl), 1,4-di-acetyl-(2E,4E)-16-Heptadecene-1,2,4-triol | P. gratissima | Leaves | - | [7] |
| (2E,5E,12Z,15Z) 1-Hydroxy-2,5,12,15-heneicosatetraen-4-one 1-Hydroxy-2,12,15-heneicosatrien-4-one | P. americana | - | - | [7] |
| Acetyl-2-nonanol | P. gratissima | Leaves | - | [7] |
| Persin Tetrahydropersin Isopersin Tetrahydropersin | P. americana | Idioblast oil cells | Surfactant and emulsifier, nutrient, membrane stabilizer, energy source, and energy storage. | [8,16,17] |
| 1-Acetoxy-2-hydroxy-16-heptadecen-4-one | P. americana | Pulp | [18] | |
| Persenone A and B | P. americana | Pulp | Nitric oxide and superoxide generation inhibitors. | [19] |
| Secosubamolide | P. americana | Bark | Cytotoxic activity | [20] |
| Phenolics | ||||
| Gallic acid 3,4-Dihydroxyphenylacetic acid 4-Hydroxybenzoic acid Vanillic acid p-Coumaric acid Ferulic acid Quercetin | P. americana | Pulp oil and varied by ripening and peeling | Antioxidant activity | [28] |
| (+)-Catechin (−)-Epicatechin Neochlorogenic acid procyanidins | P. americana | By-products | Antioxidant and neuroprotective activity. | [22] |
| Proanthocyanidins B1, B2 and A-type trimer | P. americana | Seeds | Cytotoxic to HaCat cells. | [23] |
| Tocopherols (Vitamin E) α-tocopherol γ-tocopherol | P. americana | Pulp and pulp oil varied by ripening and peeling | Antioxidant activity | [24,28] |
| (E)-Chlorogenic acid (Caffeylquinic acid, Caffetannic acid, Helianthic acid, Igasuric acid) | P. americana | - | Antioxidant, antimicrobial (antibacterial and antiviral) hepatoprotective, cardioprotective, anti-hypertension, anti-obesity, anti-inflammatory, antipyretic, neuroprotective, central nervous system stimulator. | [7,25] |
| Scopoletin | P. americana | - | Anti-oncogenic and antioxidant activity. | [7,26] |
| 4-Hydroxycinnamoylputrescine (4-Coumaroylputresine) | P. gratissima | - | Nutrient, promotes cell multiplication of tobacco explants. | [7,27] |
| Carotenoids | ||||
| Lutein zeaxanthin β-cryptoxanthin α-carotene β-carotene (pro-vitamin A, retinol) | P. americana | Pulp and pulp oil varied by ripening and peeling | Cytotoxic to prostate cancer cell lines, antioxidant, reduces the photosensitivity reactions in erythropoietic protoporphyria patients. | [24,28] |
| 10’,11’-Didehydro-5,8,11’,12’-tetrahydro-10’-apo-β-carotene-3,5,8-triol 5,8-Epoxy-5,8-dihydro-10’-apo-β,ψ-carotene-3,10’-diol | P. americana | Pulp | Surfactant and emulsifier, nutrient, membrane stabilizer, energy source and energy storage. | [8,29] |
| α-Citraurin (3-Hydroxy-8’-apo-ε-caroten-8’-al) | P. americana | Pulp | [30] | |
| Carbohydrates | ||||
| Perseulose | P. gratissima | Leaves, fruit, and seeds | Nutrient, membrane stabilizer, energy source and energy storage. | [44] |
| d-erythro-l-galacto-Nonulose | P. americana | Pulp | [45] | |
| d-erythro-l-gluco-Nonulose | P. americana | Pulp | [46] | |
| d-erythro-d-galacto-Octitol | P. gratissima | Pulp | [47] | |
| d-manno-2-Heptulose | P. gratissima P. americana | Pulp | [7,47] | |
| d-glycero-d-manno-2-Octulose | P. gratissima | Pulp | [47] | |
| Furan derivatives | ||||
| Avocadofuran B (2-Heptadecylfuran) P. americana | Pulp | Insecticidal activity | [31,32] | |
| Avocadofuran A (2-Pentadecylfuran) | P. americana | Idioblast oil cells | ||
| Avocadienofuran | P. americana P. indica | Seed oil pulp | - | [33,34] |
| Perseafuran [(E)-2-(1-Pentadecenyl) furan] | ||||
| Isoavocadienofuran | Seeds | |||
| Avocadenofuran | P. americana | Pulp | [18] | |
| Avocadynofuran | P. americana and P. indica | Pulp | [18,33] | |
| Furanone derivatives | ||||
| Obtusilactone A (Borbonol) | P. americana, P. borbonia and other Persea spp. | Idioblast oil cells | Antifungal and anticancer activity. | [35,36] |
| Isoobtusilactone A (Borbonol 2) | Persea spp | Idioblast cell oil of pulp | Antifungal and anticancer activity. | [35,37] |
| Majorynolide | P. major | - | Cytotoxic, weak antimycobacterial activity. | [33] |
| 16,17-Dihydro-Majorynolide | P. major and P. indica | - | ||
| Diterpenoids | ||||
| Perseanol Vignaticol Indicol | P. indica | Branches | Insecticidal and antifeedant activity. | [39,40] |
| Ryanodol 2,3-DidehydrocinnzeylanoneAnhydrocinnzeylanoneGarajonone | Insecticidal and toxic to mice. | [41,42,43] | ||
| Norlignans/Neolignans/Lignans | ||||
| Perseal A ((7’R,8’S)4’,7’-Dihydroxy-3,3’-dimethoxy-8,9-dinor-4,8’-oxylignan-7-al) Perseal B ((7’S,8’S) 4’,7’-Dihydroxy-3,3’-dimethoxy-8,9-dinor-4,8’-oxylignan-7-al) Obovatinal Perseal C Perseal D Perseal E ((7’S,8’S) 4,7’-Epoxy-3’,4’-dihydroxy-5,5’-dimethoxy-8,9-dinor-3,8’-lignan-7-al) ObovatenObovatifol | P. obovatifolia | Branches | Cytotoxic activity | [48,49,50,51,52] |
| Lingueresinol | P. lingue | Bark | - | [53] |
| Miscellaneous | ||||
