Multisystem Inflammatory-like Syndrome in a Child Following COVID-19 mRNA Vaccination
Abstract
:1. Introduction
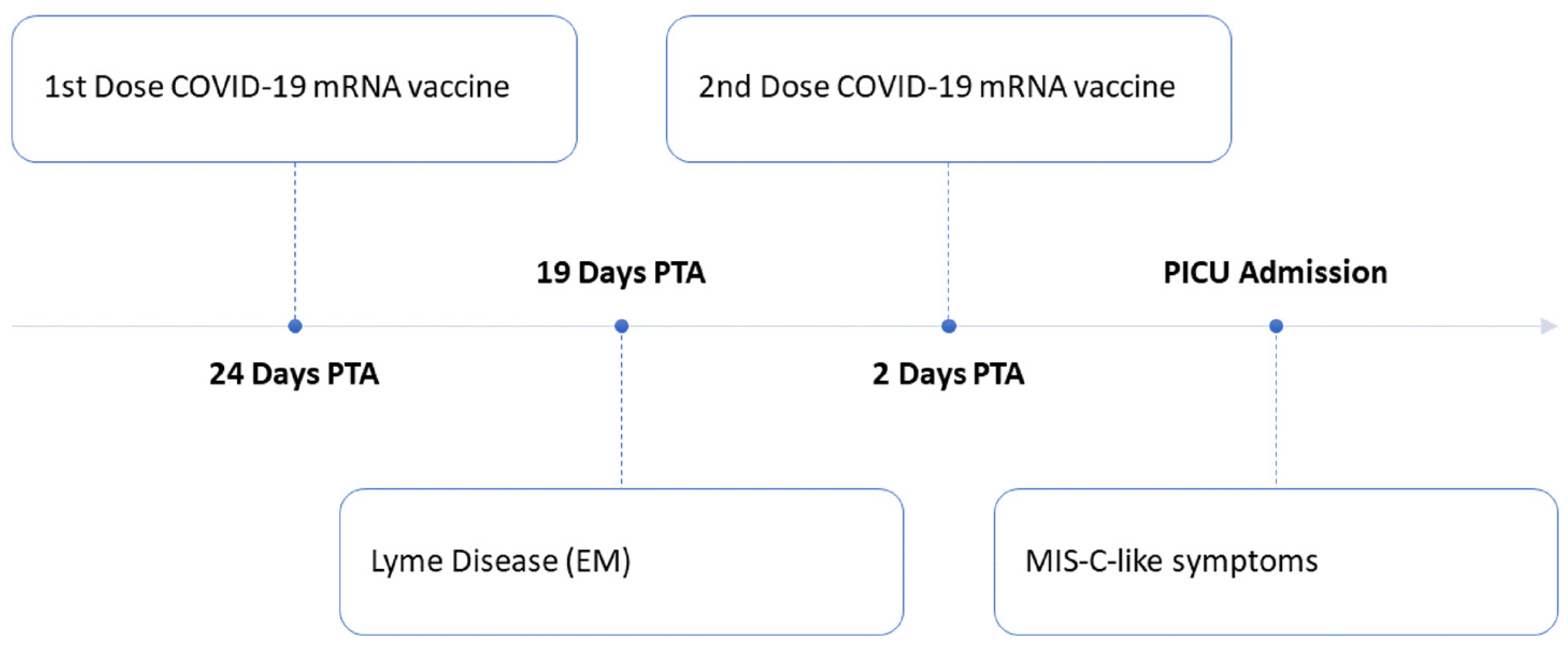
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LaRovere, K.L.; Riggs, B.J.; Poussaint, T.Y.; Young, C.C.; Newhams, M.M.; Maamari, M.; Walker, T.C.; Singh, A.R.; Dapul, H.; Hobbs, C.V.; et al. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol. 2021, 78, 536–547. [Google Scholar] [CrossRef]
- Lindan, C.E.; Mankad, K.; Ram, D.; Kociolek, L.K.; Silvera, V.M.; Boddaert, N.; Stivaros, S.M.; Palasis, S.; Group, A.P.C. Neuroimaging manifestations in children with SARS-CoV-2 infection: A multinational, multicentre collaborative study. Lancet Child. Adolesc. Health 2021, 5, 167–177. [Google Scholar] [CrossRef]
- Abdel-Mannan, O.; Eyre, M.; Lobel, U.; Bamford, A.; Eltze, C.; Hameed, B.; Hemingway, C.; Hacohen, Y. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol. 2020, 77, 1440–1445. [Google Scholar] [CrossRef]
- Starkey, J.; Kobayashi, N.; Numaguchi, Y.; Moritani, T. Cytotoxic Lesions of the Corpus Callosum That Show Restricted Diffusion: Mechanisms, Causes, and Manifestations. Radiographics 2017, 37, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, M.; Tanabe, T.; Shimakawa, S.; Nakamura, M.; Murata, S.; Shabana, K.; Shinohara, J.; Odanaka, Y.; Matsumura, H.; Maki, K.; et al. Clinico-radiological spectrum of reversible splenial lesions in children. Brain Dev. 2014, 36, 330–336. [Google Scholar] [CrossRef]
- Chen, W.X.; Liu, H.S.; Yang, S.D.; Zeng, S.H.; Gao, Y.Y.; Du, Z.H.; Li, X.J.; Lin, H.S.; Liang, H.C.; Mai, J.N. Reversible splenial lesion syndrome in children: Retrospective study and summary of case series. Brain Dev. 2016, 38, 915–927. [Google Scholar] [CrossRef]
- Takanashi, J.; Shiihara, T.; Hasegawa, T.; Takayanagi, M.; Hara, M.; Okumura, A.; Mizuguchi, M. Clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. J. Neurol. Sci. 2015, 349, 226–228. [Google Scholar] [CrossRef]
- Hara, M.; Mizuochi, T.; Kawano, G.; Koike, T.; Shibuya, I.; Ohya, T.; Ohbu, K.; Nagai, K.; Nagamitsu, S.; Yamashita, Y.; et al. A case of clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. Brain Dev. 2011, 33, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). Available online: https://www.cdc.gov/mis-c/hcp/ (accessed on 26 December 2021).
- Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome (MIS). Available online: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed on 26 December 2021).
- Vogel, T.P.; Top, K.A.; Karatzios, C.; Hilmers, D.C.; Tapia, L.I.; Moceri, P.; Giovannini-Chami, L.; Wood, N.; Chandler, R.E.; Klein, N.P.; et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3037–3049. [Google Scholar] [CrossRef]
