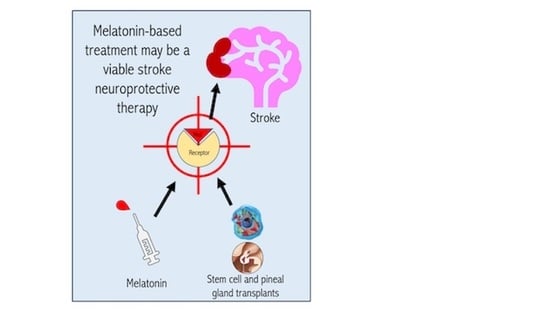
Melatonin—A Potent Therapeutic for Stroke and Stroke-Related Dementia
Abstract
:1. Introduction
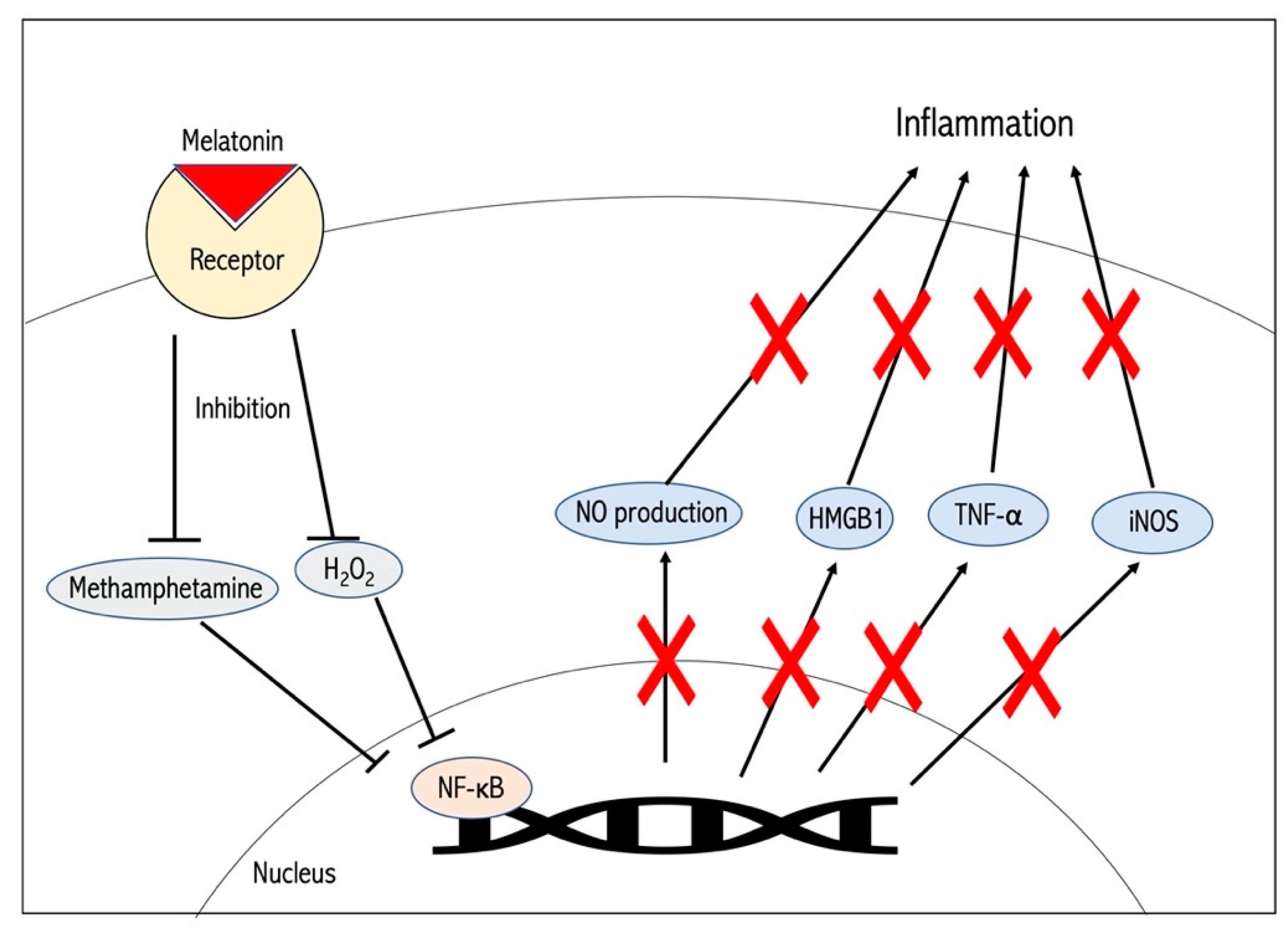
2. Mechanisms Behind Melatonin-Induced Neuroprotection in Experimental Stroke Models
3. The Use of Pineal Gland Grafts in Melatonin-Based Stroke Therapies
4. The Role of Melatonin Receptors in Stem Cell Therapeutics under Stroke Conditions
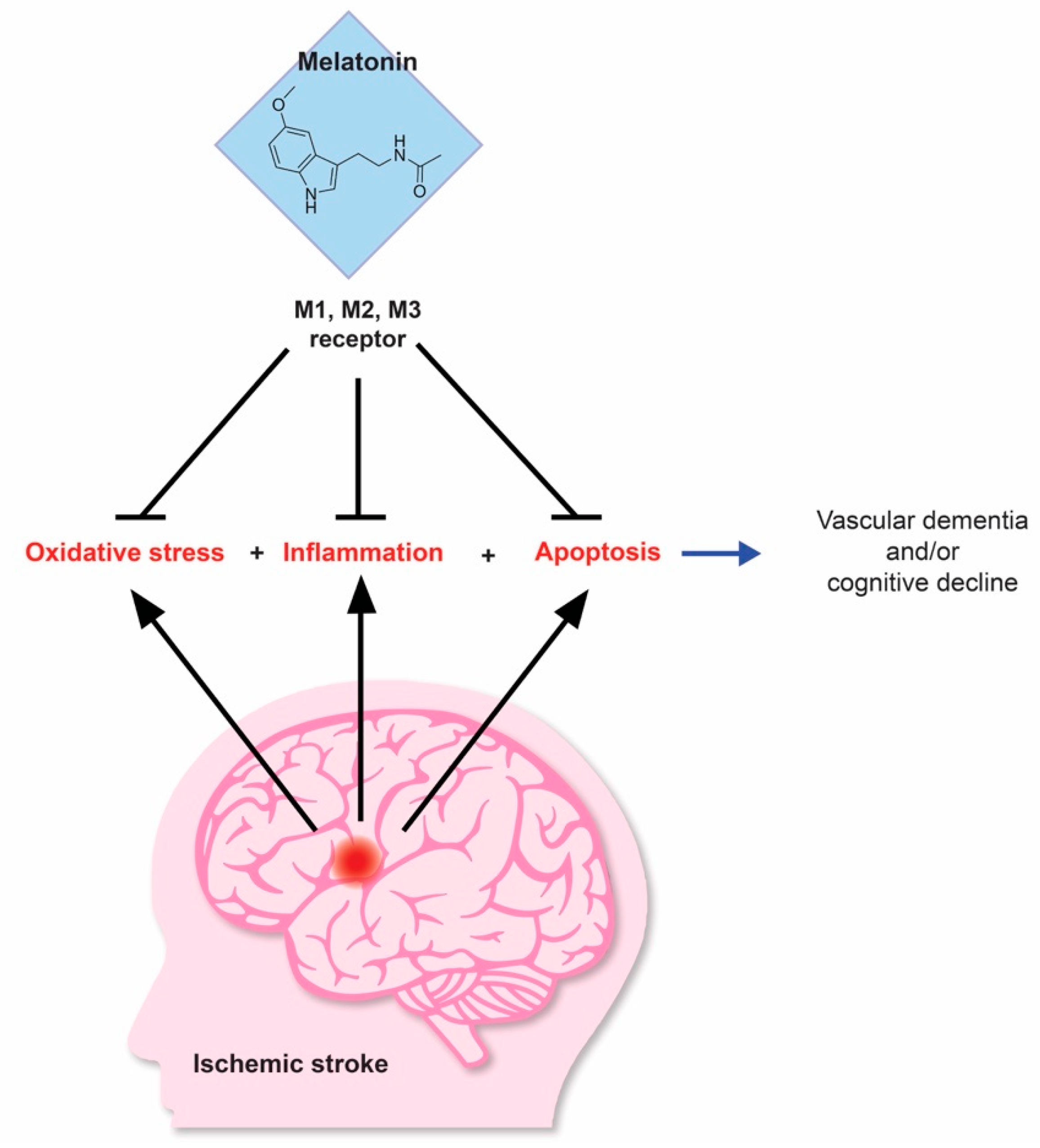
5. Stroke-Induced Dementia as a Potential Target for Melatonin-Based Therapeutics
6. Novel Evidence Supporting Melatonin as an Effective Therapeutic Agent in Stroke
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, D.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Hanes, M.A.; Farley, N.J. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999, 65, 2523–2529. [Google Scholar] [CrossRef]
- Skinner, D.C.; Malpaux, B. High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology 1999, 140, 4399–4405. [Google Scholar] [CrossRef]
- Yu, H.S.; Yee, R.W.; Howes, K.A.; Reiter, R.J. Diurnal rhythms of immunoreactive melatonin in the aqueous humor and serum of male pigmented rabbits. Neurosci. Lett. 1990, 116, 309–314. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [CrossRef]
- Reiter, R.J. Oxidative damage in the central nervous system: Protection by melatonin. Prog. Neurobiol. 1998, 56, 359–384. [Google Scholar] [CrossRef]
- Cutler, R.G. Oxidative stress: Its potential relevance to human disease and longevity determinants. AGE 1995, 18, 91–96. [Google Scholar] [CrossRef]
- Leker, R.R.; Teichner, A.; Lavie, G.; Shohami, E.; Lamensdorf, I.; Ovadia, H. The nitroxide antioxidant tempol is cerebroprotective against focal cerebral ischemia in spontaneously hypertensive rats. Exp. Neurol. 2002, 176, 355–363. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Costantino, G.; Caputi, A.P. Protective effect of melatonin on cellular energy depletion mediated by peroxynitrite and poly (ADP-ribose) synthetase activation in a non-septic shock model induced by zymosan in the rat. J. Pineal Res. 1998, 25, 78–85. [Google Scholar] [CrossRef]
- Cagnoli, C.M.; Atabay, C.; Kharlamova, E.; Manev, H. Melatonin protects neurons from singlet oxygen-induced apoptosis. J. Pineal Res. 1995, 18, 222–226. [Google Scholar] [CrossRef]
- Kilic, E.; Ozdemir, Y.G.; Bolay, H.; Kelestimur, H.; Dalkara, T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Lee, M.Y.; Chen, H.Y.; Hsu, Y.S.; Wu, T.S.; Chen, S.T.; Chang, G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005, 38, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Pang, S.F.; Cheung, R.T. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2002, 32, 168–172. [Google Scholar] [CrossRef]
- Kaneko, Y.; Hayashi, T.; Yu, S.; Tajiri, N.; Solomita, M.A.; Chheda, S.H.; Weinbren, N.L.; Parolini, O.; Borlongan, C.V. Human amniotic epithelial cells express melatonin receptor MT1 but not melatonin receptor MT2: A new perspective to neuroprotection. J. Pineal Res. 2011, 50, 272–280. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kuan, Y.H.; Chen, H.Y.; Chen, T.Y.; Chen, S.T.; Huang, C.C.; Yang, I.P.; Hsu, Y.S.; Wu, T.S.; Lee, E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res. 2007, 42, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Shinozuka, K.; Staples, M.; Borlongan, C.V. Melatonin-based therapeutics for neuroprotection in stroke. Int. J. Mol. Sci. 2013, 14, 8924–8947. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.Z.; Chiou, A.L.; Williams, L.R.; Hoffer, B.J. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J. Neurosci. 1997, 17, 4341–4348. [Google Scholar] [CrossRef]