| (6S,7E,9Z) Abscisic acid-13-Hydroxy, 13-O-β-D-glucopyranoside | P. americana | Seeds | Derivative of abscisic acid (plant hormone involved in seed and bud dormancy). | [7] |
| Dimethyl sciadinonate | P. americana | - | Growth inhibitor of silkworm larvae. | [7,54] |
| (3β,5α,24R) Stigmast-7-en-3-ol; (Schottenol, 22-Dihydrochondrillasterol, 22,23-Dihydro-α-spinasterol, Poriferast-7-en-3-ol) | P. americana | Pulp oil | Protective role by cholesterol metabolism modulation (liver x receptor agonist). | [55] |
| Perseapicroside A | P. mexicana | - | - | [56] |
| Glutathione | P. americana | - | Anticancer and antioxidant activity. | [57,58,59] |
| 12-Tridecenal | P. bombycina | Essential oil | - | [60] |
| Nutritional Composition | Unit | Value Per 100 g | 1 Fruit 136 g | 1 Serving 30 g |
|---|---|---|---|---|
| 1. Proximate | ||||
| Water | g | 72.3 | 98.4 | 21.7 |
| Energy | kcal | 167 | 227 | 50 |
| Energy (insoluble fiber adjusted) | kcal | 148 | 201 | 44 |
| Protein | g | 1.96 | 2.67 | 0.59 |
| Total lipid (fat) | g | 15.41 | 21 | 4.62 |
| Ash | g | 1.66 | 2.26 | 0.5 |
| Carbohydrate | g | 8.64 | 11.8 | 2.59 |
| Fiber | g | 6.8 | 9.2 | 2 |
| Sugars | g | 0.3 | 0.41 | 0.09 |
| Starch | g | 0.11 | 0.15 | 0.03 |
| 2. Minerals | ||||
| Calcium | mg | 13 | 18 | 4 |
| Iron | mg | 0.61 | 0.83 | 0.18 |
| Magnesium | mg | 29 | 39 | 9 |
| Phosphorus | mg | 54 | 73 | 16 |
| Potassium | mg | 507 | 690 | 152 |
| Sodium | mg | 8 | 11 | 2 |
| Zinc | mg | 0.68 | 0.92 | 0.2 |
| Copper | mg | 0.17 | 0.23 | 0.05 |
| Manganese | mg | 0.15 | 0.2 | 0.05 |
| Selenium | ug | 0.4 | 0.5 | 0.1 |
| 3. Vitamins and Phytochemicals | ||||
| Vitamin C | mg | 8.8 | 12 | 2.6 |
| Thiamine | mg | 0.08 | 0.1 | 0.02 |
| Riboflavin | mg | 0.14 | 0.19 | 0.04 |
| Niacin | mg | 1.91 | 2.6 | 0.57 |
| Pantothenic acid | mg | 1.46 | 2 | 0.44 |
| Vitamin B-6 | mg | 0.29 | 0.39 | 0.09 |
| Folate, dietary folate equivalents | μg | 89 | 121 | 27 |
| Choline total | mg | 14.2 | 19.3 | 4.3 |
| Betaine | mg | 0.7 | 1 | 0.2 |
| Vitamin B-12 | μg | 0 | 0 | 0 |
| Vitamin A | μg | 7 | 10 | 2 |
| β-Carotene | μg | 63 | 86 | 19 |
| α-Carotene | μg | 24 | 33 | 7 |
| β-Cryptoxanthin | μg | 27 | 37 | 8 |
| Lutein + zeaxanthin | μg | 271 | 369 | 81 |
| Vitamin E (α-tocopherol) | mg | 1.97 | 2.68 | 0.59 |
| Tocopherol β | mg | 0.04 | 0.05 | 0.01 |
| Tocopherol γ | mg | 0.32 | 0.44 | 0.1 |
| Tocopherol δ | mg | 0.02 | 0.03 | 0.01 |
| Vitamin K1 (phylloquinone) | μg | 21 | 28.6 | 6.3 |
| 4. Lipids | ||||
| Fatty acids, total monounsaturated | g | 9.799 | 13.3 | 2.94 |
| 16:1 | g | 0.698 | ||
| 17:1 | g | 0.01 | ||
| 18:1 | g | 9.066 | ||
| 20:1 | g | 0.025 | ||
| Fatty acids, total saturated | g | 2.126 | 2.9 | 0.64 |
| 8:0 | g | 0.001 | ||
| 16:0 | g | 2.075 | ||
| 18:0 | g | 0.049 | ||
| Fatty acids, total polyunsaturated | g | 1.816 | 2.47 | 0.55 |
| 18:2 | g | 1.674 | ||
| 18:3 | g | 0.125 | ||
| 18:3 n-3 c,c,c (ALA) | g | 0.111 | ||
| 18:3 n-6 c,c,c | g | 0.015 | ||
| 20:3 | g | 0.016 | ||
| Cholesterol | mg | 0 | 0 | 0 |
| Stigmasterol | mg | 2 | 3 | 1 |
| Campesterol | mg | 5 | 7 | 2 |
| β-sitosterol | mg | 76 | 103 | 23 |
| Variety | Part Studied | Types of Extract | Detection Assays | Major Findings | Type of Antioxidants | References |
|---|---|---|---|---|---|---|
| Hass | Pulp and peel + pulp | Expeller pressed oils | ABTS and HPLC-PDA | Higher antioxidant capacity, α-tocopherol and β-carotene content were observed in oils from the unpeeled microwave-dried pulp of ripe and unripe avocado. | Oils from the pulp of ripe unpeeled microwave-dried avocado had significantly greater phenolic acid and quercetin contents. | [28] |
| Hass | Peel | 50% (v/v) ethanol using accelerated solvent extraction | HPLC coupled to ultra-high-definition accurate-mass-QTOF | Sixty-one compounds belonging to 11 families were identified. | Procyanidins, flavonols, hydroxybenzoic, and hydroxycinnamic acids. | [90] |
| Hass | Seeds and seed coat | Accelerated solvent extraction | DPPH, TEAC, ORAC, HPLC-DAD-ESI-QTOF-MS | Significant antioxidant activity was observed in both seed and seed coat extracts. A total of 84 compounds were identified, among which 45 were phenolic compounds. | Condensed tannins, phenolic acids, and flavonoids. | [91] |
| Hass | Pulp | Oil extracted with or without ultrasound | HPLC | Similar quantities of α, β, γ, and δ-tocopherols and phenolic compounds were detected both with and without ultrasound extractions. | Tocopherols and phenols. | [109] |
| Hass | Seeds | Methanol and 50% (v/v) ethanol | HPLC, ABTS, FRAP, ORAC and methoxy radical scavenging activity by EPR | 50% (v/v) ethanol extract displayed greater antioxidant capacity in the ORAC, FRAP, and ABTS assays. | Chlorogenic acid, (−)-epicatechin, catechins and procyanidins. | [2] |