- Chai, Q.; Nygaard, U.; Schmidt, R.C.; Zaremba, T.; Moller, A.M.; Thorvig, C.M. Multisystem inflammatory syndrome in a male adolescent after his second Pfizer-BioNTech COVID-19 vaccine. Acta Paediatr. 2022, 111, 125. [Google Scholar] [CrossRef] [PubMed]
- Buchhorn, R.; Meyer, C.; Schulze-Forster, K.; Junker, J.; Heidecke, H. Autoantibody Release in Children after Corona Virus mRNA Vaccination: A Risk Factor of Multisystem Inflammatory Syndrome? Vaccines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Dionne, A.; Sperotto, F.; Chamberlain, S.; Baker, A.L.; Powell, A.J.; Prakash, A.; Castellanos, D.A.; Saleeb, S.F.; de Ferranti, S.D.; Newburger, J.W.; et al. Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021, 6, 1446–1450. [Google Scholar] [CrossRef]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices-United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Youn, T.; Yang, H. Cytotoxic Lesion of the Corpus Callosum (CLOCCs) after SARS-CoV-2 mRNA Vaccination. J. Korean Med. Sci. 2021, 36, e228. [Google Scholar] [CrossRef] [PubMed]
- Ka, A.; Britton, P.; Troedson, C.; Webster, R.; Procopis, P.; Ging, J.; Chua, Y.W.; Buckmaster, A.; Wood, N.; Jones, C.; et al. Mild encephalopathy with reversible splenial lesion: An important differential of encephalitis. Eur. J. Paediatr. Neurol. 2015, 19, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.F.; Shen, J.; Mao, S.S.; Yu, Y.L.; Xu, L.; Jiang, P.F.; Gao, F.; Xia, Z.Z. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion associated with Mycoplasma pneumoniae infection. BMC Infect. Dis. 2016, 16, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluss, J.; Ferey, S.; Menache-Starobinski, C.; Delavelle, J.; Van Bogaert, P.; Vargas, M.I. Mild influenza-associated encephalopathy/encephalitis with a reversible splenial lesion in a Caucasian child with additional cerebellar features. Eur. J. Paediatr. Neurol. 2010, 14, 97–100. [Google Scholar] [CrossRef]
- Tsuji, M.; Yoshida, T.; Miyakoshi, C.; Haruta, T. Is a reversible splenial lesion a sign of encephalopathy? Pediatr. Neurol. 2009, 41, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Bulakbasi, N.; Kocaoglu, M.; Tayfun, C.; Ucoz, T. Transient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. Am. J. Neuroradiol. 2006, 27, 1983–1986. [Google Scholar]
- Pan, J.J.; Zhao, Y.Y.; Lu, C.; Hu, Y.H.; Yang, Y. Mild encephalitis/encephalopathy with a reversible splenial lesion: Five cases and a literature review. Neurol. Sci. 2015, 36, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.; Niculescu, I.; Patel, S.; Krishnan, A. COVID-19 and Involvement of the Corpus Callosum: Potential Effect of the Cytokine Storm? Am. J. Neuroradiol. 2020, 41, 1625–1628. [Google Scholar] [CrossRef]
