Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta
Abstract
:1. Introduction
2. Dietary Phenolic Compounds
2.1. Structure
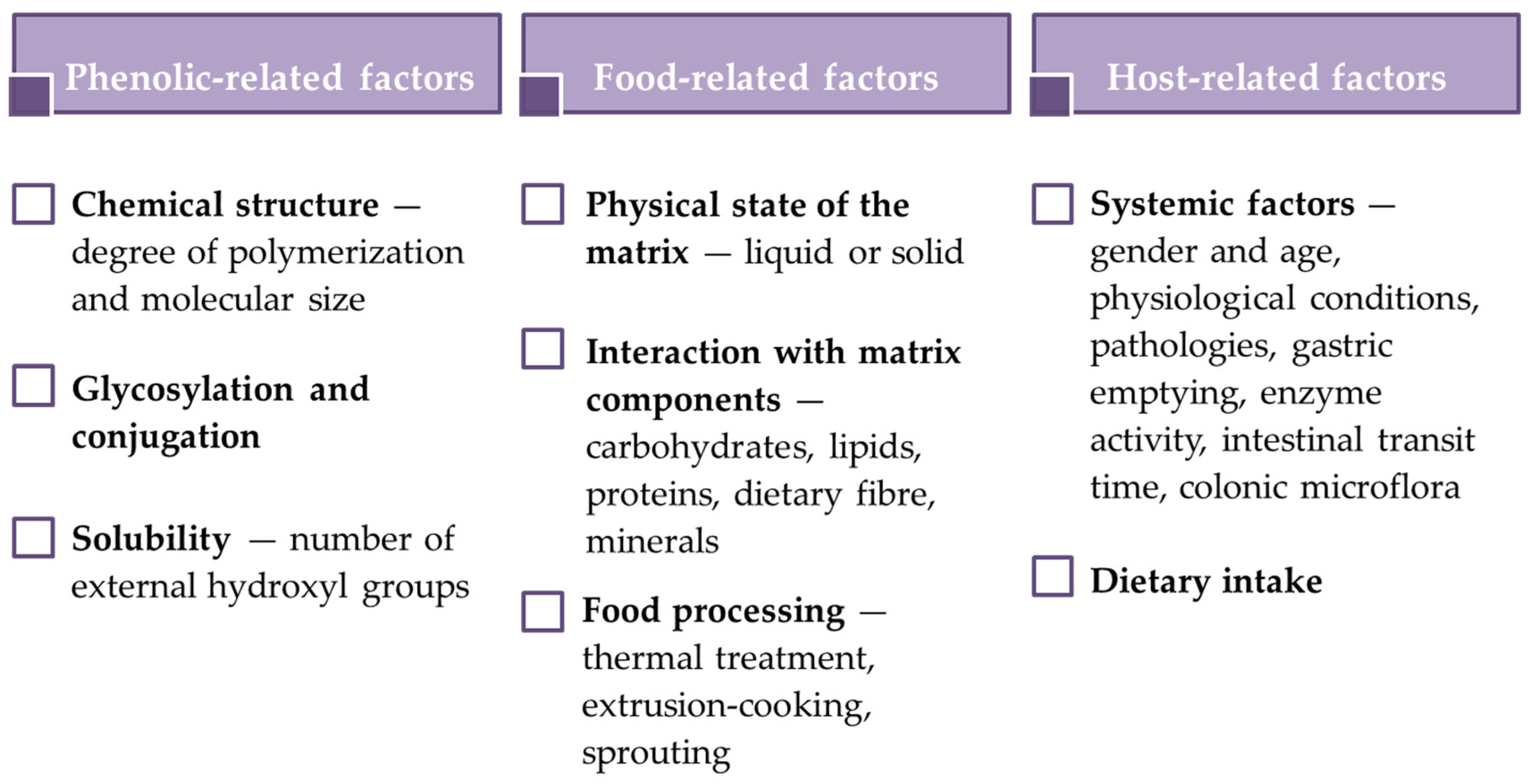
2.2. Bioaccessibility, Biotransformation and Bioavailability
Methods to Evaluate Phenolic Compound Bioaccessibility and Bioavailability
3. Strategies to Modulate Phenolic Compound Content in Pasta
3.1. Use of Functional Ingredients in Pasta-Making
3.1.1. Whole Grain Flours
3.1.2. Composite Flours
3.1.3. Powders and Extracts from Plant Foods and Food By-Products
3.2. Raw Material Processing, Pasta-Making and Pasta Cooking
4. Bioaccessibility of Phenolic Compounds in Pasta
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 59, pp. 79–108. [Google Scholar]
- Jelena, C.H.; Giorgio, R.; Justyna, G.; Neda, M.D.; Natasa, S.; Artur, B.; Giuseppe, G. Beneficial effects of polyphenols on chronic diseases and ageing. In Polyphenols: Properties, Recovery and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 69–102. ISBN 9780128135723. [Google Scholar]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://fdc.nal.usda.gov/ (accessed on 2 March 2020).