| Hass | Peel and seeds | Aqueous extract | ORAC | Peel extract showed higher antioxidant capacity than seed extract. | Epicatechin and chlorogenic acid were found in both extracts. | [101] |
| Hass | Pulp, peel, and seeds | Hexane to eliminate lipids and 80% methanol for phenolic extraction | HPLC-DAD-ESI-QTOF-MS | Higher concentrations of phenolic compounds were detected in the pulp and seed extract of overripe than in pulp and seed of optimally ripe fruit. The concentration of procyanidins increased after ripening. | Nine compounds in pulp, three in peel and three in seed. Procyanidins to degree of polymerization 2 to 6, and 13 were identified and quantified. | [96] |
| Hass | Peel, pulp, and seeds | Ultrasonic extraction with 80% (v/v) ethanol | DPPH, and ABTS | Seed and peel extracts exhibited greater antioxidant values and phenolic content than the pulp extract. | - | [102] |
| Hass | Peel, pulp, and seeds | Different solvents for different assays | DPPH and spectroscopic | All extracts exhibited significant antioxidant capacity. The seed extract had the greatest antioxidant activity, total phenolic content, and flavonoids compared to that of peel and pulp. | Carotenoids, phenolic compounds, flavonoids, vitamin c and tocopheryl acetate were detected in all extracts. | [106] |
| Hass | Pulp | Aqueous and ethanolic | FRAP and DPPH | Harvesting seasons affected the antioxidant capacity. | Positive correlations between FRAP and total phenolics, DPPH and total phenolics | [85] |
| Hass | Pulp | Hydrophilic and lipophilic extracts | DPPH, TEAC and ORAC | Higher antioxidant capacity values were obtained from lipophilic extracts compared to hydrophilic extracts. | A positive correlation was observed between DPPH/TEAC assays with palmitoleic, oleic, linoleic, α-linolenic acids. | [108] |
| Hass | Pulp | Acetone with 2,6-ditert-butyl-4-methylphenol, sodium carbonate, and sodium sulfate | HPLC-PDA | Seasonal variations in carotenoid were observed and α-tocopherol was detected. | Carotenoid such as: All-trans-neoxanthin; all-trans-violaxanthin; all-transneochrome; 9-cis-neoxanthin; all-trans-lutein-5,6-epoxide; chrysanthemaxanthin; lutein; zeaxanthin; β-cryptoxanthin; α-carotene; β-carotene were identified along with α-tocopherol. | [110] |
| Hass | Pulp | Tetrahydrofuran | DPPH | Low antioxidant activity. | A slight positive correlation against stearic acid content. | [111] |
| Hass | Leaves, pulp, peel, and seeds | Freeze-dried samples | FRAP, 4-dinitrophenylhydrazine and HPLC | The leaf, peel, and seed extracts had greater antioxidant capacity than that the pulp extracts. C7 sugars such as mannoheptulose and perseitol contributed to the antioxidant capacity of the pulp. | Vitamin C, anthocyanin, and C7 sugars. | [100] |
| Hass and Fuerte | Peel and seeds | 80% (v/v) ethanol with ultrasonic extraction | ABTS, DPPH, FRAP, and HPLC-ABTS | Peel extracts of both varieties displayed higher antioxidant capacity in the ABTS and FRAP assays compared to their seed extracts, whereas in the DPPH assay, seed extracts showed greater antioxidant activity. | Peel: procyanidin B2 and epicatechin Seed: trans-5-O-caffeoyl-D-quinic acid, procyanidin B1, catechin, and epicatechin. | [97] |
| Hass and Fuerte | Pulp, peel, and seeds | Ethyl acetate, 70% (v/v) acetone, and 70% (v/v) methanol | CUPRAC, DPPH, and ABTS | Acetone (70% v/v) was found to be the most effective solvent for extracting antioxidants. Peel and seed extracts exhibited greater antioxidant values in all three assays compared to pulp. | Peels and seeds: catechins, procyanidins, and hydroxycinnamic acids Pulp: hydroxybenzoic and hydroxycinnamic acids and procyanidin. | [104] |
| Hass and Shepard | Seeds and peel | 80% (v/v) methanol | HPLC-PAD, HPLC-ESI-MS, DPPH, ABTS and ORAC | The peel extracts displayed a higher total phenolic compound content and antioxidant activity in comparison to the seed extracts. Hass variety had a higher antioxidant capacity, which might be attributed to its procyanidin dimers and catechins than the Shepard variety. | Seed and peel extracts contained flavanol monomers, proanthocyanidins, and hydroxycinnamic acids. In addition, flavonol glycosides were detected in seed extracts. | [94] |
| Hass, Lamb-Hass, and Rugoro | Pulp | Methanol, ethanol, acetone, and ethyl acetate | HPLC-DAD-ESI-TOF | Seventeen compounds were identified using standards. Twenty-five compounds were tentatively identified. | Quinic acid, succinic acid, pantothenic acid, p-coumaroyl-D-glucose, abscisic acid, pentadecylfuran, avocado furan, and oleic acid were the most common compounds among the three avocado varieties. | [92] |