- Moonis, G.; Filippi, C.G.; Kirsch, C.F.E.; Mohan, S.; Stein, E.G.; Hirsch, J.A.; Mahajan, A. The Spectrum of Neuroimaging findings on CT and MRI in Adults with Coronavirus Disease (COVID-19). Am. J. Roentgenol. 2020, 217, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; Tanuma, N.; Hayashi, M.; Imamura, T.; Takanashi, J.; Nagata, R.; Okumura, A.; Kashii, H.; Tomita, S.; Kumada, S.; et al. Oxidative stress in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Brain Dev. 2012, 34, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Kometani, H.; Kawatani, M.; Ohta, G.; Okazaki, S.; Ogura, K.; Yasutomi, M.; Tanizawa, A.; Ohshima, Y. Marked elevation of interleukin-6 in mild encephalopathy with a reversible splenial lesion (MERS) associated with acute focal bacterial nephritis caused by Enterococcus faecalis. Brain Dev. 2014, 36, 551–553. [Google Scholar] [CrossRef]
- Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines among Adolescents and Young Adults. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (accessed on 10 December 2021).
- Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html (accessed on 10 December 2021).
- Olivotto, S.; Basso, E.; Lavatelli, R.; Previtali, R.; Parenti, L.; Fiori, L.; Dilillo, D.; Zuccotti, G.V.; Veggiotti, P.; Bova, S.M. Acute encephalitis in pediatric multisystem inflammatory syndrome associated with COVID-19. Eur. J. Paediatr. Neurol. 2021, 34, 84–90. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for Lyme Disease-United States, 2008–2015. MMWR Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef]
- Aguero-Rosenfeld, M.E.; Nowakowski, J.; McKenna, D.F.; Carbonaro, C.A.; Wormser, G.P. Serodiagnosis in early Lyme disease. J. Clin. Microbiol. 1993, 31, 3090–3095. [Google Scholar] [CrossRef] [Green Version]
- Steere, A.C.; McHugh, G.; Damle, N.; Sikand, V.K. Prospective study of serologic tests for lyme disease. Clin. Infect. Dis. 2008, 47, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin. Infect. Dis. 2021, 72, e1–e48. [Google Scholar] [CrossRef]
- Rebman, A.W.; Crowder, L.A.; Kirkpatrick, A.; Aucott, J.N. Characteristics of seroconversion and implications for diagnosis of post-treatment Lyme disease syndrome: Acute and convalescent serology among a prospective cohort of early Lyme disease patients. Clin. Rheumatol. 2015, 34, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]

| Laboratory Studies | Source | Result (Normal Range) |
|---|---|---|
| Infectious Disease | ||
| SARS-CoV2 PCR | NP swab | Negative |
| Anti-SARS-CoV2 nucleocapsid antibodies | Blood | Negative |
| Culture and gram stain | CSF | Negative |
| Adenovirus PCR | Blood, NP swab | Negative |
| Enterovirus PCR | Blood, CSF | Negative |
| Herpes Simplex Virus type 1 and type 2 | CSF | Negative |
| Varicella Zoster Virus PCR | CSF | Negative |
| Epstein Barr Virus PCR | Blood | Negative |
| Coxsackie A9 antibodies | Blood | Negative |
| Ehrlichia and Anaplasma PCR | Blood | Negative |
