Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strain and Virus
2.2. Epitope and Antigencity Prediction in the Antigen (IBDV)
2.3. Cloning of the Desired Flagellin and IBDV Antigenic Segments and Expression of Antigen-Adjuvant Recombinant Proteins
2.4. Vaccine Preparation and Immunization of Chickens
2.5. Analysis of Antibody Immune Response
2.6. Analysis of Cell-Mediated Immune Response
2.7. Th1 and Th2-Type Cytokine mRNA Expression Level
2.8. IBD Virus Neutralization Assay
2.9. Statistical Analysis
3. Results
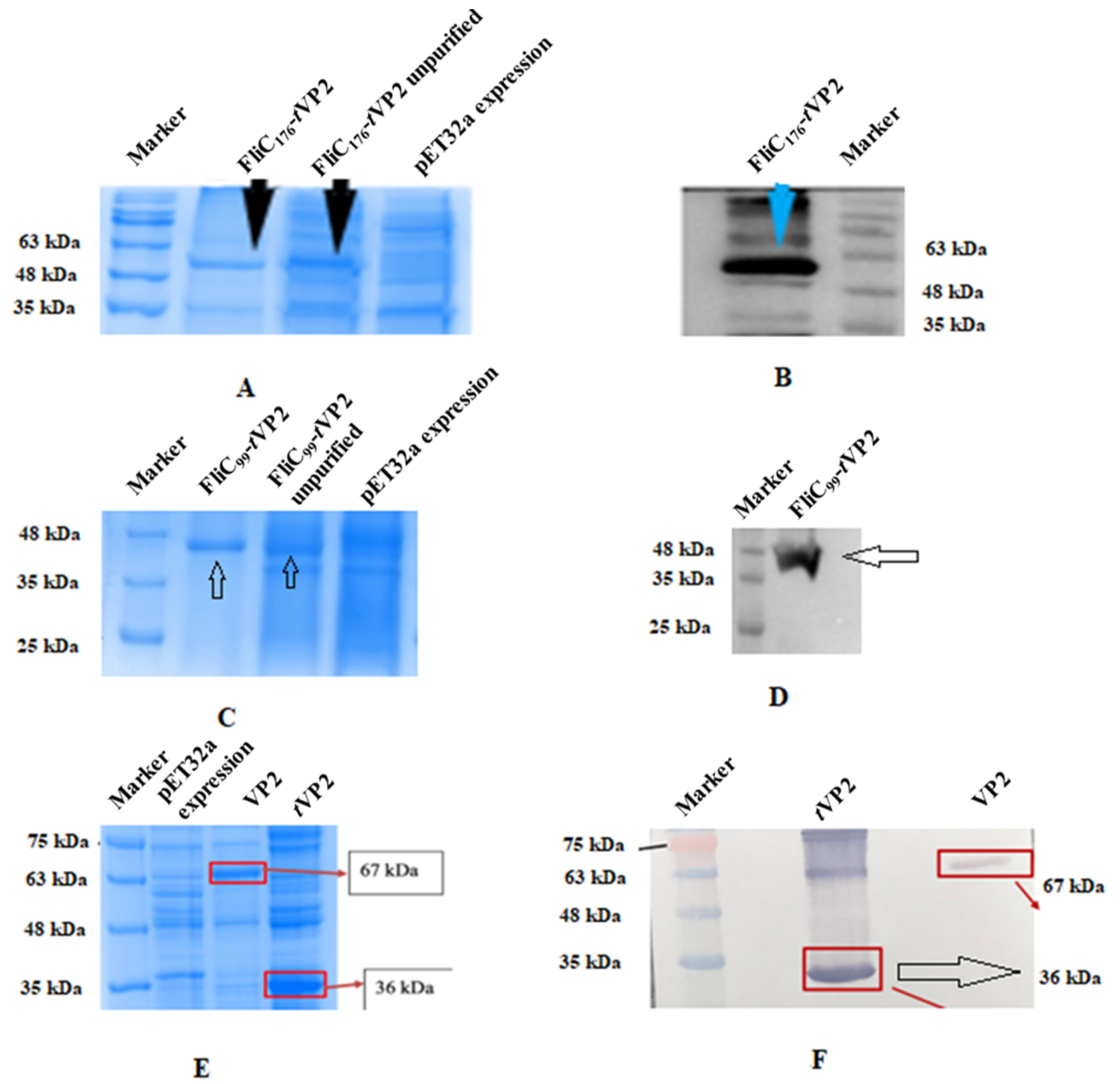
3.1. Antigen-Adjuvant Chimeric Proteins Were Formulated as Subunit Vaccines
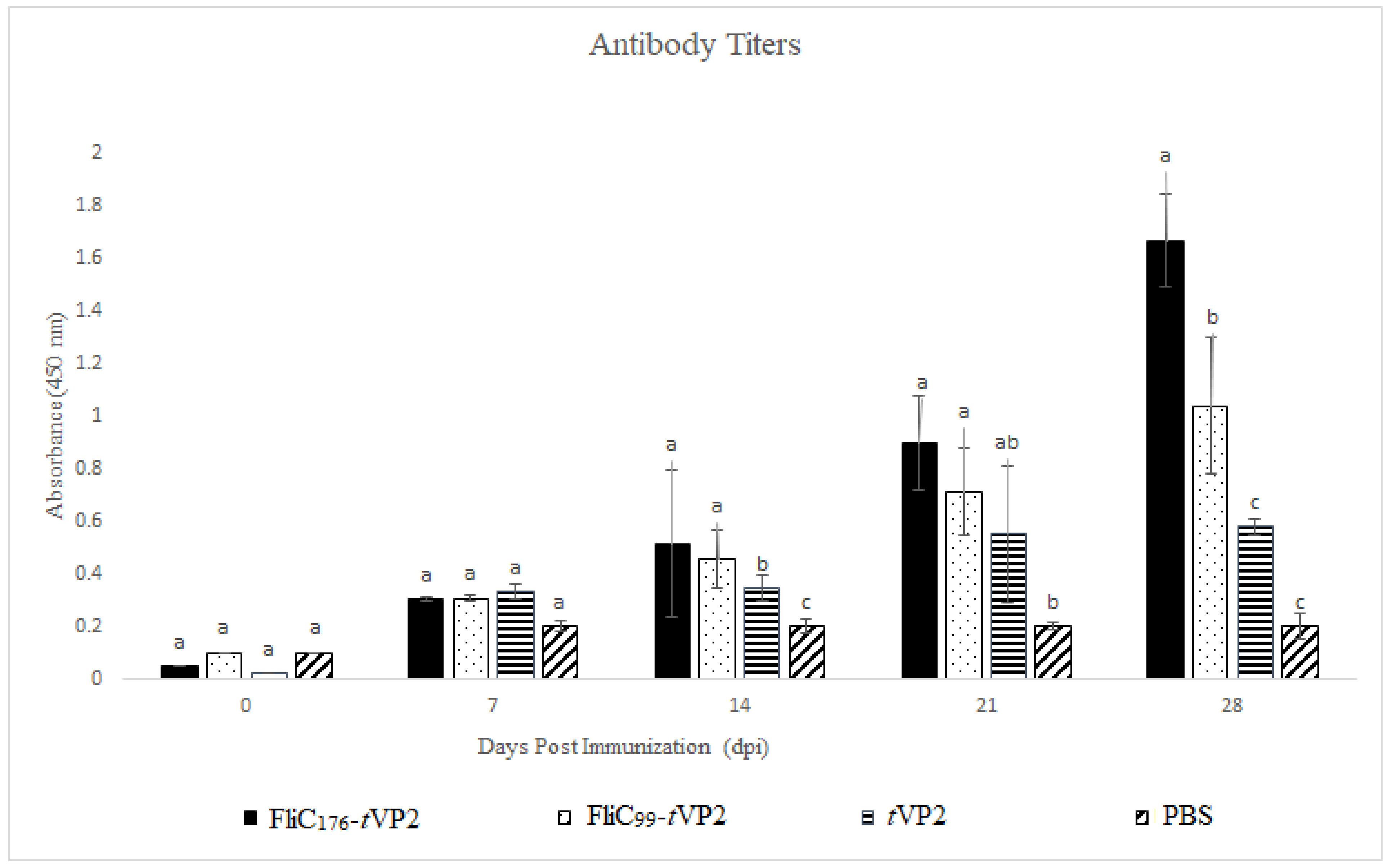
3.2. Both FliC176 and FliC99 Resulted in an Increase in Antibody Levels
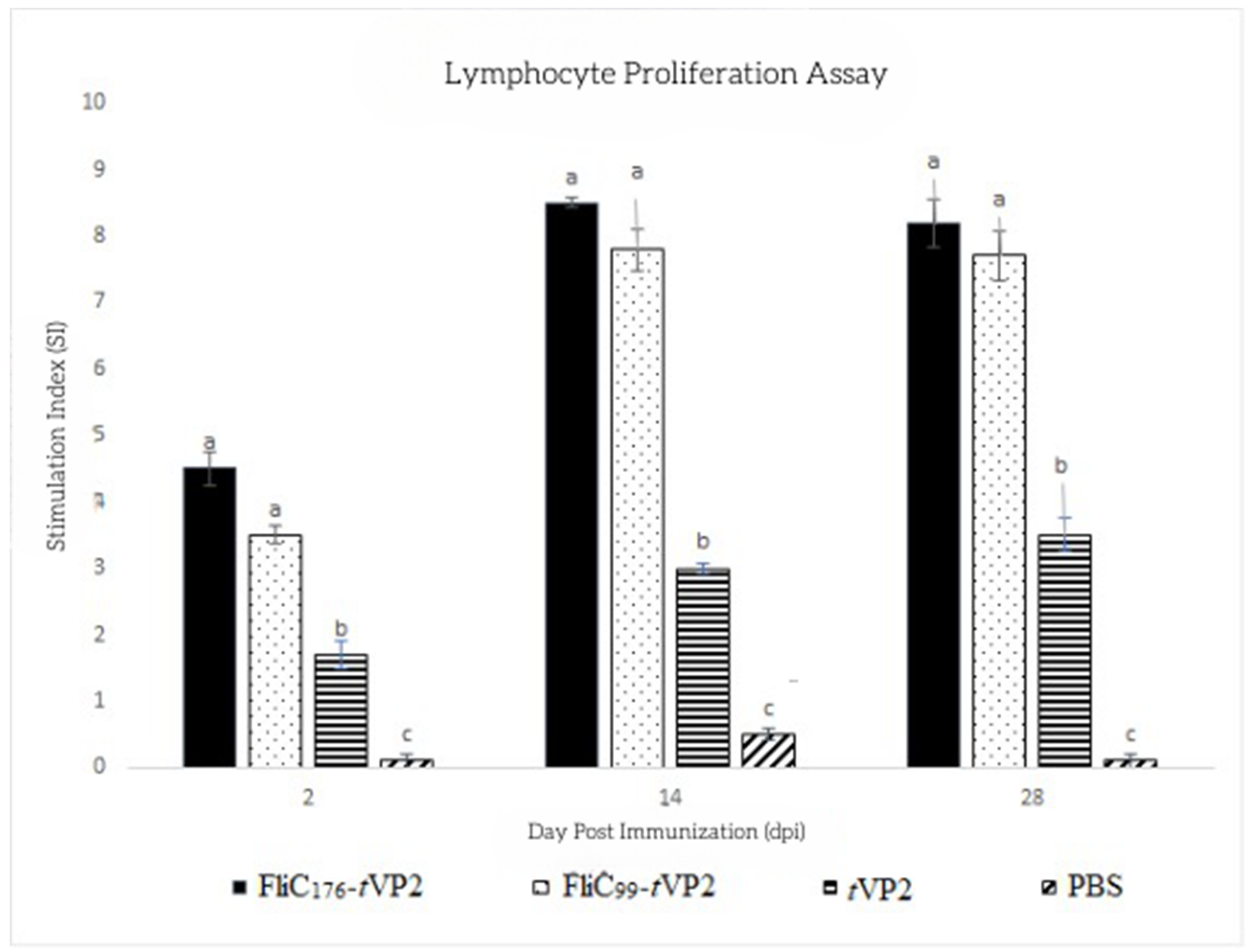
3.3. Both FliC176 and FliC99 Increased the Proliferation of the Lymphocytes
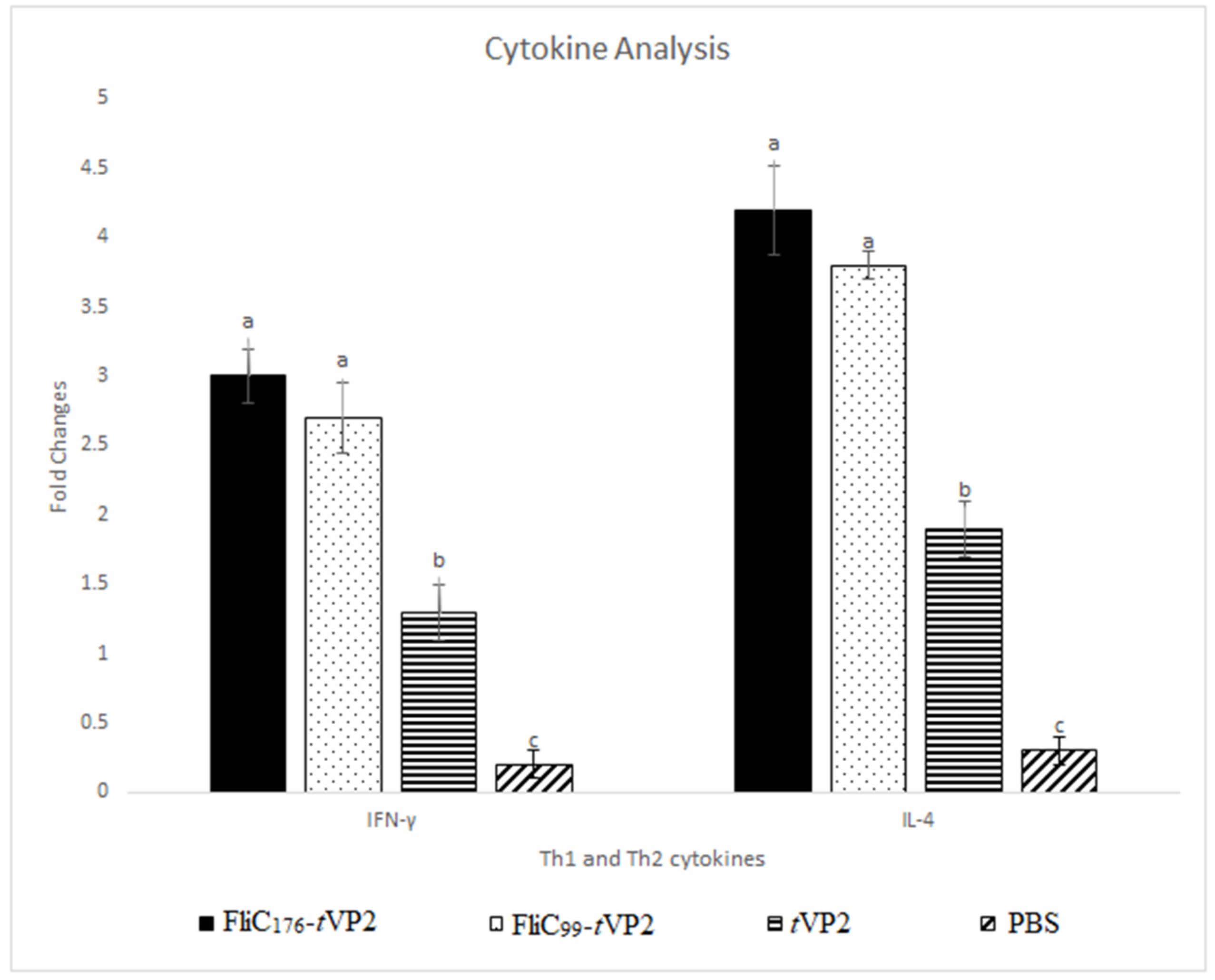
3.4. Both FliC176 and FliC99 Raised the Gene Expression of the Cytokines
3.5. Neutralization Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, D.R. The Evolution of Eukaryotic Cilia and Flagella as Motile and Sensory Organelles. Adv. Exp. Med. Biol. 2007, 607, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Van Maele, L.; Marqués, J.M.; Rial, A.; Sirard, J.C.; Chabalgoity, J.A. Mucosal Administration of Flagellin Protects Mice from Streptococcus Pneumoniae Lung Infection. Infect. Immun. 