Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials
Abstract
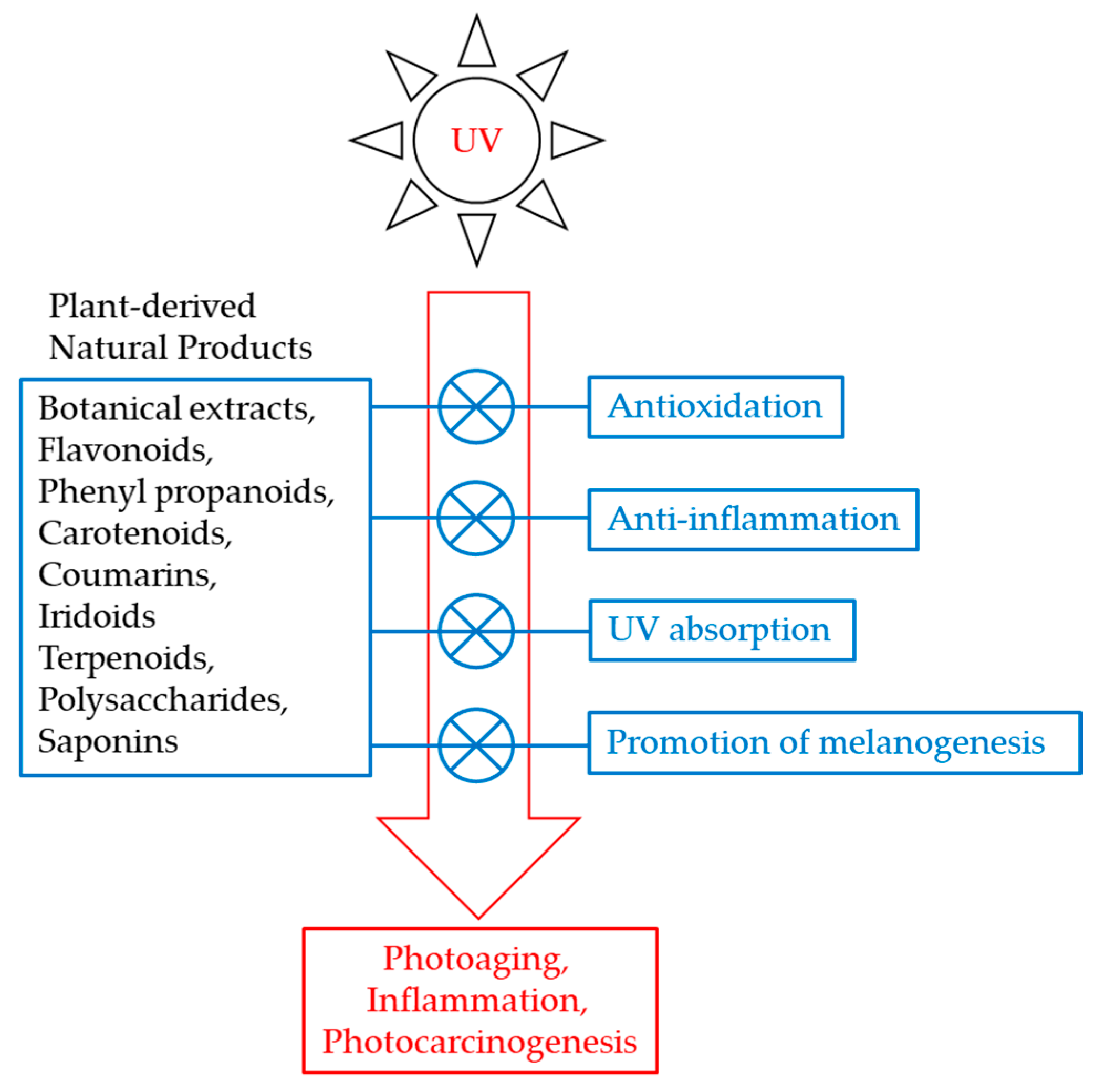
:1. Introduction
2. UV-Induced Toxicity in the Skin
3. Melanin as an Endogenous UV Filter
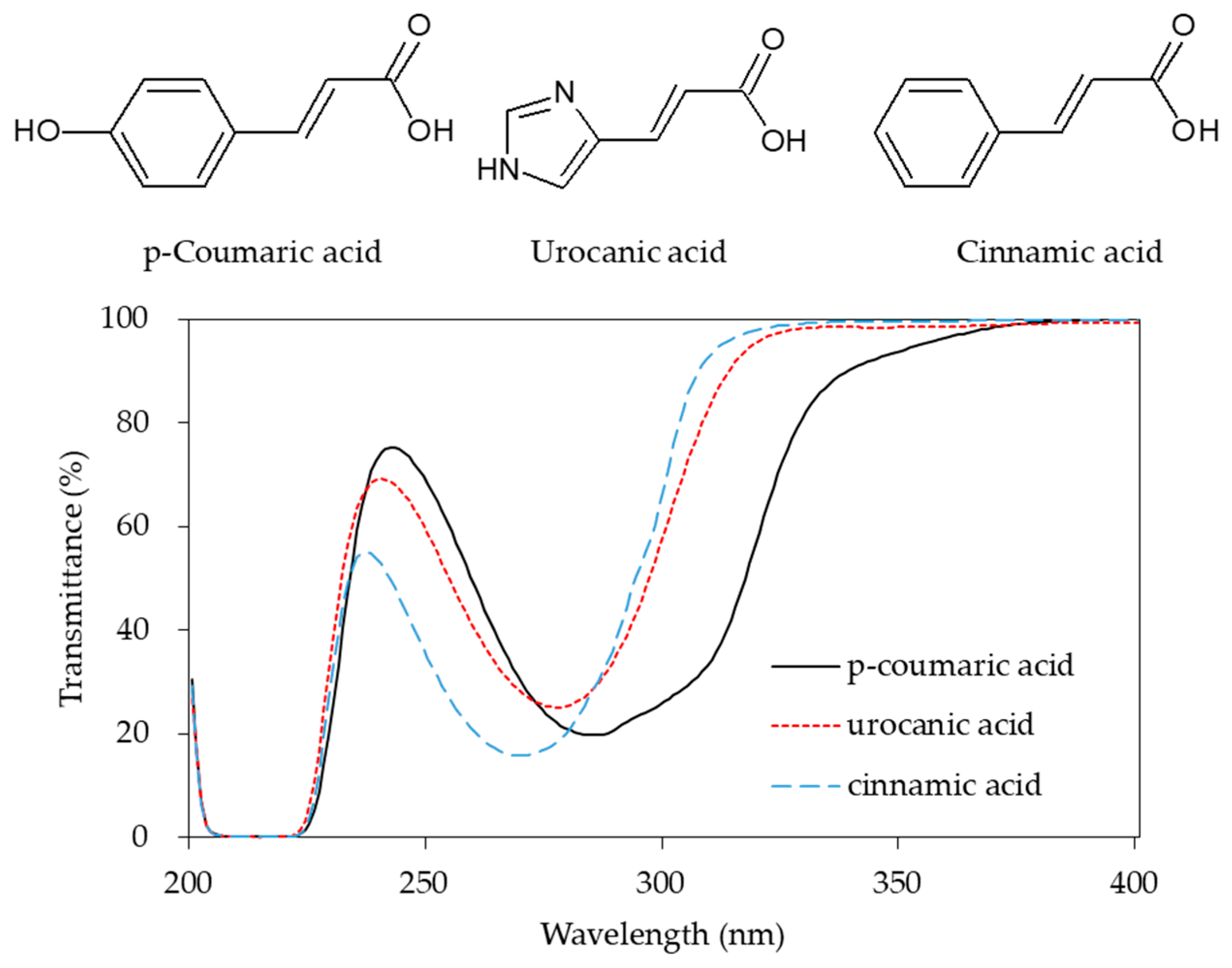
4. Trans-Urocanic Acid and Sunscreen Products
5. UV Protection by Botanical Extracts
6. Plant-Derived Antioxidants That Protect Melanocytes
7. UV Absorption by Phenyl Propanoids
8. Anti-inflammatory and Anticarcinogenic Effects of Quercetin
9. Synthesis of Melanin
10. Use of MC1R Agonists to Stimulate Melanin Synthesis
11. Plant-Derived Materials that Stimulate Melanin Synthesis
12. Plant-Derived Materials that Attenuate Extrinsic Skin Aging
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP-1 | activator protein-1 |
| COX | cyclooxygenase |
| CREB | cAMP-responsive element-binding protein |
| DAG | diacyl glycerol |
| DOPA | dihydroxyphenylalanine |
| DCT | dopachrome tautomerase |
| ERK | extracellular signal-regulated kinase |
| FITC | fluorescein isothiocyanate |
| GSK3β | glycogen synthase kinase 3β |
| IL | interleukin |
| JNK | c-Jun-N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| MC1R | melanocortin 1 receptor |
| MITF | microphthalmia-associated transcription factor |
| MSH | melanocyte stimulating hormone |
| NO | nitric oxide |
| NF-κB | nuclear factor-κB |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PLGA | poly(D,L-lactide-co-glycolide) |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PKG | protein kinase G |
| PM | particulate matter |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 |
| ROS | reactive oxygen species |
| SCF | stem cell factor |
| siRNA | small interfering RNA |
| SPF | sun protection factor |
| TNF-α | tumor necrosis factor-α |
| TPGS | tocopheryl polyethylene glycol 1000 succinate |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| TYR | tyrosinase |
| TYRP1 | tyrosinase-related protein 1 |
| UV | ultraviolet |
| UVA-PF | UVA-protection factor |
References
- Rapf, R.J.; Vaida, V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys. Chem. Chem. Phys. 2016, 18, 20067–20084. [Google Scholar] [CrossRef]
- Lucas, R.M.; Yazar, S.; Young, A.R.; Norval, M.; de Gruijl, F.R.; Takizawa, Y.; Rhodes, L.E.; Sinclair, C.A.; Neale, R.E. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019, 18, 641–680. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, G.H.; Neale, R.E.; Barnes, P.W.; Neale, P.J.; Zepp, R.G.; Wilson, S.R.; Andrady, A.L.; Bais, A.F.; McKenzie, R.L.; Aucamp, P.J.; et al. Environmental effects of stratospheric ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2019. Photochem. Photobiol. Sci. 2020, 19, 542–584. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamaguchi, Y.; Hoashi, T.; Hearing, V.J. Melanin content and DNA damage in normal human skin in response to chronic ultraviolet radiation. J. Investig. Dermatol. 2005, 124, A134. [Google Scholar]
- Stremnitzer, C.; Barresi, C.; Mlitz, V.; Kezic, S.; Kammeyer, A.; Ghannadan, M.; Posa-Markaryan, K.; Selden, C.; Tschachler, E.; Eckhart, L. Endogenous and exogenous urocanic acid protects against ultraviolet B-induced DNA damage. J. Investig. Dermatol. 2010, 130, S136. [Google Scholar]
- Markova, N.; Yarosh, D.; Smiles, K.; Karaman-Jurukovska, N. The natural antioxidant L-ergothioneine is integral to the skin’s defense against ultraviolet-induced oxidative damage. J. Am. Acad. Dermatol. 2009, 60, Ab156. [Google Scholar]
- Hammiller, B.; Karuturi, B.V.K.; Miller, C.; Holmes, M.; Labhasetwar, V.; Madsen, G.; Hansen, L.A. Delivery of antioxidant enzymes for prevention of ultraviolet irradiation-induced epidermal damage. J. Dermatol. Sci. 2017, 88, 373–375. [Google Scholar] [CrossRef]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar]
- Kozma, B.; Eide, M.J. Photocarcinogenesis: An epidemiologic perspective on ultraviolet light and skin cancer. Dermatol. Clin. 2014, 32, 301–313. [Google Scholar] [CrossRef]
- Epstein, J.H. Photocarcinogenesis, skin cancer, and aging. J. Am. Acad. Dermatol. 1983, 9, 487–502. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Soter, N.A. Acute effects of ultraviolet radiation on the skin. Semin. Dermatol. 1990, 9, 11–15. [Google Scholar] [PubMed]
- Afaq, F.; Mukhtar, H. Effects of solar radiation on cutaneous detoxification pathways. J. Photochem. Photobiol. B 2001, 63, 61–69. [Google Scholar] [CrossRef]
- Aitken, G.R.; Henderson, J.R.; Chang, S.C.; McNeil, C.J.; Birch-Machin, M.A. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin. Exp. Dermatol. 2007, 32, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Jonason, A.S.; Leffell, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and p53 in the onset of skin cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef]
- Van Laethem, A.; Claerhout, S.; Garmyn, M.; Agostinis, P. The sunburn cell: Regulation of death and survival of the keratinocyte. Int. J. Biochem. Cell Biol. 2005, 37, 1547–1553. [Google Scholar] [CrossRef]
- D’Agostini, F.; Balansky, R.M.; Camoirano, A.; De Flora, S. Modulation of light-induced skin tumors by N-acetylcysteine and/or ascorbic acid in hairless mice. Carcinogenesis 2005, 26, 657–664. [Google Scholar] [CrossRef]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Photoaging: Prevention and topical treatments. Am. J. Clin. Dermatol. 2010, 11, 95–102. [Google Scholar] [CrossRef]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef]