| Hass, Quintal, Margarida, and Fortuna | Peel, pulp, and seeds | Ethanol | ABTS, DPPH, FRAP | Peel extract of the Quintal variety showed the highest antioxidant capacity in all three assays. A similar trend was observed in terms of total phenolic and flavonoid contents. | Phenolics and flavonoids might contribute to the antioxidant capacity. | [99] |
| Hass, Bacon, Fuerte, Pinkerton, Rincon, and Orotawa | Pulp | Methanol | UHPLC-HE-MS | Pulp extracts had 19 individual phenolic compounds. A decrease in concentration of epicatechin concentration was observed with fruit ripening. | Gallic acid, sinapinic acid, vanillin, p-coumaric acid, gentisic acid, protocatechuic acid, 4-hydroxybenzoic acid, chlorogenic acid, and benzoic acid. | [89] |
| Hass, Hass Motril, ColinV 33, Gem, Harvest, Jiménez 1, Jiménez 2, Lamb Hass, Marvel, Nobel, Pinkerton, Sir Prize and Tacambaro | Pulp | Methanol | GC coupled to APCI-TOF MS and FID | Twenty-seven compounds were quantified by GC-APCI-MS. Seven compounds are quantified by GC-FID. The concentration of organic acids, flavonoids, and vitamins decreased, whereas phenolic acids, ferulic acids, or p-coumaric acids increased with the ripening process. | Quinic, ferulic, chlorogenic and p-coumaric acids, epicatechin, and quercetin. | [93] |
| Booth 7 | Pulp | Sodium acetate | ABTS | Total antioxidant capacity gradually increased with the ripening process. Treatment with aqueous 1-methylcyclopropene (1-MCP) significantly delayed the accumulation of total soluble phenolics, flavonoids, and total antioxidant capacity. | - | [112] |
| Collinson | Pulp | 80% methanol and acetone | ABTS, DPPH, and FRAP | Lipophilic extracts displayed greater antioxidant capacity in the ABTS and DPPH assays compared to hydrophilic extracts. The opposite trend was observed in the FRAP assay. | - | [113] |
| Fortuna | Fresh and dried seeds | Water, 70% (v/v) ethanol, 70% (v/v) methanol, and partition with n-hexane chloroform, ethyl acetate, and n-butanol | Spectroscopic and HPLC | Ethanol extract of dried seed showed 50, 38, and 24 mg/g of dry matter of total phenol, condensed tannins, and flavonoid contents, respectively. HPLC study revealed epicatechin (4.7 μg/mL), rutin (2.8 μg/mL), and chlorogenic acid (1.4 μg/mL) and quercetin in the extract. | Epicatechin, rutin, chlorogenic acid, quercetin. | [114] |
| Fortuna | Pulp | Oil extracted with SCO2 and compressed LPG | DPPH | The SCO2-extracted oil displayed higher antioxidant activity in the range of 17.4–82.5% compared to LPG-compressed oil. | - | [115] |
| Fortuna | Pulp | Lyophilized and cold pressed oil | GC-FID and GC-MS | A greater concentration of α-tocopherol and squalene were achieved with cold pressing. | α-tocopherol and squalene. | [116] |
| Fuerte | Pulp | Different solvents | FRAP, SOD and HPLC | Increase in the total antioxidant activity, SOD activity, and α-tocopherol content was observed in the presence of 1-MCP and low O2. | - | [117] |
| Lula | Pulp | Oil extracted with water at high temperatures | HPLC and spectroscopic assays | Greater quantity of α-tocopherol was detected compared to β, γ, and δ-tocopherols. In addition, sterols and carotenoids were also reported. | Tocopherols, sterols, and carotenoids were potent antioxidants. | [118] |
| Mexican landrace | Peel | Methanol | DPPH | Antioxidant values in the range of 53.31–307.33 mmol trolox equivalents/fresh weight were reported. | Activity can be attributed to anthocyanins. | [119] |
| Slimcado, Booth 7, Booth 8, Choquette, Loretta, Simmonds, and Tonnage | Pulp, peel, and seeds | Acetone, water, acetic acid | HPLC-MS, ORAC and DPPH | Seed extracts exerted the highest antioxidant activity, phenolic content, and procyanidins followed by peel and pulp. Significant correlations were observed among antioxidant capacities, phenolic contents, and procyanidins. Antioxidant activity can be attributed to the procyanidin content. | Catechin, epicatechin, A- and B-type dimers, A- and B-type trimers, tetramers, pentamers and hexamers were identified in peels and seeds. | [84] |
| - | Pulp | Supercritical CO2/ ethanol extracts | HPLC | Supercritical CO2 + ethanol at 200 bar and at 40 °C and 60 °C yielded significantly higher α-tocopherol content. | α-tocopherol | [120] |
| - | Seeds and pulp | Lipid | ABTS and DPPH | Seed extracts exhibited significantly greater antioxidant activity in both assays. Dose-dependent antioxidant activity was observed for both extracts. | - | [98] |
| - | Pulp | Oil extracted with mechanical pressing | DPPH | Greater antioxidant values were observed when the avocado pulp was dried at 60 °C under ventilation, and mechanical pressing was used for the oil extraction compared to vacuum oven and Soxhlet extraction. | α-tocopherol, phenolic compounds, carotenoids. | [121] |
| - | Seeds | Ultrasonic extraction with water | ORAC | Total antioxidant capacity increased with an increase in ultrasonic power. Positive correlation was observed between total polyphenolic content and antioxidant capacity. | - | [86] |