2010, 78, 4226–4233. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, J.; Zhang, E.; Zhong, M.; Xiao, Y.; Yu, J.; Zhou, D.; Cao, Y.; Yang, Y.; Li, Y.; et al. Activation of NLRC4 Downregulates TLR5-Mediated Antibody Immune Responses against Flagellin. Cell. Mol. Immunol. 2016, 13, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Bargieri, D.Y.; Rosa, D.S.; Braga, C.J.M.; Carvalho, B.O.; Costa, F.T.M.; Maria, N.; José, A.; Soares, I.S.; Ferreira, L.C.S.; Rodrigues, M.M. New Malaria Vaccine Candidates Based on the Plasmodium Vivax Merozoite Surface Protein-1 and the TLR-5 Agonist Salmonella Typhimurium FliC Flagellin. Vaccine 2008, 26, 6132–6142. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Hong, S.H.; Sin, J.I.; Vu, H.V.D.; Jeong, K.; Cho, K.O.; Uematsu, S.; Akira, S.; Lee, S.E.; Rhee, J.H. Flagellin Enhances Tumor-Specific CD8+ T Cell Immune Responses Through TLR5 Stimulation in a Therapeutic Cancer Vaccine Model. Vaccine 2013, 31, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Jeon, Y.J.; Namgung, B.; Hong, M.; Yoon, S. Il A Conserved TLR5 Binding and Activation Hot Spot on Flagellin. Sci. Rep. 2017, 7, 40878. [Google Scholar] [CrossRef] [PubMed]