- Kahari, V.M.; Saarialho-Kere, U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell. Mol. Life Sci. 1999, 55, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Lee, M.W. Anti-oxidative, anti-inflammatory and whitening effects of phenolic compounds from Bambusae caulis in Liquamen. Nat. Prod. Res. 2012, 26, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, Y.K.; Kim, K.H.; Park, S.J.; Kim, S.J.; Chung, J.H. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J. Cell. Physiol. 2009, 219, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Scharffetter, K.; Wlaschek, M.; Hogg, A.; Bolsen, K.; Schothorst, A.; Goerz, G.; Krieg, T.; Plewig, G. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch. Dermatol. Res. 1991, 283, 506–511. [Google Scholar] [CrossRef]
- Brenneisen, P.; Oh, J.; Wlaschek, M.; Wenk, J.; Briviba, K.; Hommel, C.; Herrmann, G.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem. Photobiol. 1996, 64, 649–657. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Investig. 1998, 101, 1432–1440. [Google Scholar] [CrossRef]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef]
- Westermarck, J.; Kahari, V.M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999, 13, 781–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Turka, L.A. Keratinocytes: Key immunocytes of the integument. Am. J. Pathol. 1993, 143, 325–331. [Google Scholar]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, K.; Itoh, T.; Akao, Y.; Inoue, H.; Nozawa, Y.; Ito, M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br. J. Dermatol. 2010, 163, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Fagot, D.; Asselineau, D.; Bernerd, F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch. Dermatol. Res. 2002, 293, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Ghahary, A.; Karimi-Busheri, F.; Marcoux, Y.; Li, Y.; Tredget, E.E.; Taghi Kilani, R.; Li, L.; Zheng, J.; Karami, A.; Keller, B.O.; et al. Keratinocyte-releasable stratifin functions as a potent collagenase-stimulating factor in fibroblasts. J. Investig. Dermatol. 2004, 122, 1188–1197. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [Green Version]
- Pustisek, N.; Situm, M. UV-radiation, apoptosis and skin. Coll Antropol. 2011, 35 (Suppl. 2), 339–341. [Google Scholar]
- Sitailo, L.A.; Tibudan, S.S.; Denning, M.F. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J. Biol. Chem. 2002, 277, 19346–19352. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Seok, J.K.; Suh, H.J.; Boo, Y.C. Gardenia jasminoides extract attenuates the UVB-induced expressions of cytokines in keratinocytes and indirectly Inhibits matrix metalloproteinase-1 expression in human dermal fibroblasts. Evid. Based Complement. Altern. Med. 2014, 2014, 429246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, J.K.; Kwak, J.Y.; Seo, H.H.; Suh, H.J.; Boo, Y.C. Effects of Bambusae caulis in Taeniam extract on UVB-induced cell death, oxidative stress and matrix metalloproteinase 1 expression in keratinocytes. J. Soc. Cosmet. Sci. Korea 2015, 41, 9–20. [Google Scholar]
- Seok, J.K.; Boo, Y.C. p-Coumaric Acid Attenuates UVB-Induced Release of Stratifin from Keratinocytes and Indirectly Regulates Matrix Metalloproteinase 1 Release from Fibroblasts. Korean J. Physiol. Pharm. 2015, 19, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell. Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell. Biol. 2012, 212, 1–115. [Google Scholar]
- Iwata, M.; Corn, T.; Iwata, S.; Everett, M.A.; Fuller, B.B. The relationship between tyrosinase activity and skin color in human foreskins. J. Investig. Dermatol. 1990, 95, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, T.; Yamaguchi, Y.; Batzer, J.; Coelho, S.G.; Zmudzka, B.Z.; Miller, S.A.; Wolber, R.; Beer, J.Z.; Hearing, V.J. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Investig. Dermatol. 2005, 124, 1326–1332. [Google Scholar] [CrossRef] [Green Version]
- Haltaufderhyde, K.D.; Oancea, E. Genome-wide transcriptome analysis of human epidermal melanocytes. Genomics 2014, 104, 482–489. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A2. Int. J. Leg. Med. 2007, 121, 36–39. [Google Scholar] [CrossRef]
- Ginger, R.S.; Askew, S.E.; Ogborne, R.M.; Wilson, S.; Ferdinando, D.; Dadd, T.; Smith, A.M.; Kazi, S.; Szerencsei, R.T.; Winkfein, R.J.; et al. SLC24A5 encodes a trans-Golgi network protein with potassium-dependent sodium-calcium exchange activity that regulates human epidermal melanogenesis. J. Biol. Chem. 2008, 283, 5486–5495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, A.L.; Chen, W.; Thurber, A.E.; Smit, D.J.; Smith, A.G.; Bladen, T.G.; Brown, D.L.; Duffy, D.L.; Pastorino, L.; Bianchi-Scarra, G.; et al. Analysis of cultured human melanocytes based on polymorphisms within the SLC45A2/MATP, SLC24A5/NCKX5, and OCA2/P loci. J. Investig. Dermatol. 2009, 129, 392–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Fistarol, S.K.; Itin, P.H. Disorders of pigmentation. J. Dtsch. Dermatol. Ges. 2010, 8, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.T. Pigmentary disorders. Med. Clin. N. Am. 2009, 93, 1225–1239. [Google Scholar] [CrossRef]