- Luscher, C.; Malenka, R.C.; Nicoll, R.A. Monitoring glutamate release during LTP with glial transporter currents. Neuron 1998, 21, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Redmond, D.E. Cellular replacement therapy for Parkinson’s disease—Where we are today? Neuroscientist 2002, 8, 457–488. [Google Scholar] [CrossRef]
- Kondziolka, D.; Wechsler, L.; Goldstein, S.; Meltzer, C.; Thulborn, K.R.; Gebel, J.; Jannetta, P.; DeCesare, S.; Elder, E.M.; McGrogan, M.; et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000, 55, 565–569. [Google Scholar] [CrossRef]
- Kondoh, T.; Uneyama, H.; Nishino, H.; Torii, K. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci. 2002, 72, 583–590. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Sumaya, I.; Moss, D.; Kumazaki, M.; Sakurai, T.; Hida, H.; Nishino, H. Melatonin-secreting pineal gland: A novel tissue source for neural transplantation therapy in stroke. Cell Transplant. 2003, 12, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.K.; Chaudhary, G.; Sinha, K. Enhanced protection by melatonin and meloxicam combination in a middle cerebral artery occlusion model of acute ischemic stroke in rat. Can. J. Physiol. Pharmacol. 2002, 80, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, J.; Hong, Y.; Lee, M.; Kim, K.; Lee, S.R.; Chang, K.T.; Hong, Y. Beneficial effects of melatonin on stroke-induced muscle atrophy in focal cerebral ischemic rats. Lab. Anim. Res. 2012, 28, 47–54. [Google Scholar] [CrossRef]
- Kahan, B.D.; Gholerial, R. Immunosuppressive agents. Surg. Clin. N. Am. 1994, 74, 1029–1054. [Google Scholar] [CrossRef]
- Ben-Nathan, D.; Maestroni, G.J.M.; Lustig, S.; Conti, S. Protective effects of melatonin in mice infected with encephalitis virus. Arch. Virol. 1995, 140, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nathan, D.; Maestroni, G.J.M.; Conti, A. The protective effect of melatonin in viral and bacterial infections. In Therapeutic Potential of Melatonin; Maestroni, G.J.M., Conti, A., Reiter, R.J., Eds.; Karger: Basel, Switzerland, 1997; pp. 72–80. [Google Scholar] [CrossRef]
- Maestroni, G.J. The immunoendocrine role of melatonin. J. Pineal Res. 1993, 14, 1–10. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Sanberg, P.R. Neural transplantation for treatment of Parkinson’s disease. Drug Discov. Today 2002, 7, 674–682. [Google Scholar] [CrossRef]
- Sharma, R.; McMillan, C.R.; Niles, L.P. Neural stem cell transplantation and melatonin treatment in a 6-hydroxydopamine model of Parkinson’s disease. J. Pineal Res. 2007, 43, 245–254. [Google Scholar] [CrossRef]
- Niles, L.P.; Armstrong, K.J.; Castro, L.M.; Dao, C.V.; Sharma, R.; McMillan, C.R.; Doering, L.C.; Kirkham, D.L. Neural stem cells express melatonin receptors and neurotrophic factors: Colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Beni, S.M.; Kohen, R.; Reiter, R.J.; Tan, D.X.; Shohami, E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-κB and AP-1. FASEB J. 2004, 18, 149–151. [Google Scholar] [CrossRef] [Green Version]