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, M.; Jensen, M.G.; Riboldi, G.; Petronio, M.; Bügel, S.; Toubro, S.; Tetens, I.; Astrup, A. Wholegrain vs. refined wheat bread and pasta. Effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite 2010, 54, 163–169. [Google Scholar] [CrossRef]
- Zou, W.; Sissons, M.; Warren, F.J.; Gidley, M.J.; Gilbert, R.G. Compact structure and proteins of pasta retard in vitro digestive evolution of branched starch molecular structure. Carbohydr. Polym. 2016, 152, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Korczak, R.; Timm, D.; Ahnen, R.; Thomas, W.; Slavin, J.L. High Protein Pasta is Not More Satiating than High Fiber Pasta at a Lunch Meal, Nor Does it Decrease Mid-Afternoon Snacking in Healthy Men and Women. J. Food Sci. 2016, 81, S2240–S2245. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Bailey, R. Association of Pasta Consumption with Diet Quality and Nutrients of Public Health Concern in Adults: National Health and Nutrition Examination Survey 2009–2012. Curr. Dev. Nutr. 2017, 1, e001271. [Google Scholar] [CrossRef] [Green Version]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Yeo, J.D. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Katzung, B.G. Basic & Clinical Pharmacology, 14th ed.; Weitz, M., Boyle, P., Eds.; McGraw Hill Education: New York, NY, USA, 2014; ISBN 978-1-260-28817-9. [Google Scholar]
- Schönfeldt, H.C.; Pretorius, B.; Hall, N. Bioavailability of Nutrients. In Encyclopedia of Food and Health; Elsevier Ltd: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Galanakis, C.M. Nutraceuticals and Natural Product Pharmaceuticals; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128164501. [Google Scholar]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Lafarga, T.; Villaró, S.; Bobo, G.; Simó, J.; Aguiló-Aguayo, I. Bioaccessibility and antioxidant activity of phenolic compounds in cooked pulses. Int. J. Food Sci. Technol. 2019, 54, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The profile and bioaccessibility of phenolic compounds in cereals influenced by improved extrusion cooking treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Li, Y.; Hou, D.X.; Wu, S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [Green Version]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minekus, M.; Marteau, P.; Havenaar, R.; Huis in’t Veld, J.; Minekus, M.; Huis in’t Veld, J.; Huisintveld, J.A. Multicompartmental Dynamic Computer-Controlled Model Simulating the Stomach and Small Intestine. ATLA 1995, 23, 197–209. [Google Scholar]
- Nicoletti, M. Nutraceuticals and botanicals: Overview and perspectives. Int. J. Food Sci. Nutr. 2012, 63, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.; Oniszczuk, A.; Kasprzak, K.; Olech, M.; Mitrus, M.; Oniszczuk, T. Chemical composition and selected quality characteristics of new types of precooked wheat and spelt pasta products. Food Chem. 2020, 309, 125673. [Google Scholar] [CrossRef]
- Chen, C.Y.O.; Kamil, A.; Blumberg, J.B. Phytochemical composition and antioxidant capacity of whole wheat products. Int. J. Food Sci. Nutr. 2015, 66, 63–70. [Google Scholar] [CrossRef]
- Hirawan, R.; Ser, W.Y.; Arntfield, S.D.; Beta, T. Antioxidant properties of commercial, regular- and whole-wheat spaghetti. Food Chem. 2010, 119, 258–264. [Google Scholar] [CrossRef]
- Turco, I.; Bacchetti, T.; Bender, C.; Zimmermann, B.; Oboh, G.; Ferretti, G. Polyphenol content and glycemic load of pasta enriched with Faba bean flour. Funct. Foods Heal. Dis. 2016, 6, 291. [Google Scholar] [CrossRef] [Green Version]
- Cota-Gastélum, A.G.; Salazar-García, M.G.; Espinoza-López, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Wong-Corral, F.J.; Del-Toro-Sánchez, C.L. Characterization of pasta with the addition of Cicer arietinum and Salvia hispanica flours on quality and antioxidant parameters. Ital. J. Food Sci. 2019, 31, 626–643. [Google Scholar]
- Sȩczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Effect of carob (Ceratonia siliqua L.) flour on the antioxidant potential, nutritional quality, and sensory characteristics of fortified durum wheat pasta. Food Chem. 2016, 194, 637–642. [Google Scholar]
- Cárdenas-Hernández, A.; Beta, T.; Loarca-Piña, G.; Castaño-Tostado, E.; Nieto-Barrera, J.O.; Mendoza, S. Improved functional properties of pasta: Enrichment with amaranth seed flour and dried amaranth leaves. J. Cereal Sci. 2016, 72, 84–90. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Tovar, J.; Bello-Pérez, L.A. Influence of blue maize flour on gluten-free pasta quality and antioxidant retention characteristics. J. Food Sci. Technol. 2018, 55, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Palavecino, P.M.; Ribotta, P.D.; León, A.E.; Bustos, M.C. Gluten-free sorghum pasta: Starch digestibility and antioxidant capacity compared with commercial products. J. Sci. Food Agric. 2019, 99, 1351–1357. [Google Scholar] [CrossRef]