| - | Pulp | Acetone and its fractions | ORAC, HPLC-PDA/MS-TOF | Fractions with lipophilic acetogenins exhibited the highest antioxidant capacity. | 1-acetoxy-2,4-dihydroxy-n-heptadeca-16-ene; Persediene; Persenone-C; Persenone-A; Persenone-B; Persin, and 1-acetoxy-2,4-dihydroxy-heneicosa-12,15-diene. | [122] |
| - | Leaves | 50% ethanol extract | Spectroscopic, LC–ESI-MS, LCMS-IT-TOF | Glycosylated flavonoids were detected. | Quercetin-3-glucoside and quercetin-3-rhamnoside. | [95] |
| - | Seeds | Different concentrations of ethanol | ORAC | The antioxidant values increased with temperature. However, it was negatively impacted by ethanol concentration. | - | [123] |
| - | Leaves, pulp, peel, and seeds | 1M HCL and methanol | DPPH and FRAP | Greater DPPH radical scavenging activity, total phenol and flavonoid content were observed in leaf extracts. The peel extract showed the greatest FRAP value. | - | [103] |
| - | Pulp and seeds | 50% (v/v) ethanol | DPPH and FRAP | Seeds extracts showed significantly greater antioxidant values compared to that of pulp in both assays. Similar trend was observed for total phenolic content. | - | [105] |
| - | Peel | Different concentrations of ethanol | DPPH | Maximum antioxidant activity when extraction was performed with 48% (v/v) ethanol under agitation for 20 min at 70 °C and solvent-to-solid ratio (v/w) 20. | Positive correlation was observed between total phenolic content and antioxidants. | [88] |
| - | Seeds | Different concentrations of ethanol | DPPH | Extraction for 60 min with 30% (v/v) ethanol at 70 °C with a solvent to-solid material ratio of 8 yielded the maximum antioxidant capacity. | Positive correlation was observed between total phenolic content and antioxidants. | [87] |
| - | Leaves | Methanol, ethanol, cold and hot water | DPPH, FRAP, and hydroxyl radical scavenging ability | Significant antioxidant activity was observed in all three assays. | Antioxidant activity might be contributed by the phenolics and flavonoids. | [124] |
| - | Pulp | Oils extracted using Soxhlet, subcritical CO2 (SCO2) and ultrasound | ABTS, FRAP, and β-carotene bleaching | SCO2-extracted oil displayed significantly greater (p < 0.05) antioxidant capacity in all three assays compared to Soxhlet or ultrasound-extracted oils. | Strong positive correlations (p < 0.01) were found between α and γ tocopherols and antioxidant activity. | [125] |
| - | Leaves | Powdered leaves | Spectroscopic | Vitamin C, tannins, alkaloids and phenolic content were reported. | - | [126] |
| - | Pulp | Lipid-soluble bioactive | DPPH, reducing power, metal chelating, nitric oxide scavenging, hydrogen peroxide scavenging, hemoglobin-induced linoleic acid system | Exhibited lower antioxidant properties compared to vitamin C. | - | [127] |
| - | Pulp | Methanol + water | ABTS and TBARS | Lower antioxidant activity was reported compared to other fruits tested in the study. | - | [128] |
| - | Leaves and seeds | Water | DPPH, NO radical scavenging activity, inhibition of degradation of deoxyribose, Fe (II) chelating ability | Higher phenolic content and radical scavenging activity were observed in leaf extract. However, it showed lower iron chelation activity compared to the seed extract. | - | [129] |
| - | Seeds | Different solvents and fractions | DPPH | One fraction exhibited a radical scavenging activity of 81.6%. | - | [130] |
- ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt.
- TEAC: Trolox equivalent antioxidant capacity.
- DPPH: 2,2-Diphenyl-1-picrylhydrazyl.
- ORAC: Oxygen radical absorbance capacity.
- HPLC-PDA: High-performance liquid chromatography–photodiode array.
- HPLC-DAD-ESI-QTOF-MS: High-performance liquid chromatography–diode array detector–electrospray ionization–quadrupole time-of-flight mass spectrometry.
- HPLC-ESI-QTOF-MS: High-performance liquid chromatography–electrospray ionization–quadrupole time-of-flight mass spectrometry.
- FRAP: Ferric reducing ability of plasma.
- CUPRAC: Cupric reducing antioxidant capacity.
- SOD: Superoxide dismutase.
- HPLC-MS: High-performance liquid chromatography mass spectrometry.
- HPLC-ESI-MS: High-performance liquid chromatography–electrospray ionization–mass spectrometry.
- LC–ESI-MS: Liquid chromatography–electrospray ionization–mass spectrometry.
- UHPLC-HE-MS: Ultra high-performance liquid chromatography–heated electrospray–mass spectrometry.
- TBARS: Thiobarbituric acid reactive substances.
- HPLC-DAD-ESI-TOF: High performance liquid chromatography–diode array detector–electrospray ionization–time of flight.
- GC-APCI-TOF-MS: Gas chromatography–atmospheric pressure chemical ionization–time-of-flight mass spectrometry.