- López-Yglesias, A.H.; Lu, C.-C.; Zhao, X.; Chou, T.; VandenBos, T.; Strong, R.K.; Smith, K.D. FliC’s Hypervariable D3 Domain Is Required for Robust Anti-Flagellin Primary Antibody Responses. ImmunoHorizons 2019, 3, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Nempont, C.; Cayet, D.; Rumbo, M.; Villeret, V.; Sirard, J.; Cayet, D.; Rumbo, M.; Bompard, C.; Villeret, V.; Sirard, J. Deletion of Flagellin’s Hypervariable Region Abrogates Antibody-Mediated Neutralization and Systemic Activation of TLR5-Dependent Immunity. J. Immunol. 2008, 181, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.D.; Wang, H.Y.; Ke, G.M.; Cheng, L.T. N-Terminus of Flagellin Fused to an Antigen Improves Vaccine Efficacy against Pasteurella Multocida Infection in Chickens. Vaccines 2020, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.-N.; Cheng, L.-T. Development of a Subunit Vaccine against Duck Hepatitis A Virus Serotype 3. Vaccines 2022, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Chuekwon, K.; Chu, C.-Y.; Cheng, L.-T. N-Terminus of Flagellin Enhances Vaccine Efficacy against Actinobacillus Pleuropneumoniae. BMC Vet. Res. 2022, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Pathak, D.; Ramamurthy, N.; Maity, H.K.; Chellappa, M.M. Infectious Bursal Disease Virus in Chickens: Prevalence, Impact, and Management Strategies. Vet. Med. Res. Rep. 2019, 10, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M.; Kim, I.J.; Rautenschlein, S.; Yeh, H.Y. Infectious Bursal Disease Virus of Chickens: Pathogenesis and Immunosuppression. Dev. Comp. Immunol. 2000, 24, 223–235. [Google Scholar] [CrossRef]