- Jalgaonkar, K.; Jha, S.K.; Mahawar, M.K. Influence of incorporating defatted soy flour, carrot powder, mango peel powder, and moringa leaves powder on quality characteristics of wheat semolina-pearl millet pasta. J. Food Process. Preserv. 2018, 42, e13575. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef]
- Michalak-Majewska, M.; Teterycz, D.; Muszyński, S.; Radzki, W.; Sykut-Domańska, E. Influence of onion skin powder on nutritional and quality attributes of wheat pasta. PLoS ONE 2020, 15, e0227942. [Google Scholar] [CrossRef] [Green Version]
- Padalino, L.; D’Antuono, I.; Durante, M.; Conte, A.; Cardinali, A.; Linsalata, V.; Mita, G.; Logrieco, A.; Del Nobile, M. Use of Olive Oil Industrial By-Product for Pasta Enrichment. Antioxidants 2018, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Marinelli, V.; Padalino, L.; Nardiello, D.; Del Nobile, M.A.; Conte, A. New Approach to Enrich Pasta with Polyphenols from Grape Marc. J. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Pasqualone, A.; Delvecchio, L.N.; Gambacorta, G.; Laddomada, B.; Urso, V.; Mazzaglia, A.; Ruisi, P.; Di Miceli, G. Effect of Supplementation with Wheat Bran Aqueous Extracts Obtained by Ultrasound-Assisted Technologies on the Sensory Properties and the Antioxidant Activity of Dry Pasta. Nat. Prod. Commun. 2015, 10, 1739–1742. [Google Scholar] [CrossRef] [Green Version]
- Menga, V.; Amato, M.; Phillips, T.D.; Angelino, D.; Morreale, F.; Fares, C. Gluten-free pasta incorporating chia (Salvia hispanica L.) as thickening agent: An approach to naturally improve the nutritional profile and the in vitro carbohydrate digestibility. Food Chem. 2017, 221, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia Fruits as Food Enriching Ingredient, the First Step towards New Functional Food Products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef] [Green Version]
- Oniszczuk; Widelska; Wójtowicz; Oniszczuk; Wojtunik-Kulesza; Dib; Matwijczuk Content of Phenolic Compounds and Antioxidant Activity of New Gluten-Free Pasta with the Addition of Chestnut Flour. Molecules 2019, 24, 2623. [CrossRef] [Green Version]
- Abbasi Parizad, P.; Marengo, M.; Bonomi, F.; Scarafoni, A.; Cecchini, C.; Pagani, M.A.; Marti, A.; Iametti, S. Bio-Functional and Structural Properties of Pasta Enriched with a Debranning Fraction from Purple Wheat. Foods 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanoletti, M.; Abbasi Parizad, P.; Lavelli, V.; Cecchini, C.; Menesatti, P.; Marti, A.; Pagani, M.A. Debranning of purple wheat: Recovery of anthocyanin-rich fractions and their use in pasta production. LWT—Food Sci. Technol. 2017, 75, 663–669. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Taddei, F.; Nicoletti, I.; Gazza, L.; Corradini, D.; D’Egidio, M.G.; Martini, D. Use of bran fractions and debranned kernels for the development of pasta with high nutritional and healthy potential. Food Chem. 2017, 225, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Ciccoritti, R.; Nicoletti, I.; Nocente, F.; Corradini, D.; D’Egidio, M.G.; Taddei, F. From seed to cooked pasta: Influence of traditional and non-conventional transformation processes on total antioxidant capacity and phenolic acid content. Int. J. Food Sci. Nutr. 2018, 69, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Merendino, N.; Molinari, R.; Costantini, L.; Mazzucato, A.; Pucci, A.; Bonafaccia, F.; Esti, M.; Ceccantoni, B.; Papeschi, C.; Bonafaccia, G. A new “functional” pasta containing tartary buckwheat sprouts as an ingredient improves the oxidative status and normalizes some blood pressure parameters in spontaneously hypertensive rats. Food Funct. 2014, 5, 1017–1026. [Google Scholar] [CrossRef]
- Bruno, J.A.; Konas, D.W.; Matthews, E.L.; Feldman, C.H.; Pinsley, K.M.; Kerrihard, A.L. Sprouted and Non-Sprouted Chickpea Flours: Effects on Sensory Traits in Pasta and Antioxidant Capacity. Polish J. Food Nutr. Sci. 2019, 69, 203–209. [Google Scholar] [CrossRef]