- Callender, V.D.; St Surin-Lord, S.; Davis, E.C.; Maclin, M. Postinflammatory hyperpigmentation: Etiologic and therapeutic considerations. Am. J. Clin. Dermatol. 2011, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ganju, P.; Nagpal, S.; Mohammed, M.H.; Nishal Kumar, P.; Pandey, R.; Natarajan, V.T.; Mande, S.S.; Gokhale, R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016, 6, 18761. [Google Scholar] [CrossRef] [Green Version]
- Spritz, R.A.; Andersen, G.H. Genetics of Vitiligo. Dermatol. Clin. 2017, 35, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Kim, T.K.; Brozyna, A.A.; Janjetovic, Z.; Brooks, D.L.; Schwab, L.P.; Skobowiat, C.; Jozwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1alpha expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [Green Version]
- An, S.M.; Koh, J.S.; Boo, Y.C. Inhibition of melanogenesis by tyrosinase siRNA in human melanocytes. BMB Rep. 2009, 42, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Beer, J.Z.; Hearing, V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: Factors influencing the incidence of skin cancer. Arch. Dermatol. Res. 2008, 300 (Suppl. 1), 43–50. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, J. Urocanic acid, the major acid-soluble, ultraviolet-absorbing compound in guinea pig epidermis. Arch. Biochem. Biophys. 1957, 70, 295–298. [Google Scholar] [CrossRef]
- Gibbs, N.K.; Norval, M.; Traynor, N.J.; Wolf, M.; Johnson, B.E.; Crosby, J. Action spectra for the trans to cis photoisomerisation of urocanic acid in vitro and in mouse skin. Photochem. Photobiol. 1993, 57, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Hou, X.Y.; Shi, C.M.; Nagata, D.; Walsh, K.; Cohen, R.A. Modulation by peroxynitrite of AKt- and AMP-activated kinase-dependent serine phosphorylation of endothelial nitric oxide synthase. J. Biol. Chem. 2002, 277, 32552–32557. [Google Scholar] [CrossRef] [Green Version]
- Brookman, J.; Chacon, J.N.; Sinclair, R.S. Some photophysical studies of cis- and trans-urocanic acid. Photochem. Photobiol. Sci. 2002, 1, 327–332. [Google Scholar] [CrossRef]
- Barresi, C.; Stremnitzer, C.; Mlitz, V.; Kezic, S.; Kammeyer, A.; Ghannadan, M.; Posa-Markaryan, K.; Selden, C.; Tschachler, E.; Eckhart, L. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J. Investig. Dermatol. 2011, 131, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, N.K.; Norval, M. Urocanic acid in the skin: A mixed blessing? J. Investig. Dermatol. 2011, 131, 14–17. [Google Scholar] [CrossRef]
- De Fabo, E.C.; Noonan, F.P. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 1983, 158, 84–98. [Google Scholar] [CrossRef]
- Reeve, V.E.; Greenoak, G.E.; Canfield, P.J.; Boehm-Wilcox, C.; Gallagher, C.H. Topical urocanic acid enhances UV-induced tumour yield and malignancy in the hairless mouse. Photochem. Photobiol. 1989, 49, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.R.; Hughes, M.C.; Green, A.C. Effects of sunscreen on skin cancer and photoaging. Photodermatol. Photoimmunol. Photomed. 2014, 30, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, A.; Psotova, J.; Walterova, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med. FAC Univ. Palacky Olomouc Czech. Repub. 2003, 147, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, S.K.; Korman, N.J.; Mukhtar, H.; Agarwal, R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997, 89, 556–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.M.; Lee, S.I.; Choi, S.W.; Moon, S.W.; Boo, Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by alpha-melanocyte stimulating hormone. Br. J. Dermatol. 2008, 159, 292–299. [Google Scholar] [CrossRef]
- Cole, N.; Sou, P.W.; Ngo, A.; Tsang, K.H.; Severino, J.A.; Arun, S.J.; Duke, C.C.; Reeve, V.E. Topical ‘Sydney’ propolis protects against UV-radiation-induced inflammation, lipid peroxidation and immune suppression in mouse skin. Int. Arch. Allergy Immunol. 2010, 152, 87–97. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Benedict, A.L.; Jenkins, S.N.; Ye, L.; Wehage, S.L.; Talalay, P. Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice. Photochem. Photobiol. Sci. 2010, 9, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Murapa, P.; Dai, J.; Chung, M.; Mumper, R.J.; D’Orazio, J. Anthocyanin-rich fractions of blackberry extracts reduce UV-induced free radicals and oxidative damage in keratinocytes. Phytother. Res. 2012, 26, 106–112. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.H.; Park, C.; Lee, J.S.; Kim, Y.H. Portulaca oleracea extracts protect human keratinocytes and fibroblasts from UV-induced apoptosis. Exp. Dermatol. 2014, 23 (Suppl. 1), 13–17. [Google Scholar] [CrossRef]
- Perez-Sanchez, A.; Barrajon-Catalan, E.; Caturla, N.; Castillo, J.; Benavente-Garcia, O.; Alcaraz, M.; Micol, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. B 2014, 136, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kwak, J.Y.; Choi, G.W.; An, S.M.; Kwak, J.H.; Seo, H.H.; Suh, H.J.; Boo, Y.C. Scutellaria radix Extract as a Natural UV Protectant for Human Skin. Phytother. Res. 2016, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Karapetsas, A.; Voulgaridou, G.P.; Konialis, M.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.I.; Stathopoulou, K.; Aligiannis, N.; Bozidis, P.; et al. Propolis Extracts Inhibit UV-Induced Photodamage in Human Experimental In Vitro Skin Models. Antioxidants 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.M.; Lee, S.J.; Koh, J.S.; Park, K.; Boo, Y.C. Effects of plant extract-containing creams on UVB radiation-induced inflammatory responses in mice. J. Soc. Cosmet. Sci. Korea 2010, 36, 271–280. [Google Scholar]