- Moriya, T.; Horie, N.; Mitome, M.; Shinohara, K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J. Pineal Res. 2007, 42, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Lekic, T.; Hartman, R.; Rojas, H.; Manaenko, A.; Chen, W.; Ayer, R.; Tang, J.; Zhang, J.H. Protective effect of melatonin upon neuropathology, striatal function and memory ability after intracerebral hemorrhage in rats. J. Neurotrauma 2010, 27, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.W.; Lee, E.J. Effects of melatonin in experimental stroke models in acute, sub-acute and chronic stages. Neuropsychiatr. Dis. Treat. 2009, 5, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Li, X.; Cai, Z.; Yang, N.; Liu, Y.; Shu, J.; Pan, L.; Zuo, P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell. Mol. Neurobiol. 2008, 28, 569–579. [Google Scholar] [CrossRef]
- Kilic, E.; Kilic, U.; Bacigaluppi, M.; Guo, A.; Abdallah, N.B.; Wolfer, D.P.; Reiter, R.J.; Hermann, D.M.; Bassetti, C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 2008, 45, 142–148. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Klempin, F.; Babu, H.; Benitez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef]
- Xu, S.C.; He, M.D.; Zhong, M.; Zhang, Y.W.; Wang, Y.; Yang, J.; Yu, Z.P.; Zhou, Z. Melatonin protects against Nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. J. Pineal Res. 2010, 49, 86–94. [Google Scholar] [CrossRef]
- Romeu, L.R.; da Motta, E.L.; Maganhin, C.C.; Oshima, C.T.; Fonseca, M.C.; Barrueco, K.F.; Simoes, R.S.; Pellegrino, R.; Baracat, E.C.; Soares-Junior, J.M. Effects of melatonin on histomorphology and on the expression of steroid receptors, VEGF and PCNA in ovaries of pinealectomized female rats. Fertil. Steril. 2011, 95, 1379–1384. [Google Scholar] [CrossRef]
- Imbesi, M.; Uz, T.; Manev, H. Role of melatonin receptors in the effects of melatonin on BDNF and neuroprotection in mouse cerebellar neurons. J. Neural Transm. 2008, 115, 1495–1499. [Google Scholar] [CrossRef]
- Lee, C.H.; Yoo, K.Y.; Choi, J.H.; Park, O.K.; Hwang, I.K.; Kwon, Y.G.; Kim, Y.M.; Won, M.H. Melatonin’s protective action against ischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. J. Neurosci. Res. 2010, 88, 2630–2640. [Google Scholar] [CrossRef]
- Mao, L.L.; Cheng, Q.; Guardiola-Lemaitre, B.; Schuster-Klein, C.; Dong, C.; Lai, L.; Hill, S.M. In vitro and in vivo antitumor activity of melatonin receptor agonists. J. Pineal Res. 2010, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Rivara, S.; Pala, D.; Bedini, A.; Spadoni, G.; Tarzia, G. Recent advances in the development of melatonin MT1 and MT2 receptor agonists. Expert Opin. Ther. Pat. 2010, 20, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Poeggeler, B.; Srinivasan, V.; Trakht, I.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonergic drugs in clinical practice. Arzneimittelforschung 2008, 58, 1–10. [Google Scholar] [CrossRef]
- Simpson, D.; Curran, M.P. Ramelteon—A review of its use in insomnia. Drugs 2008, 68, 1901–1919. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Risk factors and neurodegenerative mechanisms in stroke related dementia. Panminerva Med. 2012, 54, 139–148. [Google Scholar] [PubMed]