- GC-APCI-TOF-FID: Gas chromatography–atmospheric pressure chemical ionization–time-of-flight–flame ionization detector.
| Preclinical Studies | ||||||
|---|---|---|---|---|---|---|
| Variety | Parts | Type of Extracts | Bioactive Compounds | Type of Cell Lines | Major Findings and Molecular Mechanisms of Action | References |
| Hass | Seeds | Methanol | - | MCF-7 breast, H1299 lung, HT29 colon, and LNCaP prostate cancer cells | Dose-dependent inhibition of all cells with IC50 values 19–132 µg/mL after 48 h of treatment. In LNCaP prostate cancer cells, the induction of caspase 3-mediated apoptosis, PARP cleavage, downregulation of cyclin D1 and E2, cell cycle arrest at G0/G1 phase and reduction of nuclear translocation of nuclear factor kappa B (NF-κB) were observed. | [140] |
| Hass | Seeds | High-speed countercurrent chromatographic fraction of methanol-water partition (M7) | Proanthocyanidins B1, B2 and A-type trimer. Traces of abscisic acid glucosides. | HaCaT immortalized nontumorigenic human epidermal cells | Significant inhibition of cell proliferation, increased LDH activity. Molecular mechanisms of action were not investigated. | [23] |
| Hass | Pulp | Chloroform-soluble | Two aliphatic acetogenins- (2S,4S)-2,4-dihydroxyheptadec 16-enyl acetate] and 2 [(2S,4S)-2,4-dihydroxyheptadec-16-ynyl acetate. | 83–01-82CA human oral cancer cell line, MEK overexpressing cell line 83–01-82CA/MEKCA | The two aliphatic acetogenins targeted the EGFR/RAS/RAF/MEK/ERK1/2 cancer pathway by synergistically inhibiting c-RAF (Ser338) and ERK1/2 (Thr202/Tyr204) phosphorylation. | [165] |
| Hass | Pulp | Chloroform | - | 83-01-82CA human oral cancer and TE1177 normal epithelial cell lines | In the oral cancer cells, the extract induced apoptosis by increasing the levels of reactive oxygen species by twofold to threefold. Apoptosis was not induced in the normal cell line. | [141,142] |
| Hass | Pulp | Acetone | Lutein, zeaxanthin, β-cryptoxanthin, α-carotene, and β-carotene, α-tocopherol and γ-tocopherol. | LNCaP androgen-dependent and PC-3 androgen-independent prostate cancer cell lines | Inhibited the growth of both the prostate cancer cell lines. Arrested PC-3 cells at the G2/M phase and increased the expression of p27 protein. | [24] |
| Lulu | Unripe fruit pulp | 95% (v/v) ethanol extracts and its fractions | 1,2,4-Trihydroxynonadecane, 1,2,4-Trihydroxyheptadec-16-ene and 1,2,4-Trihydroxyheptadec-16-yne. | A-549 human lung, MCF-7 human breast, HT-29 human colon, A-498 human Kidney, MIA PaCa-2 human pancreatic carcinoma, PC-3 human prostate cancer cells | All three compounds were active against six human tumor cell lines and exhibited selectivity against PC-3 cells. Molecular mechanisms were not studied. | [21] |
| - | Seeds | Ethanol extract and its hexane and dichloromethane fractions | - | Lung A549 and gastric BGC823 cancer cells | Growth inhibition at 200 μg/mL. The IC50 values and molecular mechanisms of action were not investigated. | [166] |
| - | Pulp and seed extracts | Lipids | Fatty acids, hydrocarbon, and sterols. | HCT116 colon and HePG2 liver cancer cell lines | Seed extract showed greater activity against HCT116 (IC50 < 4 µg/mL) and HePG2 (IC50 < 20 µg/mL) cell lines compared to the pulp extract. Molecular mechanisms of action were not investigated. | [98] |
| - | Seeds | Chloroform extracts and its soluble methanol fraction (FML) and non-soluble methanol fraction (FTML). | - | MCF-7 breast cancer cell line | Chloroform extract, FML, and FTML inhibited cell growth in a dose-dependent manner and displayed IC50 values of 94.87, 34.52, and 66.03 µg/mL, respectively. FML induced apoptosis and arrested cells at the subG1/G0 phase. | [167] |
| - | Leaves | Silver nanoparticles | MCF-7 breast and HeLa cervical cancer cells | Dose-dependent cytotoxicity was observed at concentrations above 50 μM in MCF-7 but not in HeLa cells. Downregulation of p53 expression was observed in both cell lines. | [168] | |
| - | Leaves | Aqueous-ethanol (5% v/v) | - | Larynx cancer tissue | Significant increase in adenosine deaminase activity in cancerous tissues derived from 13 patients who underwent surgery for larynx cancer (median age of 57 years) compared to noncancerous (r = 0.60, p = 0.029) tissues. | [169] |
| - | Seeds | Fraction of ethanol extract | Triterpenoid | MCF-7 breast and HepG2 liver cancer cells | Inhibited MCF-7 (IC50 = 62 µg/mL) and HepG2 (IC50 = 12 µg/mL) cells with no activity against normal cells. Molecular mechanisms of action were not investigated. | [170] |
| - | Pulp | Ethanol, chloroform, ethyl acetate, and petroleum. | - | Esophageal squamous cell carcinoma and colon adenocarcinoma cell line | Moderate activity. The IC50 values and molecular mechanisms of action were not investigated. | [171] |
| - | Pulp | Aqueous | - | A549 lung, HepG-2 liver, HT-29 colon, and MCF-7 breast cancer cells. | Exhibited LC50 values in the range of 13.3–54.5 µg/mL against the tested cell lines. Molecular mechanisms of action were not investigated. | [172] |
| - | Root bark | Methanol extract and its fractions. | 4-hydroxy-5-methylene-3-undecyclidenedihydrofuran-2 (3H)- one | MCF-7 breast cancer cell line | Antiproliferative activity with an IC50 value of 20.48 μg/mL with induction of apoptosis. | [36] |