- Lee, M.J.; Kim, M.J.; Song, Y.S.; Song, Y.O.; Moon, G.S. Bamboo culm extract supplementation elevates HDL-cholesterol and ameliorates oxidative stress in C57BL/6 mice fed atherogenic diet. J. Med. Food 2008, 11, 69–77. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, Y.; Lou, D.; Wu, X. Antihyperlipidemic and antihypertensive effect of a triterpenoid-rich extract from bamboo shavings and vasodilator effect of friedelin on phenylephrine-induced vasoconstriction in thoracic aortas of rats. Phytother. Res. 2007, 21, 1135–1141. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, X.; Bao, B. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother. Res. 2006, 20, 872–876. [Google Scholar] [CrossRef]
- Sun, J.; Yu, J.; Zhang, P.C.; Tang, F.; Yue, Y.D.; Yang, Y.N.; Feng, Z.M.; Guo, X.F. Isolation and identification of lignans from Caulis bambusae in Taenia with antioxidant properties. J. Agric. Food Chem. 2013, 61, 4556–4562. [Google Scholar] [CrossRef]
- Watanabe, T.; Terabe, S. Analysis of natural food pigments by capillary electrophoresis. J. Chromatogr. A 2000, 880, 311–322. [Google Scholar] [CrossRef]
- Hsu, J.D.; Chou, F.P.; Lee, M.J.; Chiang, H.C.; Lin, Y.L.; Shiow, S.J.; Wang, C.J. Suppression of the TPA-induced expression of nuclear-protooncogenes in mouse epidermis by crocetin via antioxidant activity. Anticancer Res. 1999, 19, 4221–4227. [Google Scholar]
- Pham, T.Q.; Cormier, F.; Farnworth, E.; Tong, V.H.; Van Calsteren, M.R. Antioxidant properties of crocin from Gardenia jasminoides Ellis and study of the reactions of crocin with linoleic acid and crocin with oxygen. J. Agric. Food Chem. 2000, 48, 1455–1461. [Google Scholar] [CrossRef]
- Jianjun, J.; Huiru, D. Preparation of high-purity baicalein from Scutellaria baicalensis Georgi. Nat. Prod. Res. 2008, 22, 1410–1412. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Li, W.F.; Li, W.W.; Ren, K.H.; Fan, C.M.; Chen, Y.Y.; Shen, Y.L. Protective effects of the aqueous extract of Scutellaria baicalensis against acrolein-induced oxidative stress in cultured human umbilical vein endothelial cells. Pharm. Biol. 2011, 49, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.; No, R.H.; Kwon, H.S.; Lee, H.Y. Enhancement of skin anti-inflammatory activities of Scutellaria baicalensis extract using a nanoencapsulation process. J. Cosmet. Laser 2014, 16, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Lin, X.F.; Miao, X.; Wang, B.T.; Yang, Z.L.; Luo, D. Inhibitory effects of Baicalin on ultraviolet B-induced photo-damage in keratinocyte cell line. Am. J. Chin. Med. 2008, 36, 745–760. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, S.F.; Lee, Y.M.; Chuang, C.L.; Bau, D.T.; Lin, S.S. Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity. In Vivo 2013, 27, 707–714. [Google Scholar]
- Kimura, Y.; Sumiyoshi, M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur. J. Pharm. 2011, 661, 124–132. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Sforcin, J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H. Effects of Propolis Extract and Propolis-Derived Compounds on Obesity and Diabetes: Knowledge from Cellular and Animal Models. Molecules 2019, 24, 4394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, A.J.; Km, C.J.; Cho, Y.J.; Kim, Y.; Cha, J.Y.; Hwang, J.K.; Kim, I.H.; Kim, C.T. Characterization of polysaccharides obtained from purslane (Portulaca olerace L.) using different solvents and enzymes. Food Sci. Biotechnol. 2007, 16, 928–934. [Google Scholar]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, M.A.; Cassidy, P.; Grossman, D. NAC protects melanocytes against oxidative stress/damage and delays onset of UV-induced melanoma in mice. J. Investig. Dermatol. 2007, 127, S151. [Google Scholar]
- Wang, Y.; Li, S.; Li, C. Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. 2019, 25, 1017–1023. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Choi, Y.G.; Kim, D.S.; Park, S.H.; Yoon, J.A.; Kwon, S.B.; Park, E.S.; Park, K.C. Cytoprotective effect of green tea extract and quercetin against hydrogen peroxide-induced oxidative stress. Arch. Pharm. Res. 2005, 28, 1251–1256. [Google Scholar] [CrossRef]
- Guan, C.; Xu, W.; Hong, W.; Zhou, M.; Lin, F.; Fu, L.; Liu, D.; Xu, A. Quercetin attenuates the effects of H2O2 on endoplasmic reticulum morphology and tyrosinase export from the endoplasmic reticulum in melanocytes. Mol. Med. Rep. 2015, 11, 4285–4290. [Google Scholar] [CrossRef]
- Lin, M.; Lu, S.S.; Wang, A.X.; Qi, X.Y.; Zhao, D.; Wang, Z.H.; Man, M.Q.; Tu, C.X. Apigenin attenuates dopamine-induced apoptosis in melanocytes via oxidative stress-related p38, c-Jun NH2-terminal kinase and Akt signaling. J. Dermatol. Sci. 2011, 63, 10–16. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Q.; Yang, X.; Yan, H.B.; Lu, Q.P. Hyperoside protects human primary melanocytes against H2O2-induced oxidative damage. Mol. Med. Rep. 2016, 13, 4613–4619. [Google Scholar] [CrossRef] [Green Version]