- Zhang, X.; Bi, X. Post-Stroke Cognitive Impairment: A Review Focusing on Molecular Biomarkers. J. Mol. Neurosci. 2020, 70, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, M.; He, W.; Cai, Z. Minocycline upregulates cyclic AMP response element binding protein and brain-derived neurotrophic factor in the hippocampus of cerebral ischemia rats and improves behavioral deficits. Neuropsychiatr. Dis. Treat. 2015, 11, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Goulay, R.; Romo, L.M.; Hol, E.M.; Dijkhuizen, R.M. From Stroke to Dementia: A Comprehensive Review Exposing Tight Interactions between Stroke and Amyloid-β Formation. Transl. Stroke Res. 2020, 11, 601–614. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Fukushima, H.; Mukawa, T.; Toyoda, H.; Wu, L.J.; Zhao, M.G.; Xu, H.; Shang, Y.; Endoh, K.; Iwamoto, T.; et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J. Neurosci. 2011, 31, 8786–8802. [Google Scholar] [CrossRef]
- Gumuslu, E.; Mutlu, O.; Sunnetci, D.; Ulak, G.; Celikyurt, I.K.; Cine, N.; Akar, F.; Savli, H.; Erden, F. The antidepressant agomelatine improves memory deterioration and upregulates CREB and BDNF gene expression levels in unpredictable chronic mild stress (UCMS)-exposed mice. Drug Target Insights 2014, 8, 11–21. [Google Scholar] [CrossRef]
- Yang, C.L.; Guo, H.; Zhou, H.; Suo, D.Q.; Li, W.J.; Zhou, Y.; Zhao, Y.; Yang, W.S.; Jin, X. Chronic oleoylethanolamide treatment improves spatial cognitive deficits through enhancing hippocampal neurogenesis after transient focal cerebral ischemia. Biochem. Pharmacol. 2015, 94, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qiu, R.; Li, L.; He, D.; Lv, H.; Wu, X.; Gu, N. The role of exogenous neural stem cells transplantation in cerebral ischemic stroke. J. Biomed. Nanotechnol. 2014, 10, 3219–3230. [Google Scholar] [CrossRef]
- Jeong, H.C.; Kim, M.S.; Lim, Y.J.; Ryu, C.H.; Jun, J.A.; Jeun, S.S. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed. Res. Int. 2014, 2014, 129145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Zhi, P.K.; Luo, Z.K.; Shi, J. Severe instead of mild hyperglycemia inhibits neurogenesis in the subventricular zone of adult rats after transient focal cerebral ischemia. Neuroscience 2015, 303, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kako, E.; Sawamoto, K. Enhancement of ventricular-subventricular zone-derived neurogenesis and oligodendrogenesis by erythropoietin and its derivatives. Front. Cell. Neurosci. 2013, 7, 235. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.Y.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Pharmacological Effects of Melatonin as Neuroprotectant in Rodent Model: A Review on the Current Biological Evidence. Cell. Mol. Neurobiol. 2020, 40, 25–51. [Google Scholar] [CrossRef]
- Liu, Z.J.; Ran, Y.Y.; Qie, S.Y.; Wei-Jun, G.; Fu-Hai, G.; Zi-Tong, D.; Jia-Ning, X. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci. Ther. 2019, 25, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar] [CrossRef]
- Motallebzade, E.; Tameh, A.A.; Zavareh, S.A.T.; Farhood, B.; Aliasgharzedeh, A.; Mohseni, M. Neuroprotective effect of melatonin on radiation-induced oxidative stress and apoptosis in the brainstem of rats. J. Cell. Physiol. 2020, 1–8. [Google Scholar] [CrossRef]
- Azedi, F.; Mehrpour, M.; Talebi, S.; Zendedel, A.; Kazemnejad, S.; Mousavizadeh, K.; Beyer, C.; Zarnani, A.H.; Joghataei, M.T. Melatonin regulates neuroinflammation ischemic stroke damage through interactions with microglia in reperfusion phase. Brain Res. 2019, 1723, 146401. [Google Scholar] [CrossRef]