- Bouasla, A.; Wójtowicz, A.; Zidoune, M.N.; Olech, M.; Nowak, R.; Mitrus, M.; Oniszczuk, A. Gluten-Free Precooked Rice-Yellow Pea Pasta: Effect of Extrusion-Cooking Conditions on Phenolic Acids Composition, Selected Properties and Microstructure. J. Food Sci. 2016, 81, C1070–C1079. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Olech, M. The Impact of Processing Parameters on the Content of Phenolic Compounds in New Gluten-Free Precooked Buckwheat Pasta. Molecules 2019, 24, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula, R.; Rabalski, I.; Messia, M.C.; Abdel-Aal, E.S.M.; Marconi, E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res. Int. 2017, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Podio, N.S.; Baroni, M.V.; Pérez, G.T.; Wunderlin, D.A. Assessment of bioactive compounds and their in vitro bioaccessibility in whole-wheat flour pasta. Food Chem. 2019, 293, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Montesano, D.; Masoero, F.; Trevisan, M. Impact of boiling on free and bound phenolic profile and antioxidant activity of commercial gluten-free pasta. Food Res. Int. 2017, 100, 69–77. [Google Scholar] [CrossRef]
- Van Der Kamp, J.W.; Poutanen, K.; Seal, C.J.; Richardson, D.P. The HEALTHGRAIN definition of “whole grain”. Food Nutr. Res. 2014, 58, 22100. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.B.; van der Kamp, J.W.; King, R.; Lê, K.A.; Mejborn, H.; Seal, C.J.; Thielecke, F. Perspective: A definition for whole-grain food products—Recommendations from the Healthgrain Forum. Adv. Nutr. 2017, 8, 525–531. [Google Scholar]
- Slavin, J.; Tucker, M.; Harriman, C.; Jonnalagadda, S.S. Whole grains: Definition, dietary recommendations, and health benefits. Cereal Foods World 2013, 58, 191–198. [Google Scholar] [CrossRef]
- Philip Karl, J.; McKeown, N.M. Whole Grains in the Prevention and Treatment of Abdominal Obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 515–528. ISBN 9780124078697. [Google Scholar]
- Kris-Etherton, P.M.; Ohlson, M.; Bagshaw, D.; Stone, N.J. Dietary Patterns for the Prevention and Treatment of Cardiovascular Disease. In Clinical Lipidology: A Companion to Braunwald’s Heart Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 217–231. ISBN 9781416054696. [Google Scholar]
- Wang, L.; Sikand, G.; Wong, N.D. Nutrition, Diet Quality, and Cardiovascular Health. In Molecular Basis of Nutrition and Aging: A Volume in the Molecular Nutrition Series; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 315–330. ISBN 9780128018279. [Google Scholar]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J. Food Sci. Technol. 2015, 52, 3681–3688. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Schoenlechner, R. Quinoa: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains: Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 105–129. ISBN 9780081008911. [Google Scholar]
- D’Amico, S.; Schoenlechner, R. Amaranth: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains: Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 131–159. ISBN 9780081008911. [Google Scholar]
- Alencar, N.M.M.; de Carvalho Oliveira, L. Advances in Pseudocereals: Crop Cultivation, Food Application, and Consumer Perception; Springer: Cham, Switzerland, 2019; pp. 1695–1713. [Google Scholar]
- Inglett, G.E.; Chen, D.; Liu, S.X. Physical properties of gluten-free sugar cookies made from amaranth-oat composites. LWT—Food Sci. Technol. 2015, 63, 214–220. [Google Scholar] [CrossRef]
- Chauhan, A.; Saxena, D.C.; Singh, S. Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT—Food Sci. Technol. 2015, 63, 939–945. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ruzzi, M. Current and Forward-Looking Approaches to Technological and Nutritional Improvements of Gluten-Free Bread with Legume Flours: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1101–1122. [Google Scholar] [CrossRef]
- Siyuan, S.; Tong, L.; Liu, R. Corn phytochemicals and their health benefits. Food Sci. Hum. Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Althwab, S.; Carr, T.P.; Weller, C.L.; Dweikat, I.M.; Schlegel, V. Advances in grain sorghum and its co-products as a human health promoting dietary system. Food Res. Int. 2015, 77, 349–359. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Schieber, A. Side Streams of Plant Food Processing As a Source of Valuable Compounds: Selected Examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. LWT-Food Sci. Technol. 2015, 66, 41–46. [Google Scholar] [CrossRef]
- Pandiella, S.S.; Mousia, Z.; Laca, A.; Díaz, M.; Webb, C. DEBRANNING TECHNOLOGY TO IMPROVE CEREAL-BASED FOODS. In Using Cereal Science and Technology for the Benefit of Consumers; Elsevier: Amsterdam, The Netherlands, 2005; pp. 241–244. [Google Scholar]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Kim-Anne, L.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [Green Version]
- Świeca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Ha, K.S.; Jo, S.H.; Mannam, V.; Kwon, Y.I.; Apostolidis, E. Stimulation of Phenolics, Antioxidant and α-Glucosidase Inhibitory Activities during Barley (Hordeum vulgare L.) Seed Germination. Plant Foods Hum. Nutr. 2016, 71, 211–217. [Google Scholar] [CrossRef]