| - | Endocarp, whole seed, seed and leaves | Ethanol | - | Jurkat lymphoblastic leukemia cells | Induced significant oxidative stress-dependent apoptosis via mitochondrial membrane depolarization. Activated transcription factor p53, protease caspase-3, and apoptosis-inducing factor (APAF). | [138] |
| - | Pulp | 50% (v/v) Methanol | - | Human lymphocyte cells | Chemoprotective against cyclophosphamide-induced chromosomal aberrations at 200 mg/kg body weight. | [158] |
| - | Seeds and peel | Methanol | - | MDA-MB-231 breast cancer cells | Apoptosis due to activation of caspase-3 and its target protein, PARP. | [144] |
| - | Leaves | - | Persin | In vitro: MDA-MB-231, MCF-7, and T-47D breast cancer cells In vivo: Quackenbush lactating mice | In vitro: Persin selectively arrested cells at the G2/M phase and induced caspase-dependent apoptosis. Apoptosis was dependent on the expression of Bim protein, which also indicated the microtubule-stabilizing properties of persin. Overall, MCF-7 and T-47D cells were more sensitive to persin compared to MDA-MB-231. In vivo: Persin exerted cytotoxicity in the lactating mammary epithelium. | [139] |
| MCF-7, T-47D, and SK-Br3 breast cancer and MCF-10A human mammary epithelial cells. | Synergistic interaction between tamoxifen and persin against the tested breast cancer cells was observed. Significant reduction of IC50 values of tamoxifen when combined with 13.8 μmol/L of persin. The synergistic cytotoxicity was Bim-dependent and mediated by the modulation of ceramide metabolism. | [149] | ||||
| - | Fruit | - | Persenone A | In vitro: RAW 264.7 mouse macrophage cells In vivo: Female ICR mice (7 weeks old) | Downregulated the expression of iNOS/COX-2 (nitric oxide synthase/cyclooxygenase-2) in macrophage cells. When applied topically, reduced the generation of H2O2 in mouse skin. | [173] |
| - | Fruit | - | (2R)-(12Z,15Z)-2-hydroxy-4-oxoheneicosa-12,15-dien-1-yl acetate (1), persenone A (2) and B (3) | HL-60 acute promyelocytic leukemia and RAW 264.7 mouse macrophage cells. | Suppressed the growth of HL-60 cells (compound 1, IC50 = 33.7; compound 2, IC50 = 1.4; compound 33, IC50 = 1.8 μM). Inhibited nitric oxide generation induced by lipopolysaccharide in combination with interferon-γ in RAW 264.7 cells. | [19] |
| - | - | - | Scopoletin | In vivo: Skin papilloma in mice induced by 7,12-dimethylbenz(a)anthracene and croton oil | Reduced carcinogen-induced toxicity and led to decrease in the size of skin papilloma. Downregulated AhR, CYP1A1, PCNA, stat-3, survivin, MMP-2, cyclin D1, and c-myc, and upregulated p53, caspase-3, and TIMP-2. | [26] |
| Chemical synthesis | Type of cell lines | Major findings and molecular mechanisms of action | References | |||
| Antimicrobial peptide-PaDef defensin | K562 chronic myeloid leukemia cells | Cytotoxic with an IC50 value of 97.3 μg/mL. Activated caspase-8 and induced the expression of TNF-α. | [153] | |||
| MCF-7 breast cancer cell line | Inhibited the growth in a concentration-dependent manner (IC50 = 141.62 µg/mL). Induced cytochrome c, APAF-1, and the caspase 7 and 9 expressions, loss of mitochondrial Δψm and enhanced the phosphorylation of MAPK p38. | [143] | ||||
| Persin and tetrahydropersin | Breast cancer: MCF-7, T-47D, MDA-MB-468, MDA-MB-157, SkBr3, Hs578T, MDA-MB-231 cells, normal mammary epithelial MCF-10A cells, Ovarian cancer: OVCAR3 and IGROV-1 cells Prostate cancer: PC-3 and LNCaP cells | Persin was more potent compared to tetrahydropersin against most of the tested cancer cell lines with IC50 values in the range 15.1 ± 1.3 to more than 39 μM. Molecular mechanisms of action was not studied. | [154] | |||
| β-Hydroxy-α,β-unsaturated ketones | A2780 human ovarian, SW1573 lung, HBL-100 human breast, T-47D human breast and WiDr colorectal cancer cells. | GI50 values in the range of 0.5–3.9 μM. Induced apoptosis and dose-dependent cell cycle arrest in the S and G2/M phase. | [145] | |||
| Case-control studies | ||||||
| Type of cancer | Major findings | References | ||||
| Prostate cancer | A study involving 243 men with prostate cancer and 273 controls in Jamaica reported that monounsaturated fat from avocado was associated with reduced risk of prostate cancer. | [160] | ||||
| Bioactive Compounds | Type of Cancer | Type of Study | Major Findings | References |
|---|---|---|---|---|
| Carotenoids- α-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin | Breast cancer | A nested case-control study in women consisting of 604 breast cancer cases and 626 controls. | In women with high mammographic density, plasma levels of carotenoids reduced breast cancer risk significantly (40–50% reduction, p < 0.05). | [162] |
| An ancillary study involving 207 women ages 18 to 70 years who had been successfully treated for early-stage breast cancer. | An inverse association between total plasma carotenoid concentrations and the oxidative stress biomarkers (urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostaglandin-F2α) was observed. | [163] | ||
| Larynx, pharynx and oral cancers | The study population involving 52 patients curatively treated for early-stage larynx, pharynx or oral cavity during 1997–2001. | An inverse association was observed between individual/grouped xanthophylls and urinary F2-isoprostanes (F2-IsoPs), a biomarker of oxidative stress. However, individual/grouped carotenes did not show such association with F2-IsoPs. | [161] | |