- Michalska, P.; Buendia, I.; Duarte, P.; Mendivil, C.F. Melatonin-sulforaphane hybrid ITH12674 attenuates glial response in vivo by blocking LPS binding to MD2 and receptor oligomerization. Pharmacol. Res. 2020, 152, 104597. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Pu, Y.; Yang, Z.; Pan, Y.; Liu, L. Therapeutic effects of melatonin on cerebral ischemia reperfusion injury: Role of Yap-OPA1 signaling pathway and mitochondrial fusion. Biomed. Pharmacother. 2019, 110, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jana, T.; Tzveta, S.; Zlatina, N.; Natasha, I.; Dimitrinka, A.; Milena, A.; Katerina, G. Effect of endurance training on diurnal rhythms of superoxide dismutase activity, glutathione and lipid peroxidation in plasma of pinealectomized rats. Neurosci. Lett. 2020, 716, 134637. [Google Scholar] [CrossRef]
- Doğanlar, Z.B.; Güçlü, H.; Öztopuz, Ö.; Türkön, H.; Dogan, A.; Uzun, M.; Doğanlar, O. The Role of Melatonin in Oxidative Stress, DNA Damage, Apoptosis and Angiogenesis in Fetal Eye under Preeclampsia and Melatonin Deficiency Stress. Curr. Eye Res. 2019, 44, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Komaki, A.; Hashemi-Firouzi, N.; Mortezaee, K.; Faraji, N.; Golipoor, Z. Therapeutic effects of melatonin-treated bone marrow mesenchymal stem cells (BMSC) in a rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2020, 108, 101804. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Govitrapong, P. Melatonin receptor as a drug target for neuroprotection. Curr. Mol. Pharmacol. 2020, 13. [Google Scholar] [CrossRef]
- Sinha, B.; Wu, Q.; Li, W.; Tu, Y.; Sirianni, A.C.; Chen, Y.; Jiang, J.; Zhang, X.; Chen, W.; Zhou, S.; et al. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through MT1 receptor. J. Pineal Res. 2018, 64, e12443. [Google Scholar] [CrossRef] [Green Version]
- Shahrokhi, N.; Khaksari, M.; AsadiKaram, G.; Soltani, Z.; Shahrokhi, N. Role of melatonin receptors in the effect of estrogen on brain edema, intracranial pressure and expression of aquaporin 4 after traumatic brain injury. Iran J. Basic Med. Sci. 2018, 21, 301–308. [Google Scholar] [CrossRef]
- Tang, H.; Ma, M.; Wu, Y.; Deng, M.F.; Hu, F.; Almansoub, H.A.; Huang, H.Z.; Wang, D.Q.; Zhou, L.T.; Wei, N.; et al. Activation of MT2 receptor ameliorates dendritic abnormalities in Alzheimer’s disease via C/EBPα/miR-125b pathway. Aging Cell 2019, 18, e12902. [Google Scholar] [CrossRef]
- Wu, X.L.; Lu, S.S.; Liu, M.R.; Tang, W.D.; Chen, J.Z.; Zheng, Y.R.; Ahsan, A.; Cao, M.; Jiang, L.; Hu, W.W.; et al. Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury. Acta Pharmacol. Sin. 2020. [Google Scholar] [CrossRef]
- Phonchai, R.; Phermthai, T.; Kitiyanant, N.; Suwanjang, W.; Kotchabhakdi, N.; Chetsawang, B. Potential effects and molecular mechanisms of melatonin on the dopaminergic neuronal differentiation of human amniotic fluid mesenchymal stem cells. Neurochem. Int. 2019, 124, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Qiu, X.; Wang, Y.; Liu, J.; Li, Q.; Jiang, H.; Li, S.; Song, C. Long-term oral melatonin alleviates memory deficits, reduces amyloid-β deposition associated with downregulation of BACE1 and mitophagy in APP/PS1 transgenic mice. Neurosci. Lett. 2020, 735, 135192. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, L.Y.; Chen, Y.; Bai, Y.P.; Jia, J.; Feng, J.G.; Liu, K.X.; Zhou, J. Melatonin alleviates intestinal injury, neuroinflammation and cognitive dysfunction caused by intestinal ischemia/reperfusion. Int. Immunopharmacol. 2020, 85, 106596. [Google Scholar] [CrossRef]