- Ning, W.; Wang, S.; Liu, D.; Fu, L.; Jin, R.; Xu, A. Potent effects of peracetylated (−)-epigallocatechin-3-gallate against hydrogen peroxide-induced damage in human epidermal melanocytes via attenuation of oxidative stress and apoptosis. Clin. Exp. Dermatol. 2016, 41, 616–624. [Google Scholar] [CrossRef]
- Jung, E.; Kim, J.H.; Kim, M.O.; Lee, S.Y.; Lee, J. Melanocyte-protective effect of afzelin is mediated by the Nrf2-ARE signalling pathway via GSK-3beta inactivation. Exp. Dermatol. 2017, 26, 764–770. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Zhu, L.; Guo, S.; Yi, X.; Cui, T.; He, Y.; Chang, Y.; Liu, B.; Li, C.; et al. Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic. Biol. Med. 2018, 129, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhao, Y.; Kong, Y.; Zhang, W.; Ma, W.; Li, W.; Wang, K. Geniposide prevents H2O2-induced oxidative damage in melanocytes by activating the PI3K-Akt signalling pathway. Clin. Exp. Dermatol. 2018, 43, 667–674. [Google Scholar] [CrossRef]
- Lu, L.; Wang, S.; Fu, L.; Liu, D.; Zhu, Y.; Xu, A. Bilobalide protection of normal human melanocytes from hydrogen peroxide-induced oxidative damage via promotion of antioxidase expression and inhibition of endoplasmic reticulum stress. Clin. Exp. Dermatol. 2016, 41, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mildner, M.; Jin, J.; Eckhart, L.; Kezic, S.; Gruber, F.; Barresi, C.; Stremnitzer, C.; Buchberger, M.; Mlitz, V.; Ballaun, C.; et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J. Investig. Dermatol. 2010, 130, 2286–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, R.R.; Hodgins, D.S.; Abell, C.W. Phenylalanine ammonia-lyase. Induction and purification from yeast and clearance in mammals. J. Biol. Chem. 1976, 251, 4646–4650. [Google Scholar] [PubMed]
- Turner, N.J. Ammonia lyases and aminomutases as biocatalysts for the synthesis of alpha-amino and beta-amino acids. Curr. Opin. Chem. Biol. 2011, 15, 234–240. [Google Scholar] [CrossRef]
- Lee, S.J.; Mun, G.I.; An, S.M.; Boo, Y.C. Evidence for the association of peroxidases with the antioxidant effect of p-coumaric acid in endothelial cells exposed to high glucose plus arachidonic acid. BMB Rep. 2009, 42, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. Cell. Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.I.; An, S.M.; Mun, G.I.; Lee, S.J.; Park, K.M.; Park, S.H.; Boo, Y.C. Protective effect of Sasa quelpaertensis and p-coumaric acid on ethanol-induced hepatotoxicity in mice. J. Appl. Biol. Chem. 2008, 51, 148–154. [Google Scholar] [CrossRef] [Green Version]
- An, S.M.; Koh, J.S.; Boo, Y.C. P-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother. Res. 2010, 24, 1175–1180. [Google Scholar]
- Song, K.; Boo, Y.C. UVB shielding Effects of para-Coumaric acid. J. Soc. Cosmet. Sci. Korea 2012, 38, 263–273. [Google Scholar]
- Song, K.; An, S.M.; Kim, M.; Koh, J.S.; Boo, Y.C. Comparison of the antimelanogenic effects of p-coumaric acid and its methyl ester and their skin permeabilities. J. Dermatol. Sci. 2011, 63, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.K.; Kim, S.J.; Boo, Y.C.; Baek, J.H.; Lee, S.H.; Koh, J.S. Effects of p-coumaric acid on erythema and pigmentation of human skin exposed to ultraviolet radiation. Clin. Exp. Dermatol. 2011, 36, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Fagot, D.; Asselineau, D.; Bernerd, F. Matrix metalloproteinase-1 production observed after solar-simulated radiation exposure is assumed by dermal fibroblasts but involves a paracrine activation through epidermal keratinocytes. Photochem. Photobiol. 2004, 79, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ghahary, A.; Marcoux, Y.; Karimi-Busheri, F.; Li, Y.; Tredget, E.E.; Kilani, R.T.; Lam, E.; Weinfeld, M. Differentiated keratinocyte-releasable stratifin (14-3-3 sigma) stimulates MMP-1 expression in dermal fibroblasts. J. Investig. Dermatol. 2005, 124, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Lam, E.; Kilani, R.T.; Li, Y.; Tredget, E.E.; Ghahary, A. Stratifin-induced matrix metalloproteinase-1 in fibroblast is mediated by c-fos and p38 mitogen-activated protein kinase activation. J. Investig. Dermatol. 2005, 125, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Hseu, Y.C.; Korivi, M.; Lin, F.Y.; Li, M.L.; Lin, R.W.; Wu, J.J.; Yang, H.L. Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblasts. J. Dermatol. Sci. 2018, 90, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Staniforth, V.; Chiu, L.T.; Yang, N.S. Caffeic acid suppresses UVB radiation-induced expression of interleukin-10 and activation of mitogen-activated protein kinases in mouse. Carcinogenesis 2006, 27, 1803–1811. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.; Chen, H.; Li, W.; Lim, D.Y.; et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef] [Green Version]