- Hithamani, G.; Srinivasan, K. Bioaccessibility of polyphenols from selected cereal grains and legumes as influenced by food acidulants. J. Sci. Food Agric. 2017, 97, 621–628. [Google Scholar] [CrossRef]
- Pal, P.; Singh, N.; Kaur, P.; Kaur, A.; Virdi, A.S.; Parmar, N. Comparison of Composition, Protein, Pasting, and Phenolic Compounds of Brown Rice and Germinated Brown Rice from Different Cultivars. Cereal Chem. J. 2016, 93, 584–592. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Ohm, J.-B.; Lee, C.W.; Cho, K. Germinated Wheat: Phytochemical Composition and Mixing Characteristics. Cereal Chem. J. 2016, 93, 612–617. [Google Scholar] [CrossRef]
- Ti, H.; Zhang, R.; Zhang, M.; Li, Q.; Wei, Z.; Zhang, Y.; Tang, X.; Deng, Y.; Liu, L.; Ma, Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef]
- Erba, D.; Angelino, D.; Marti, A.; Manini, F.; Faoro, F.; Morreale, F.; Pellegrini, N.; Casiraghi, M.C. Effect of sprouting on nutritional quality of pulses. Int. J. Food Sci. Nutr. 2019, 70, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, M.; Coda, R.; Rizzello, C.G. Recent Advances in the Use of Sourdough Biotechnology in Pasta Making. Foods 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, N.Y.A.; Jamaluddin, A.; Ghani, A.A.; Razak, D.I.A.; Jonit, J.; Mansor, A.; Manan, M.A. Quantification of phenolic compounds changes by Aspergillus oryzae on rice bran fermentation. Food Res. 2018, 3, 133–137. [Google Scholar] [CrossRef]
- Dey, T.B.; Kuhad, R.C. Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 2014, 4, 120–127. [Google Scholar]
- Călinoiu, L.F.; Cătoi, A.F.; Vodnar, D.C. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and Extruded Products: Changes in Quality Attributes as Affected by Extrusion Process Parameters: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Pigni, N.B.; Aranibar, C.; Lucini Mas, A.; Aguirre, A.; Borneo, R.; Wunderlin, D.; Baroni, M.V. Chemical profile and bioaccessibility of polyphenols from wheat pasta supplemented with partially-deoiled chia flour. LWT 2020, 124, 109134. [Google Scholar] [CrossRef]
- Marinelli, V.; Padalino, L.; Conte, A.; Del Nobile, M.A.; Briviba, K. Red grape marc flour as food ingredient in durum wheat spaghetti: Nutritional evaluation and bioaccessibility of bioactive compounds. Food Sci. Technol. Res. 2018, 24, 1093–1100. [Google Scholar] [CrossRef]
- Bustos, M.C.; Vignola, M.B.; Paesani, C.; León, A.E. Berry fruits-enriched pasta: Effect of processing and in vitro digestion on phenolics and its antioxidant activity, bioaccessibility and potential bioavailability. Int. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Rosell, C.M.; Perea-Flores, M.D.J.; Bello-Pérez, L.A. Starch and antioxidant compound release during in vitro gastrointestinal digestion of gluten-free pasta. Food Chem. 2018, 263, 201–207. [Google Scholar]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Gallo, A.; Masoero, F.; Trevisan, M. Phenolic profile and fermentation patterns of different commercial gluten-free pasta during in vitro large intestine fermentation. Food Res. Int. 2017, 97, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Bustos, M.C.; Vignola, M.B.; Pérez, G.T.; León, A.E. In vitro digestion kinetics and bioaccessibility of starch in cereal food products. J. Cereal Sci. 2017, 77, 243–250. [Google Scholar] [CrossRef]

| Class | Subclass | Skeleton Structure | Common Representatives |
|---|---|---|---|
| Flavonoids | Flavonols |  | Kaempferol, quercetin |
| Flavan-3-ols |  | Catechin, gallocatechin, epicatechin | |
| Flavones |  | Luteolin, apingenin | |
| Isoflavones |  | Genistein, daidzein | |
| Flavanones |  | Naringenin, hesperetin | |
| Anthocyanidins |  | Cyanidin, malvidin, delphinidin | |
| Dihydrochalcones |  | Phloretin | |
| Non-Flavonoids | Phenolic acids—Hydroxybenzoic acids |  | Gallic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, protocatechuic acid, ellagic acid |
| Phenolic acids—Hydroxycinnamic acids |  | p-coumaric acid, caffeic acid, ferulic acid, sinapic acid, chlorogenic acid | |
| Stilbenes |  | Resveratrol |
| Strategy | Sub-Strategy | Pasta Products | Effect on Phenolic Compound Content/Profile | Reference |
|---|---|---|---|---|
| Use of functional ingredients in pasta-making | Whole Grain Flours | Whole grain wheat and whole grain spelt pasta (precooked) | ↑ content of protocatechuic, 4-hydroxybenzoic, vanillic, syringic, trans-p-coumaric, cis-p-coumaric, trans-ferulic and cis-ferulic acids. | Wójtowicz et al. [33] |
| Whole grain wheat products | TPAs: 226.7 µg/g | Chen et al. [34] | ||
| Whole grain spaghetti | TPC (whole wheat spaghetti): 1263–1423 µg FAE/g dm TPC (regular spaghetti): 718–927 µg FAE/g dm | Hirawan et al. [35] | ||