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, J.W.; Park, J.H.; Yoo, D.Y.; Kim, D.W.; Won, M.H. Melatonin ameliorates cuprizone-induced reduction of hippocampal neurogenesis, brain-derived neurotrophic factor and phosphorylation of cyclic AMP response element-binding protein in the mouse dentate gyrus. Brain Behav. 2019, 9, e01388. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, C.; Li, T.; Wang, W.; Ye, W.; Zeng, R.; Ni, L.; Lai, Z.; Wang, X.; Liu, C. The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: In vivo assessment and a randomized controlled trial. J. Pineal Res. 2018, 65, e12521. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, A.L.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramírez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.; Ramírez-Jirano, L.J.; Velázquez-Brizuela, I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch. Med. Res. 2018, 49, 391–398. [Google Scholar] [CrossRef]
- Albers, G.W.; Goldstein, L.B.; Hess, D.C.; Wechsler, L.R.; Fuire, K.L.; Gorelick, P.B.; Hurn, P.; Liebskind, D.S.; Nogueira, R.G.; Saver, J.L. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 2011, 42, 2645–2650. [Google Scholar] [CrossRef]
- STEPS Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009, 40, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Currier, N.L.; Sun, L.Z.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef]
- Carillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Giordano, M.; Palermo, M.S. Melatonin-induced enhancement of antibody-dependent cellular cytotoxicity. J. Pineal Res. 1991, 10, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.M.; Rahman, R.M.; Clarkson, A.N.; Sutherland, B.A.; Taurin, S.; Sammut, I.A.; Appleton, I. Melatonin treatment following stroke induction modulates L-arginine metabolism. J. Pineal Res. 2011, 51, 313–323. [Google Scholar] [CrossRef]
- Chern, C.M.; Liao, J.F.; Wang, Y.H.; Shen, Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 2012, 52, 1634–1647. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kulesh, A.A.; Drobakha, V.E.; Shestakov, V.V. The role of melatonin in the development of post-stroke cognitive impairment in elderly patients in comparison with middle-aged patients. Adv. Gerontol. 2016, 29, 651–657. [Google Scholar] [PubMed]
- Feng, D.; Wang, B.; Wang, L.; Abraham, N.; Tao, K.; Huang, L.; Shi, W.; Dong, Y.; Qu, Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J. Pineal Res. 2017, 62, e12395. [Google Scholar] [CrossRef]
- Kilic, U.; Caglayan, A.B.; Beker, M.C.; Gunal, M.Y.; Caglayan, B.; Yalcin, E.; Kelestemur, T.; Gundogdu, R.Z.; Yulug, B.; Yılmaz, B.; et al. Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol. 2017, 12, 657–665. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage and Mitochondria Injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef] [Green Version]
- Rancan, L.; Paredes, S.D.; García, C.; González, P.; Rodríguez-Bobada, C.; Calvo-Soto, M.; Hyacinthe, B.; Vara, E.; Tresguerres, J.A.F. Comparison of the Effect of Melatonin Treatment before and after Brain Ischemic Injury in the Inflammatory and Apoptotic Response in Aged Rats. Int. J. Mol. Sci. 2018, 19, 2097. [Google Scholar] [CrossRef] [Green Version]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. Serum melatonin levels are associated with mortality in patients with malignant middle cerebral artery infarction. J. Int. Med. Res. 2018, 46, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Park, J.H.; Lee, Y.L.; Kang, I.J.; Kim, D.W.; Hwang, I.K.; Lee, C.H.; Yan, B.C.; Kim, Y.M.; Lee, T.K.; et al. Melatonin improves vascular cognitive impairment induced by ischemic stroke by remyelination via activation of ERK1/2 signaling and restoration of glutamatergic synapses in the gerbil hippocampus. Biomed. Pharmacother. 2018, 108, 687–697. [Google Scholar] [CrossRef] [PubMed]