| Glutathione | Advanced colorectal carcinoma | A randomized, double blind, placebo-controlled trial in 52 patients. | Prevented of oxaliplatin-induced neuropathy without reducing the clinical efficacy of oxaliplatin. | [57] |
| Ovarian cancer | A multicenter, randomized, double-blind, parallel group design with 51 women. | Reduced the cisplatin-associated toxicity and improved the quality of life. | [58] | |
| Oral cancer | A population-based case-control study involving 1,830 Caucasian participants (855 cases and 975 controls) in during 1984–1985 in the United States. | Reduced oral cancer risk was associated with glutathione when fruit and vegetable were commonly consumed raw. | [59] |
| Variety/ies | Bacteria | Highlights | Reference |
|---|---|---|---|
| Hass Shepard Fuerte | Listeria monocytogenes Staphylococcus epidermidis Staphylococcus aureus Enterococcus faecalis Escherichia coli Salmonella Enteritidis Citrobacter freundii Pseudomonas aeruginosa Salmonella Typhimurium Enterobacter aerogenes | The antimicrobial activity of peel and seed extracts was evaluated. Ethanol extracts showed antimicrobial activity against both Gram-positive and Gram-negative bacteria (except E. coli). Aqueous extracts had antimicrobial activity against L. monocytogenes and S. epidermidis. | [176] |
| Hass Fuerte | Bacillus cereus S. aureus L. monocytogenes E. coli Pseudomonas spp. Yarrowia lipolytica | All avocado parts had antimicrobial activities. Pulp showed the highest antimicrobial activity. Gram-positive bacteria were found to be more sensitive than Gram-negative bacteria. | [104] |
| Hass | L. monocytogenes | The antilisterial properties of an enriched acetogenin extract from avocado seed were determined. Seeds had higher acetogenin content than pulp. The antimicrobial effect was probably caused by increased membrane permeability. | [177] |
| Lorena Hass | S. aureus E. coli | Extracts did not have antimicrobial activity against S. aureus ATCC 29213 and E. coli ATCC 25922 | [179] |
| Hass | Listeria innocua E. coli Lactobacillus sakei Weissella viridescens Leuconostoc mesenteroides | Peel and seed extracts did not present antimicrobial activity against any bacteria analyzed. | [101] |
| Extracts and Compounds | Key Findings and Molecular Mechanism of Action | Reference |
|---|---|---|
| Leaf aqueous extract | Reduced carrageenan-induced rat paw oedema. | [185] |
| Persenone A | Reduced inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in activated murine macrophages. | [173] |
| Avacado oil | Promoted increased collagen synthesis and decreased inflammation in wound healing on incisional and excisional cutaneous wound models in Wistar rats. | [186] |
| (2R)-(12Z,15Z)-2-hydroxy-4-oxoheneicosa-12,15-dien-1-yl acetate, persenone A and B | Decreased nitric oxide generation in activated mouse macrophages. | [19] |
| Avocado–Soybean Unsaponifiables (ASU) | Inhibited collagenase, stromelysin, IL-6, IL-8, and prostaglandin E2 (PGE2) release in activated human articular chondrocytes. | [192] |
| Stimulated glycosaminoglycan and hydroxyproline synthesis, and inhibited the production of hydroxyproline in the granulomatous tissue of mice model. | [193] | |
| Suppressed critical regulators of the inflammatory response such as PGE-2 and COX-2 in activated human chondrocytes. | [195] | |
| Decreased catabolic enzymes, matrix metalloproteinases-3 and -13 expressions via inactivating the expression of MAPKs (ERK 1/2) and nuclear factor kappa-B (NF-κB) in activated mouse or human chondrocytes. | [190] | |
| Reduced pro-inflammatory cytokines such as TNF-α, IL-1β, and iNOS expression in activated chondrocytes and THP-1 monocyte and macrophages. | [196] | |
| Exhibited a promising result on the bone repair by modulating the molecular targets of Rankl and Il1β, RANKL, TRAP in rat model. | [197] | |
| Decreased pain symptoms in patients with osteoarthritis of the temporomandibular joint. | [203] | |
| Modulated the expression of TGF-β1, TGF-β2, and BMP-2 in activated human periodontal ligament and human alveolar bone cells. | [206] | |
| ASU + Epigallocatechin gallate | Inhibited COX-2 expression and PGE2 production in activated equine chondrocytes. | [204] |
| Inhibited the gene expression of IL-1β, TNF-α, IL-6, COX-2, and IL-8 in activated equine chondrocytes. | [189] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants 2019, 8, 426. https://doi.org/10.3390/antiox8100426
Bhuyan DJ, Alsherbiny MA, Perera S, Low M, Basu A, Devi OA, Barooah MS, Li CG, Papoutsis K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants. 2019; 8(10):426. https://doi.org/10.3390/antiox8100426
Chicago/Turabian StyleBhuyan, Deep Jyoti, Muhammad A. Alsherbiny, Saumya Perera, Mitchell Low, Amrita Basu, Okram Abemsana Devi, Mridula Saikia Barooah, Chun Guang Li, and Konstantinos Papoutsis. 2019. "The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits" Antioxidants 8, no. 10: 426. https://doi.org/10.3390/antiox8100426
APA StyleBhuyan, D. J., Alsherbiny, M. A., Perera, S., Low, M., Basu, A., Devi, O. A., Barooah, M. S., Li, C. G., & Papoutsis, K. (2019). The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants, 8(10), 426. https://doi.org/10.3390/antiox8100426