| Composite Flours | Pasta formulated with wheat semolina and 35% faba bean (Vicia faba L.) flour | TPC (functional pasta): 185.3 mg GAE/100 g dm TPC (control pasta): 63.8 mg GAE/100 g dm | Turco et al. [36] | |
| Pasta with varying proportions of wheat (T. durum L.) semolina (0–100%), chickpea flour (0–90%), and chia flour (0–10%) | TPC (pasta with 10:90 chia:chickpea flour ): 16 mg GAE/g dm TPC (control pasta): 2 mg GAE/g | Cota-Gastélum et al. [37] | ||
| Pasta prepared with carob flour (1–5%) | TPC (pasta with 1% of carob flour): 5.27 mg GAE/g dm TPC (pasta with 5% carob flour): 12.12 mg GAE/g dm TPC (control pasta): 3.51 mg GAE/g dm | Sȩczyk et al. [38] | ||
| Pasta prepared with amaranth seed flours and dried amaranth leaves (35%, 50%, 55% and 70%) | TPC (amaranth-added pasta): 1.54 to 3.37 mg FAE/g dm TPC (control pasta, 100% semolina): 0.98 mg FAE/g dm The highest value was observed in pasta with a semolina: amaranth flour/leaves ratio of 65:35. | Cárdenas-Hernández et al. [39] | ||
| GF pasta (unripe plantain and chickpea flour ) added with blue maize (Zea mays L.) at 25%, 50% and 75% | Samples containing 75% of blue maize presented the highest TPC retention after extrusion and cooking (approx. 70% and 80%, respectively). In the control pasta, the phenolic retention after extrusion and cooking was approx. 52% and 60%, respectively. | Camelo-Méndez et al. [40] | ||
| GF pasta (with rice, maize and soy flour) added with white and brown sorghum | TPC (pasta with white sorghum): 2.41 g GAE/ kg TPC (pasta with brown sorghum): 2.88 g GAE/kg TPC (rice pasta): 0.37 g GAE/kg TPC (soy pasta): 1.37 g GAE/kg TPC (corn pasta): 0.52 g GAE/kg | Palavecino et al. [41] | ||
| Powders and extracts from plant foods and food by-products | Pasta from wheat semolina and pearl-millet added with carrot powder (10%), mango peel powder (5%), moringa leaves powder (3%) and defatted soy flour (15%) | TFC (control pasta): 6.30 mg/100 g dm TFC (carrot-added pasta): 7.63 mg/100 g dm TFC (mango peel-added pasta): 16.53 mg/100 g dm TFC (moringa leaves-added pasta): 17.98 mg/100 g dm TFC (defatted soy flour-added pasta): 8.03 mg/100 g dm | Jalgaonkar et al. [42] | |
| Pasta added with mushroom (white button, shiitake and porcini) powder, at 5%, 10% and 15% semolina substitution levels | TPC values in mushroom pasta were significantly higher than in control pasta, except for 5% and 10% shiitake mushroom pasta. The greatest values were found in porcini mushroom pasta samples (approximately 4–5 mg GAE/g dm). | Lu et al. [43] | ||
| Pasta added with onion powder, at 0%, 2.5%, 5% and 7.5% semolina substitution level | TPC (cooked pasta added with onion skin): approx. from 1.4 to 3 mg GAE/g dm TFC (cooked pasta added with onion skin): approx. from 0.7 to 3.8 mg QE/g dm TPC (cooked control pasta): approx. 0.5 mg GAE/g dm TFC (cooked control pasta): approx. 0.1 mg QE/g dm Cooked pasta showed TPC not significantly different from the corresponding raw sample, whichever addition level of onion skin powder. | Michalak-Majewska et al. [44] | ||
| Durum spaghetti added with olive paste powder (10%) | TPC (enriched spaghetti): 245.08 µg/g dm TPC (control pasta): 82.39 µg/g dm Control and functional pasta differed also in the phenolic profile. Increased level of flavonoids (i.e., quercetin and luteolin) in functional pasta. | Padalino et al. [45] | ||
| Spaghetti added with extracts from grape marc (grape skins, seeds, and stalks) | TPC (functional spaghetti): approx. 700 mg GAE/100g dm | Marinelli et al. [46] | ||
| Pasta prepared from semolina and bran aqueous extract | TPC (functional spaghetti): 127 mg FAE/100 g fw TPC (control pasta): 97 mg FAE/100 g fw | Pasqualone et al. [47] | ||
| GF pasta added with chia (Salvia hispanica L.) milled seeds (5% and 10% substitution levels) | In raw samples— TPA (functional GF pasta—10% sub.): 164.3 µg/g TPA (durum wheat pasta): 149.08 µg/g TPA (functional GF pasta—5% sub.): 98.40 µg/g TPA (pasta produced with commercial GF flour): 10.30 µg/g In cooked samples— ↑ TPAs in all pasta samples. TPA (functional GF pasta—10% sub.): 186.80 µg/g TPA (durum wheat pasta): 156.99 µg/g TPA (functional GF pasta—5% sub.): 123.53 µg/g TPA (pasta produced with commercial GF flour): 11.83 µg/g Control and functional pasta also differed in the phenolic profile. | Menga et al. [48] | ||
| GF pasta (from a blend of rice and field bean flour) added with pear prickly fruit (Opuntia ficus indica (L.) Mill.) in different amounts (2.5%, 5%, 7.5%, 10%, 12.5% and 15%) | Pasta samples enriched with pear prickly fruit were rich in several phenolic acids, namely protocatechuic, caffeic, syryngic, 4-OH-benzoic, vanilic, gentisic, trans-sinapic, cis-sinapic, p-coumaric, ferulic, isoferulic, m-coumaric, 3,4-dimetoxycinnamic, and salicylic acids. The higher was the addition of pear prickly fruit, the higher was the content of phenolic acids. The dominant acid was isoferulic. | Oniszczuk et al. [49] | ||