| Model | Stroke Type | Significant Findings |
|---|---|---|
| In vivo | Permanent | Modulation of L-arginine metabolism via melatonin improves stroke outcomes. Post-middle cerebral artery occlusion administration of melatonin significantly reduced nitric oxide synthase activity, nitrite levels and cyclooxygenases, all of which contribute to stroke-induced inflammation. A decrease in infarct volume and rejuvenation of mitochondrial enzymatic activity was also observed [84]. |
| In vivo | Transient | Activation of MT2 receptor via melatonin after transient middle cerebral artery injury and reperfusion significantly improved brain function and survival in mice. Free radical production and gp91(phox) cell infiltration were decreased, consequently preserving blood brain barrier function. Enhanced endogenous neurogenesis and expression of neurodevelopmental genes were also observed [85]. |
| In vivo | Permanent | Melatonin administration ameliorates ischemic reperfusion injury via activating SIRT1 signaling and improving mitochondrial function. Neuroprotective effects were demonstrated in mice upon treatment with melatonin post-ischemia including reduced infarct volume, decreased edema and improved neurological scores. Activation of SIRT1 via melatonin upregulates anti-apoptotic factor Bcl2 and lowers expression of pro-apoptotic protein Bax indicating that melatonin possesses anti-apoptotic effects [86]. |
| Clinical | Permanent | 6-sulfatoximelatonin indicates post-stroke cognitive impairment in elderly patients. The presence of 6-sulfatoximelatonin, a metabolite of melatonin, was investigated in the urine of patients during the acute phase of stroke. Increased concentration of the metabolite was linked to large ischemic lesions and hippocampal volume. Patients with the highest concentrations of the metabolite presented with dysexecutive cognitive impairment [87]. |
| In vivo | Permanent | Pre-treatment with melatonin before ischemia inhibits endoplasmic reticulum (ER) stress-dependent autophagy, shielding against cerebral ischemic/reperfusion (IR) injury. Pre-ischemic melatonin administration voided IR-associated ER stress autophagy and ameliorated autophagic flux. Melatonin also reduced edema, infarction size and apoptosis [88]. |
| In vivo | Permanent | PI3K/Akt phosphorylation reduces apoptosis and mediates melatonin’s neuroprotective effects via PDK1 and PTEN at the Thr308 site. Melatonin’s neuroprotective effects were reversed in focal ischemia murine model by PI3K/Akt inhibition, specifically reduction in infarct volume, indicating P13K/Akt are involved in melatonin’s ameliorative effects. The PI3K/Akt pathway decreased p53 phosphorylation consequently reducing apoptosis [89]. |
| In vivo | Permanent | Melatonin alleviates symptoms associated with secondary brain injury (SBI) following intracerebral hemorrhage (ICH) in rats. Administration of melatonin significantly decreased concentration of inflammatory, DNA damage, oxidative stress, blood brain barrier integrity and apoptosis markers. Mitochondrial function was maintained via decreasing membrane permeability and transition pore opening. Melatonin also ameliorated brain edema, improved behavior and upregulated antioxidant indicator expression [90]. |