- Müller, H.; Mundt, E.; Eterradossi, N.; Islam, M.R. Current Status of Vaccines against Infectious Bursal Disease. Avian Pathol. 2012, 41, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Zhang, Y.; Zhang, F.; Du, E. Incorporation of a Truncated Form of Flagellin (TFlg) into Porcine Circovirus Type 2 Virus-like Particles Enhances Immune Responses in Mice. BMC Vet. Res. 2020, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Faham, A.; Altin, J.G. Antigen-Containing Liposomes Engrafted with Flagellin-Related Peptides Are Effective Vaccines That Can Induce Potent Antitumor Immunity and Immunotherapeutic Effect. J. Immunol. 2010, 185, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Faham, A.; Herringson, T.; Parish, C.; Suhrbier, A.; Khromykh, A.A.; Altin, J.G. PDNA-Lipoplexes Engrafted with Flagellin-Related Peptide Induce Potent Immunity and Anti-Tumour Effects. Vaccine 2011, 29, 6911–6919. [Google Scholar] [CrossRef] [PubMed]
- Mett, V.; Kurnasov, O.V.; Bespalov, I.A.; Molodtsov, I.; Brackett, C.M.; Burdelya, L.G.; Purmal, A.A.; Gleiberman, A.S.; Toshkov, I.A.; Burkhart, C.A.; et al. A Deimmunized and Pharmacologically Optimized Toll-like Receptor 5 Agonist for Therapeutic Applications. Commun. Biol. 2021, 4, 466. [Google Scholar] [CrossRef] [PubMed]