- Balupillai, A.; Nagarajan, R.P.; Ramasamy, K.; Govindasamy, K.; Muthusamy, G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol. Appl. Pharm. 2018, 352, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Staniforth, V.; Huang, W.C.; Aravindaram, K.; Yang, N.S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ambothi, K.; Prasad, N.R.; Balupillai, A. Ferulic acid inhibits UVB-radiation induced photocarcinogenesis through modulating inflammatory and apoptotic signaling in Swiss albino mice. Food Chem. Toxicol. 2015, 82, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. ScientificWorldJournal 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Figueiredo, M.S.; Folador, P.; Damazio, R.G.; Pizzolatti, M.G.; Silva, F.R. Flavonoids: Prospective drug candidates. Mini Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar] [CrossRef]
- Terahara, N. Flavonoids in foods: A review. Nat. Prod. Commun. 2015, 10, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Steerenberg, P.A.; Garssen, J.; Dortant, P.M.; van der Vliet, H.; Geerse, E.; Verlaan, A.P.; Goettsch, W.G.; Sontag, Y.; Bueno-de-Mesquita, H.B.; Van Loveren, H. The effect of oral quercetin on UVB-induced tumor growth and local immunosuppression in SKH-1. Cancer Lett. 1997, 114, 187–189. [Google Scholar] [CrossRef]
- Steerenberg, P.A.; Garssen, J.; Dortant, P.; van de Vliet, H.; Geerse, L.; Verlaan, A.P.; Goettsch, W.; Sontag, Y.; Norval, M.; Gibbs, N.K.; et al. Quercetin prevents UV-induced local immunosuppression, but does not affect UV-induced tumor growth in SKH-1 hairless mice. Photochem. Photobiol. 1997, 65, 736–744. [Google Scholar] [CrossRef]
- Erden Inal, M.; Kahraman, A.; Koken, T. Beneficial effects of quercetin on oxidative stress induced by ultraviolet A. Clin. Exp. Dermatol. 2001, 26, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B 2006, 84, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, B.M.; Krol, E.S. Inhibition of UVA and UVB radiation-induced lipid oxidation by quercetin. J. Agric. Food Chem. 2009, 57, 5301–5305. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef]
- Zhu, X.B.; Li, N.; Wang, Y.L.; Ding, L.; Chen, H.J.; Yu, Y.H.; Shi, X.J. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, X.; Zhang, X.; Cao, W.; Wang, Y.; Chen, H.; Wang, T.; Tsai, H.I.; Zhang, R.; Chang, D.; et al. The effects of quercetin-loaded PLGA-TPGS nanoparticles on ultraviolet B-induced skin damages in vivo. Nanomedicine 2016, 12, 623–632. [Google Scholar] [CrossRef]
- Nan, W.H.; Ding, L.; Chen, H.J.; Khan, F.U.; Yu, L.; Sui, X.B.; Shi, X.J. Topical Use of Quercetin-Loaded Chitosan Nanoparticles Against Ultraviolet B Radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef]
- Sulaimon, S.S.; Kitchell, B.E. The biology of melanocytes. Vet. Dermatol. 2003, 14, 57–65. [Google Scholar] [CrossRef]
- Slominski, A. Coming of age of melanogenesis-related proteins. Arch. Pathol. Lab. Med. 2002, 126, 775–777. [Google Scholar]
- Steinhoff, M.; Stander, S.; Seeliger, S.; Ansel, J.C.; Schmelz, M.; Luger, T. Modern aspects of cutaneous neurogenic inflammation. Arch. Dermatol. 2003, 139, 1479–1488. [Google Scholar] [CrossRef]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Hodi, F.S.; Fisher, D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer 2012, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrzesniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. (Online) 2016, 70, 695–708. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Garratt, P.J.; Land, E.J.; Pavel, S.; Ramsden, C.A.; Riley, P.A.; Smit, N.P. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997, 272, 26226–26235. [Google Scholar] [CrossRef] [Green Version]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef]
- Cardinali, G.; Ceccarelli, S.; Kovacs, D.; Aspite, N.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J. Investig. Dermatol. 2005, 125, 1190–1199. [Google Scholar] [CrossRef] [Green Version]
- Abu Ubeid, A.; Hantash, B.M. Minireview: Peptide Analogs and Short Sequence Oligopeptides as Modulators of Skin Pigmentation. Curr. Top. Med. Chem. 2014, 14, 1418–1424. [Google Scholar] [CrossRef]
- Sawyer, T.K.; Sanfilippo, P.J.; Hruby, V.J.; Engel, M.H.; Heward, C.B.; Burnett, J.B.; Hadley, M.E. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: A highly potent alpha-melanotropin with ultralong biological activity. Proc. Natl. Acad. Sci. USA 1980, 77, 5754–5758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorr, R.T.; Ertl, G.; Levine, N.; Brooks, C.; Bangert, J.L.; Powell, M.B.; Humphrey, S.; Alberts, D.S. Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers. Arch. Dermatol. 2004, 140, 827–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreim, S.; Lautenschlager, S.; Minder, E. Safety and efficacy of an agonistic alpha-melanocyte stimulating hormone analogue, afamelanotide (Scenesse (R)), in treating patients with erythropoietic protoporphyria for 2.5 consecutive years. Br. J. Dermatol. 2011, 164, 1149. [Google Scholar]
- Abdel-Malek, Z.A.; Kadekaro, A.L.; Kavanagh, R.J.; Todorovic, A.; Koikov, L.N.; McNulty, J.C.; Jackson, P.J.; Millhauser, G.L.; Schwemberger, S.; Babcock, G.; et al. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 2006, 20, 1561. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.; Heidl, M.; Imfeld, D.; Meeus, L.; Schuetz, R.; Campiche, R. Discovery of a Highly Selective MC1R Agonists Pentapeptide to Be Used as a Skin Pigmentation Enhancer and with Potential Anti-Aging Properties. Int. J. Mol. Sci. 2019, 20, 6143. [Google Scholar] [CrossRef] [Green Version]
- Fajuyigbe, D.; Lwin, S.M.; Diffey, B.L.; Baker, R.; Tobin, D.J.; Sarkany, R.P.E.; Young, A.R. Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J. 2018, 32, 3700–3706. [Google Scholar] [CrossRef] [Green Version]