| In vivo | Permanent | Melatonin displays favorable outcomes when administered to aged rats pre-ischemia and post-ischemia. Rats subject to MCAO were treated with melatonin 24 h before pre-ischemia and data indicated reduced levels of tumor necrosis factor-α, Bcl-2-associated death promoter, interleukin-1β, Bcl-2-associated X protein glial fibrillary acidic protein in the hippocampus and cortex. Augmented levels of sirtuin 1 and B-cell lymphoma were observed in the hippocampal region. Post-ischemic treatment provided similar effects; however these were not as effective [91]. |
| Clinical | Permanent | Peroxidation status, mortality and antioxidant status are linked to melatonin concentration in patients with middle cerebral artery infarction. Non-survivors presented with significantly increased antioxidant capacity, malondialdehyde and serum melatonin levels when compared to survivors. A positive associated was observed between serum melatonin levels with total antioxidant capacity and malondialdehyde concentration [92]. |
| In vivo | Transient | Long term melatonin treatment post-transient global cerebral ischemia (tGCI) improves outcomes via activation of ERK1/2 signaling. Treatment ameliorated cognitive impairment and expanded myelin basic protein immunoreactivity and levels of Rip-immunoreactive oligodendrocytes. ERK1/2 and pERK1/2 activity was increased in oligodendrocytes. Glutamatergic synapse activity was also augmented through long term-melatonin treatment post tGCI [93]. |
| In vivo | Permanent | The shift of microglia from pro-inflammatory to anti-inflammatory polarity in STAT3-dependent manner via melatonin partially improves brain function after distal middle cerebral artery occlusion. Reduced infarct volume, improved brain function, inhibition of pro-inflammatory responses was observed after melatonin administration. Melatonin increased phosphorylated STAT3 expression in BV2 cells [58]. |
| In vitro | Permanent | Modulation of microglial action via melatonin ameliorates reperfusion phase-induced secondary injury after stroke. In vitro, melatonin treatment early in the reperfusion phase improved outcomes. GFAP, Iba1, active caspase-3 all decreased upon administration while NeuN increased. BDNF, HSPA1A and MAP2 were seen at augmented levels while VEGF mRNA was decreased. TREM2/iNOS ratio increased indicating protective forms of microglia [61]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadanandan, N.; Cozene, B.; Cho, J.; Park, Y.J.; Saft, M.; Gonzales-Portillo, B.; Borlongan, C.V. Melatonin—A Potent Therapeutic for Stroke and Stroke-Related Dementia. Antioxidants 2020, 9, 672. https://doi.org/10.3390/antiox9080672
Sadanandan N, Cozene B, Cho J, Park YJ, Saft M, Gonzales-Portillo B, Borlongan CV. Melatonin—A Potent Therapeutic for Stroke and Stroke-Related Dementia. Antioxidants. 2020; 9(8):672. https://doi.org/10.3390/antiox9080672
Chicago/Turabian StyleSadanandan, Nadia, Blaise Cozene, Justin Cho, You Jeong Park, Madeline Saft, Bella Gonzales-Portillo, and Cesar V. Borlongan. 2020. "Melatonin—A Potent Therapeutic for Stroke and Stroke-Related Dementia" Antioxidants 9, no. 8: 672. https://doi.org/10.3390/antiox9080672
APA StyleSadanandan, N., Cozene, B., Cho, J., Park, Y. J., Saft, M., Gonzales-Portillo, B., & Borlongan, C. V. (2020). Melatonin—A Potent Therapeutic for Stroke and Stroke-Related Dementia. Antioxidants, 9(8), 672. https://doi.org/10.3390/antiox9080672