| GF pasta (from a blend of rice and field bean flour) added with chestnut fruit (Castanea sativa Mill.) in different amounts (10%, 20%, 30%, 40%, and 50%) | TPA content (10%): 38.93 µg/g dm TPA content (20%): 46.98 µg/g dm TPA content (30%): 51.47 µg/g dm TPA content (40%): 56.59 µg/g dm TPA content (50%): 65.01 µg/g dm The content of each phenolic acid also increased at the higher addition of chestnut fruit, with the exception of 4-hydroxy-benzoic and salicylic acids. | Oniszczuk et al. [50] | ||
| Raw material processing, pasta-making and pasta cooking | Debranning | Pasta enriched with a debranning fraction from purple wheat (25%) | Phenolic compounds in wheat flour and semolina were negligible compared to the debranning fraction from purple wheat. In pasta samples TPC was lower than it was expected. This was possibly due to the degradation of phenolics during the pasta-making process. | Abbasi et al. [51] |
| Pasta enriched with the first and the second debranning fraction from purple wheat | Anthocyanin content (pasta enriched with the 1st debranning fraction): 67.9 µg/g dm Anthocyanin content (pasta added with the 2nd debranning fraction): 60 µg/g dm Anthocyanin content (control pasta with bran addition): 28 µg/g dm | Zanoletti et al. [52] | ||
| Spaghetti enriched (30%) with debranning fractions of durum wheat | In raw samples— Free PAs were higher in the control pasta than in functional pasta. Conjugated PAs (functional pasta): 59.4 mg/kg dm Conjugated PAs (control pasta): 21.6 mg/kg dm Bound PAs (functional pasta): 650.0 mg/kg dm Bound PAs (control pasta): 27.2 mg/kg dm Conjugated TPC (functional pasta): 110.7 mg/kg dm Conjugated TPC (control pasta): 31.4 mg/kg dm Bound TPC (functional pasta): 1308.4 mg/kg dm Bound TPC (control pasta): 156.9 mg/kg dm In cooked samples— ↑ level of PAs, whichever form was considered ↓ free and conjugated TPC ↑ level of bound phenolic compound | Ciccoritti et al. [53] | ||
| Micronization | Pasta added with micronized fractions | In raw functional pasta— Conjugated PAs: 36.8 mg/kg dm Bound PAs: 357.3 mg/kg dm Conjugated TPs: 75.8 mg/kg dm Bound TPs: 113.3 mg/kg dm In cooked functional pasta (with respect to raw samples)— ↑ free PAs and conjugated PAs ↓ bound PAs ↓ conjugated TPs ↑ bound TPs | Ciccoritti et al. [53] | |
| Pasta added with micronized fractions | Micronization preserved the content of phenolic acids, while conventional milling determined 89% decrease from seeds to cooked durum wheat pasta | Martini et al. [54] | ||
| Cereal germination | Spaghetti formulated by using 30% dry tartary buckwheat sprouts | In raw samples— TPC (raw tartary buckwheat spaghetti): 3.7 mg GAE/g TPC (100% semolina spaghetti): 0.3 mg GAE/g In cooked samples— TPC (raw tartary buckwheat spaghetti): 2.2 mg GAE/g TPC (100% semolina spaghetti): 0.2 mg GAE/g | Merendino et al. [55] | |
| Legume germination | Pasta prepared with sprouted chickpea flour | TPC (sprouted chickpea pasta): 8.4 mg GAE/g TPC (non-sprouted chickpea pasta): 7.3 mg GAE/g | Bruno et al. [56] | |
| Extrusion and Extrusion-cooking | GF precooked rice-yellow pea pasta | ↑ TPC at higher screw speed (80 rpm) | Bouasla et al. [57] | |
| GF precooked pasta from roasted buckwheat (Fagopyrum esculentum Moench and F. tataricum Gaertner) flour | Highest level of benzoic acid derivatives (i.e., gallic, protocatechuic, gentisic, 4-hydroxybenzoic and salicylic acids) at 100 rpm extruder screw speed and 32% flour moisture content. Highest content in cinnamic acid derivatives (i.e., trans-caffeic, trans-p-coumaric, cis-p-coumaric and cis-ferulic acids) at 60 rpm extruder screw speed and 30% of flour moisture | Oniszczuk et al. [58] | ||
| Barley pasta | ↓ TPC after extrusion | De Paula et al. [59] | ||
| Cooking | Barley pasta | TPAs were not greatly affected by cooking | De Paula et al. [59] | |
| Whole wheat | ↑ free TPC | Podio et al. [60] | ||
| GF pasta (i.e., pasta enriched with black rice, chickpea, red lentil, sorghum, amaranth and quinoa) | In raw GF pasta— Bound TPC > Free TPC Bound TPC (sorghum GF pasta): 7.58 mg GAE/100 g Bound TPC (quinoa GF pasta): 32.68 mg GAE/100 g In cooked GF pasta— Free TPC > Bound TPC Free TPC (black rice pasta): 27.27 mg GAE/100 g Free TPC (quinoa pasta): 19.27 mg GAE/100 g | Rocchetti et al. [61] |
| Pasta Formulation | Phenolic Compounds Analysed | In Vitro Methods | Main Findings | Reference |
|---|---|---|---|---|