- Seamon, K.B.; Padgett, W.; Daly, J.W. Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3363–3367. [Google Scholar] [CrossRef] [Green Version]
- D’Orazio, J.A.; Nobuhisa, T.; Cui, R.; Arya, M.; Spry, M.; Wakamatsu, K.; Igras, V.; Kunisada, T.; Granter, S.R.; Nishimura, E.K.; et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature 2006, 443, 340–344. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, S.; Kim, J.H.; Lee, G.S.; Lee, J.N.; Lee, N.H.; Hyun, C.G. Pratol, an O-Methylated Flavone, Induces Melanogenesis in B16F10 Melanoma Cells via p-p38 and p-JNK Upregulation. Molecules 2017, 22, 1704. [Google Scholar] [CrossRef]
- Xin, X.J.; Zou, J.H.; Zou, T.; Shang, H.L.; Sun, L.Y. A Newly Authenticated Compound from Traditional Chinese Medicine Decoction Induces Melanogenesis in B16-F10 Cells by Increasing Tyrosinase Activity. Evid. Based Complement. Altern. Med. 2018, 2018, 5198594. [Google Scholar] [CrossRef] [Green Version]
- Uto, T.; Ohta, T.; Yamashita, A.; Fujii, S.; Shoyama, Y. Liquiritin and Liquiritigenin Induce Melanogenesis via Enhancement of p38 and PKA Signaling Pathways. Medicines 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Ku, B.; Kim, D.; Choi, E.-M. Umbelliferone stimulated melanogenesis and increased glutathione level in B16F10 cells. Toxicol. Environ. Health Sci. 2017, 9, 152–160. [Google Scholar] [CrossRef]
- Tsang, T.F.; Chan, B.; Tai, W.C.; Huang, G.; Wang, J.; Li, X.; Jiang, Z.H.; Hsiao, W.L.W. Gynostemma pentaphyllum saponins induce melanogenesis and activate cAMP/PKA and Wnt/beta-catenin signaling pathways. Phytomedicine 2019, 60, 153008. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Huang, J.H.; Li, Y.X.; Jiang, L.; Ouyang, Y.J.; Li, Y.M.; Yang, L.; Zhao, X.J.; Huang, L.H.; Xiang, H.; et al. Cistanche deserticola polysaccharide induces melanogenesis in melanocytes and reduces oxidative stress via activating NRF2/HO-1 pathway. J. Cell. Mol. Med. 2020, 24, 4023–4035. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Park, S.J.; Park, S.H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Kwon, K.; Kim, J.; Kim, M.H.; Cho, J.Y.; et al. Ethanolic extract of Melia azedarach L. induces melanogenesis through the cAMP-PKA-CREB signaling pathway. Mol. Cell. Toxicol. 2019, 15, 75–83. [Google Scholar] [CrossRef]
- Makbal, R.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Argania spinosa Fruit Shell Extract-Induced Melanogenesis via cAMP Signaling Pathway Activation. Int. J. Mol. Sci. 2020, 21, 2539. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Koh, J.-S.; Ha, B.-J.; Boo, Y. Quercus glauca extract and rutin inhibit the UVB-induced expression of matrix metalloproteinase-1 in human dermalfibroblasts. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 677–684. [Google Scholar] [CrossRef]
- Shin, D.; Lee, S.; Huang, Y.H.; Lim, H.W.; Lee, Y.; Jang, K.; Cho, Y.; Park, S.J.; Kim, D.D.; Lim, C.J. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm. Biol. 2018, 56, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (−)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Ski. Pharm. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-kappaB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef]
- Kim, M.; Son, D.; Shin, S.; Park, D.; Byun, S.; Jung, E. Protective effects of Camellia japonica flower extract against urban air pollutants. BMC Complement. Altern. Med. 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxidative Med. Cell. Longev. 2018, 2018, 1454939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [Green Version]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Kyselova, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011, 4, 173. [Google Scholar] [CrossRef]

| Models | Materials | Key Findings | Literature |
|---|---|---|---|
| C57BL/6 mice, SKH-1 hairless mice | Sasa quelpaertensis | Topically applied plant extracts reduced edema and erythema in mice exposed to UV light. | [76] |
| SKH:hr-1 hairless albino mice | Propolis | The extract reduced cutaneous inflammation, immunosuppression, and lipid peroxidation induced by UV exposure. | [77] |
| SKH-1 hairless mice | Broccoli sprout | Dietary glucoraphanin-rich broccoli sprout extracts protected against UV-induced skin carcinogenesis. | [78] |
| Primary keratinocytes | Blackberry | Anthocyanin-rich fractions of blackberry extracts reduced UV-induced free radicals and oxidative damage in cells. | [79] |
| HaCaT human keratinocytes | Gardenia jasminoides | The extract displayed antioxidant, anti-inflammatory, and anti-apoptotic effects. | [41] |
| Human epidermal keratinocytes, Human dermal fibroblasts | Portulaca oleracea | The extracts protected human keratinocytes and fibroblasts from UV-induced apoptosis. | [80] |
| HaCaT human keratinocytes, Human volunteers | Citrus and Rosemary | The extracts protected UV-induced damage in a skin cell model and in human volunteers. | [81] |
| HaCaT human keratinocytes | Bambusae caulis in Taeniam | The extract enhanced the viabilities of UVB-exposed cells and reduced the number of apoptotic events. | [42] |
| HaCaT human keratinocytes, Humans volunteers | Scutellaria radix | The extract enhanced the sun protection factor (SPF) of a sunscreen product, as determined in human subjects. | [82] |