| Rickettsia rickettsia serologies, IgM, IgG | Blood | Negative |
| Eastern Equine Encephalitis IgM | Blood | Negative |
| West Nile Virus IgM | Blood | Negative |
| Pneumococcus IgG | Blood | Negative |
| Tetanus IgG | Blood | Negative |
| RVP (Adenovirus PCR, hMPV PCR, Rhinovirus PCR, Influenza A and B PCR, RSV PCR) | NP swab | Negative |
| Legionella antigen | Urine | Negative |
| Lyme antibody index | CSF | Negative |
| Neurologic | ||
| Anti-MOG antibodies | Blood, CSF | Negative |
| Autoimmune encephalitis panel | Blood, CSF | Negative |
| CNS demyelinating disease panel # | Blood | Negative |
| Cardiac | ||
| Viral Respiratory Panel (Myocarditis) | NP Swab | Negative |
| Electrocardiogram (ECG) | N/A | Widespread repolarization abnormalities, with nonspecific ST-T wave changes |
| Echocardiogram | N/A | Normal systolic and diastolic ventricular function, normal coronary dimensions, no significant valvar dysfunction, and no pericardial effusion |
| Cardiac MRI | N/A | No evidence of active myocarditis or late gadolinium enhancement |
| Immunology * | ||
| Soluble CD25, units/mL | Blood | 690 (137–838) |
| Serum inflammatory markers ^,* | ||
| D-dimer, mcg/mL FEU | Blood | 0.43 |
| Erythrocyte sedimentation rate, mm/h | Blood | 5 |
| Platelet count, K cells/μL | Blood | 313 |
| Other * | ||
| White blood, cells/mm3 | CSF | 2 with 7% neutrophils, 35% lymphocytes, 58% macrophages |
| Red blood cells | CSF | 0 |
| Protein, mg/dL | CSF | 14.9 |
| Glucose, mg/dL | CSF | 85 |
| Opening pressure, cmH2O | CSF | 21 |
| Prothrombin time, seconds | Blood | 15.7 (12.1–14.6) |
| AST, unit/L | Blood | 24 (2–40) |
| ALT, unit/L | Blood | 35 (3–30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poussaint, T.Y.; LaRovere, K.L.; Newburger, J.W.; Chou, J.; Nigrovic, L.E.; Novak, T.; Randolph, A.G. Multisystem Inflammatory-like Syndrome in a Child Following COVID-19 mRNA Vaccination. Vaccines 2022, 10, 43. https://doi.org/10.3390/vaccines10010043
Poussaint TY, LaRovere KL, Newburger JW, Chou J, Nigrovic LE, Novak T, Randolph AG. Multisystem Inflammatory-like Syndrome in a Child Following COVID-19 mRNA Vaccination. Vaccines. 2022; 10(1):43. https://doi.org/10.3390/vaccines10010043
Chicago/Turabian StylePoussaint, Tina Y., Kerri L. LaRovere, Jane W. Newburger, Janet Chou, Lise E. Nigrovic, Tanya Novak, and Adrienne G. Randolph. 2022. "Multisystem Inflammatory-like Syndrome in a Child Following COVID-19 mRNA Vaccination" Vaccines 10, no. 1: 43. https://doi.org/10.3390/vaccines10010043
APA StylePoussaint, T. Y., LaRovere, K. L., Newburger, J. W., Chou, J., Nigrovic, L. E., Novak, T., & Randolph, A. G. (2022). Multisystem Inflammatory-like Syndrome in a Child Following COVID-19 mRNA Vaccination. Vaccines, 10(1), 43. https://doi.org/10.3390/vaccines10010043