- Côté-Cyr, M.; Gauthier, L.; Zottig, X.; Bourgault, S.; Archambault, D. Recombinant Bacillus Subtilis Flagellin Hag Is a Potent Immunostimulant with Reduced Proinflammatory Properties Compared to Salmonella Enterica Serovar Typhimurium FljB. Vaccine 2022, 40, 11–17. [Google Scholar] [CrossRef] [PubMed]



| Target | Sequence (5′-3′) | RE | Gene- | DNA |
|---|---|---|---|---|
| Gene | Site | Length (bp) | Templates | |
| FliC | F 1 ggatccatgaaaaagacaatcgtagc | BamHI | 1500 | ATCC 14028 |
| R gtcgacttagaagtgtacgcgtaaac | SalI | |||
| tVP2 | F 2 ggcgggggcggcagcaccata actgcagccgatg | - | 489 | ATCC 5848 |
| R ctcgagcgtaacgacagaccctgt | Xhol | |||
| FliC176 | F ggatccaacccgctgcagaaaattg | BamHI | 543 | FliC |
| R gctgccgcccccgccacgcag taaagagaggac | - | |||
| FliC176-tVP2 | F ggatccaacccgctgcagaaaattg | BamHI | 1017 | tVP2 and FliC176 |
| R ctcgagcgtaacgacagaccctgt | Xhol | |||
| FliC99 | F gaattcatggcacaagtcattaatacaaac | EcoRI | 312 | FliC |
| R gctgccgcccccgccagactgaaccgccagttc | - | |||
| FliC99-tVP2 | F gaattcatggcacaagtcattaatacaaac | EcoRI | 786 | tVP2 and FliC99 |
| R ctcgagcgtaacgacagaccctgt | Xhol |
| Group | 1st Dose | 2nd Dose | Adjuvant | Immunization Dose | Route |
|---|---|---|---|---|---|
| FliC176-tVP2 | 0 Day | 14 Days | Summit-P101 | 50 μg | S/C |
| FliC99-tVP2 | 0 Day | 14 Days | Summit-P101 | 50 μg | S/C |