| HaCaT human keratinocytes, Reconstituted human skin tissue | Propolis | The extract inhibited UV-induced photodamage. | [83] |
| Materials | Models | Key Findings | Literature |
|---|---|---|---|
Quercetin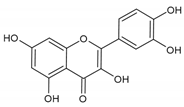 | Mel-Ab melanocytes | Quercetin reduced H2O2-induced cell death. | [107] |
| Normal human epidermal melanocytes | Quercetin attenuated ER dilation and H2O2-induced apoptosis. | [108] | |
Apigenin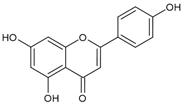 | Normal human epidermal melanocytes | Apigenin attenuated dopamine-induced apoptosis. | [109] |
Hyperoside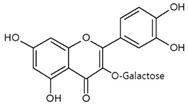 | Normal human epidermal melanocytes | Hyperoside (quercetin-3-O-galactoside) decreased apoptosis of H2O2-injured melanocytes. | [110] |
(−)-Epigallocatechin-3-gallate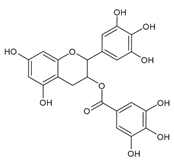 | Normal human epidermal melanocytes | (−)-Epigallocatechin-3-gallate decreased apoptosis in H2O2-injured melanocytes. | [111] |
Afzelin | Normal human epidermal melanocytes | Afzelin (kaempferol-3-O-rhamnoside) inhibited H2O2-mediated cell death. | [112] |
Baicalein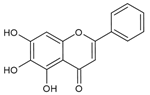 | Human vitiligo melanocytes | Baicalein inhibited H2O2-induced cytotoxicity and apoptosis. | [113] |
Geniposide | Normal human epidermal melanocytes | Geniposide (genipin-1-O-glucoside) decreased the apoptosis rate of H2O2-treated cells. | [114] |
Bilobalide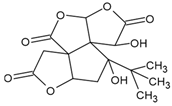 | Normal human epidermal melanocytes | Bilobalide attenuated H2O2-induced apoptosis and ER stress. | [115] |
| Compounds | Models | Key Findings | Literature |
|---|---|---|---|
Cinnamic acid | Human dermal fibroblasts | Cinnamic acid attenuated UVA-induced metalloproteinase expression through inhibition of AP-1 and activation of Nrf2. | [129] |
p-Coumaric acid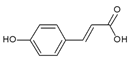 | Human epidermal melanocytes | p-Coumaric acid inhibited melanin synthesis and attenuated UVB toxicity in melanocytes. | [122] |
| Human epidermal melanocytes, Mice | p-Coumaric acid reduced erythema and pigmentation in the skin of mice exposed to UV rays. | [124] | |
| Humans | p-Coumaric acid reduced erythema and pigmentation in human skin exposed to UV rays. | [125] | |
| HaCaT human keratinocytes, Mice | p-Coumaric acid attenuated UVB toxicity in keratinocytes and reduced erythema and edema in mice skin exposed to UV rays. | [123] | |
Caffeic acid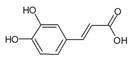 | Mice | Caffeic acid suppressed UVB radiation-induced expression of interleukin-10 and activation of MAPKs involved in contact hypersensitivity. | [130] |
| Mice | Caffeic acid targeted ERK1/2 to attenuate solar UV-induced skin carcinogenesis. | [131] | |
| Mice | Caffeic acid prevented UVB-induced photocarcinogenesis through regulation of PTEN signaling. | [132] | |
Ferulic acid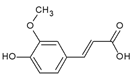 | Mice | Ferulic acid suppressed UVB-induced MMP-2 and -9 expression in mouse skin. | [133] |
| Mice | Intraperitoneal and topical administration of ferulic acid reduced the incidence of UVB-induced tumors. | [134] | |
| Humans | Ferulic acid incorporated in a sunscreen product increased SPF and UVA-PF in human skin. | [135] |
| Models | Materials | Key Findings | Literature |
|---|---|---|---|
| C57BL/6 mice | Forskolin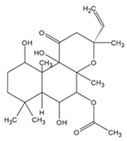 | Forskolin-induced pigmentation was protective against UV-induced cutaneous DNA damage and tumorigenesis. | [168] |
| B16F10 mouse melanoma cells | Pratol | The compound induced melanogenesis via upregulation of phospho-p38 and phospho-JNK. | [169] |
Umbelliferone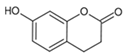 | The compound stimulated melanogenesis and increased glutathione levels in cells. | [172] | |
Apigenin-7-butylene glucoside | The compound induced melanogenesis by increasing tyrosinase activity in cells. | [170] | |
| Gynostemma pentaphyllum | Its saponins induced melanogenesis and activated the cAMP/PKA and Wnt/β-catenin signaling pathways. | [173] | |
| Argania Spinosa | Its fruit shell extract induced melanogenesis via activation of the cAMP signaling pathway. | [176] | |
| B16F10 mouse melanoma cells, Human melanoma cell lines (HMVII) | Liquiritin and liquiritigenin | The compounds induced melanogenesis via enhancement of the p38 and PKA signaling pathways. | [171] |
| B16F10 mouse melanoma cells, Human epidermal melanocytes | Melia azedarach | Its ethanolic extract induced melanogenesis through the cAMP/PKA/CREB signaling pathway. | [175] |
| Cistanche deserticola | Its polysaccharides induced melanogenesis via activation of MAPK signaling pathway and upregulation of MITF. | [174] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. https://doi.org/10.3390/antiox9070637
Boo YC. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants. 2020; 9(7):637. https://doi.org/10.3390/antiox9070637
Chicago/Turabian StyleBoo, Yong Chool. 2020. "Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials" Antioxidants 9, no. 7: 637. https://doi.org/10.3390/antiox9070637
APA StyleBoo, Y. C. (2020). Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants, 9(7), 637. https://doi.org/10.3390/antiox9070637