| Pasta produced with two varieties of whole wheat flour (Triticum aestivum L.) | TPC, 6G8AA, 8G6AA, cFA, ChDP, DFA (Isomers 1–12), FAD, HBADG, HBAG, HGPBA, pCoA, pCoFP, tFA, TFA | OD: human saliva, homogenization, pH adjustment to 2. GD: addition of pepsin solution (pepsin + 0.1 M HCl) to the homogenate; incubation with shaking for 2 h at 37 °C. ID and DIA: addition of a pancreatin/porcine bile solution and dialysis for 3 h at 37 °C. | After OD: release of 4.5–11% of TPC found in cooked supplemented pasta (depending on the variety). After GD: ↑ (344–370%) of TPC found in cooked supplemented pasta. After ID: ↑ (340–360%) of TPC found in cooked supplemented pasta. After DIA: ↑ (~140%) of TPC found in cooked supplemented pasta. Hydroxybenzoic acid diglucoside, hydroxybenzoic acid glucoside and trans-ferulic acid were the main compounds quantified in DIA samples. | Podio et al. [60] |
| Pasta from wheat flour fortified with partially-deoiled chia flour | QA, SA I/H, CTA, FTA, Try, CAH, CA, SA E/B/L, SF, RA, SA C, MeRA, MeQ | OD: human saliva; homogenization; pH adjustment to 2. GD: pepsin solution (pepsin + 0.1 M HCl) added to the homogenate; incubation with shaking for 2 h at 37 °C. ID and DIA: addition of a pancreatin/porcine bile solution and dialysis for 3 h at 37 °C. | After OD: release of 50% of the TPC found in cooked supplemented pasta. After GD and ID: ↑ (300–500%) of TPC found in cooked supplemented pasta. After DIA: ↑ (~50%) of TPC found in cooked supplemented pasta. | Pigni et al. [103] |
| Pasta produced with durum wheat semolina, red grape marc (RGM) and transglutaminase (TG) | TPC | GD: porcine pepsin; pH = 2.2–2.4; incubation with shaking for 1 h at 37 °C. ID: addition of porcine bile acid, pancreatin, α-amylase; pH = 7.2–7.6; treatment with nitrogen gas and shaking at 37 °C in a water bath for 2 h. | Bioaccessible TP in RGM/TG pasta vs control: 5.53 ± 0.61 vs. 4.16 ± 0.50 mg GAE/g dm | Marinelli et al. [104] |
| Pasta enriched with fruits from Rubus and Ribes genus | TPC | Based on the static method proposed by INFOGEST’s scientists [30] | ↑ (260%) of TPC (raspberry- and boysenberry-enriched pasta). ↑ (360%) of TPC (red- and blackcurrant enriched pasta). | Bustos et al. [105] |
| GF pasta formulated with blue maize, chickpea and unripe plantain flours | FPCs and TPC | OD: food was chewed for 15 s; each person rinsed his/her mouth with 5 mL of phosphate buffer. GD: HCl-KCl buffer; pH = 1.25; pepsin solution; incubation at 40 °C in a water bath for 60 min. ID: addition of a mixture of enzymes, incubated for 1 h at 37 °C in a water bath with constant agitation. DIA: dialysis tubing; pancreatic α-amylase solution; incubation at 37 °C. | After OD: release of FPCs. After GD: ↑ TPC release at the increase of blue maize flour percentage. After ID: release of 40% TPC. | Camelo-Méndez et al. [106] |
| GF pasta produced with white and brown sorghum | TPC | OD: simulated salivary fluid as reported in [108], sample disrupted in a Teflon pestle, incubated for 2 min at 37 °C. GD: simulated stomach fluid as reported in [108]; pH adjusted to 3; incubation for 2 h at 37°C. ID: simulated duodenal fluid as reported in [108]; pH adjusted to 7; incubation for 3 h at 37°C. | Phenolic compound bioaccessibility of white and brown sorghum GF pasta was 2.9- and 2.4-fold higher than in cooked pasta, respectively. | Palavecino et al. [41] |
| GF pasta produced with black rice, chickpea, red lentil, sorghum, amaranth and quinoa | TPC Flavonoids Lignans Stilbenes | Pre-incubation step with digestive enzymes. In vitro large intestine fermentation process. | After the large intestine fermentation process: - Flavonoid bioaccessibility: <1% - Hydroxycinnamic acid bioaccessibility: 0.6% to 8.6% (at 0 h), 0.6% to 1.6% (at 8 h) and 0.7% to 5.5% (at 24 h) - Lignan bioaccessibility: furofurans (very low); dibenzylbutyrolactones (2.7–12.2%); tyrosols and alkylresorcinols (the most bioaccessible). | Rocchetti et al. [107] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melini, V.; Melini, F.; Acquistucci, R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants 2020, 9, 343. https://doi.org/10.3390/antiox9040343
Melini V, Melini F, Acquistucci R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants. 2020; 9(4):343. https://doi.org/10.3390/antiox9040343
Chicago/Turabian StyleMelini, Valentina, Francesca Melini, and Rita Acquistucci. 2020. "Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta" Antioxidants 9, no. 4: 343. https://doi.org/10.3390/antiox9040343
APA StyleMelini, V., Melini, F., & Acquistucci, R. (2020). Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants, 9(4), 343. https://doi.org/10.3390/antiox9040343