| tVP2 | 0 Day | 14 Days | Summit-P101 | 50 μg | S/C |
| PBS | 0 Day | 14 Days | Summit-P101 | 50 μg | S/C |
| Target | Sequence (5′-3′) | Length | Annealing Temp. | GenBank |
|---|---|---|---|---|
| Gene | (bp) | (°C) | ||
| R cttcca agggatcttcattt | ||||
| IFN-γ | F gacggtggacctattatt | 255 | 50 | HQ739082 |
| R ggctttgcgctggattc | ||||
| IL-4 | F tgtgcccacgctgtgcttaca | 193 | 61 | AJ621249.1 |
| R cttgtggcagtgctggctctcc | ||||
| GAPDH | F tgctgcccagaacatcatcc | 142 | 55 | NM_204305 |
| R acggcaggtcaggtcaacaa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtaza, A.; Afzal, H.; Doan, T.-D.; Ke, G.-M.; Cheng, L.-T. Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine. Vaccines 2022, 10, 1780. https://doi.org/10.3390/vaccines10111780
Murtaza A, Afzal H, Doan T-D, Ke G-M, Cheng L-T. Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine. Vaccines. 2022; 10(11):1780. https://doi.org/10.3390/vaccines10111780
Chicago/Turabian StyleMurtaza, Asad, Haroon Afzal, Thu-Dung Doan, Guan-Ming Ke, and Li-Ting Cheng. 2022. "Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine" Vaccines 10, no. 11: 1780. https://doi.org/10.3390/vaccines10111780
APA StyleMurtaza, A., Afzal, H., Doan, T.-D., Ke, G.-M., & Cheng, L.-T. (2022). Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine. Vaccines, 10(11), 1780. https://doi.org/10.3390/vaccines10111780





