Healthcare Worker Study Cohort to Determine the Level and Durability of Cellular and Humoral Immune Responses after Two Doses of SARS-CoV-2 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Megapool Preparation
2.2. PBMC Isolation
2.3. Activation-Induced Cell Marker Assay
2.4. Serological Analysis
2.5. Statistical Analysis
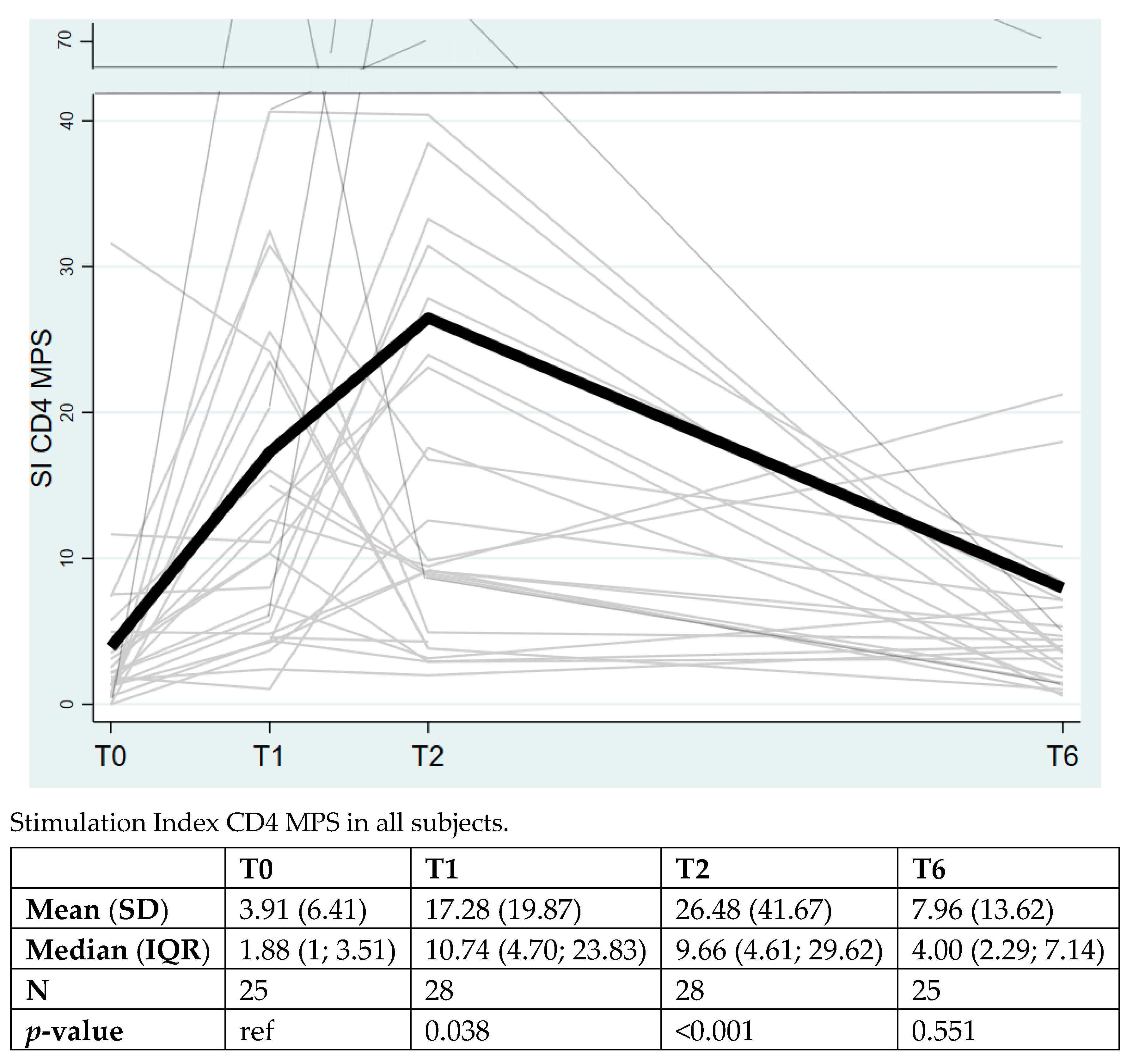
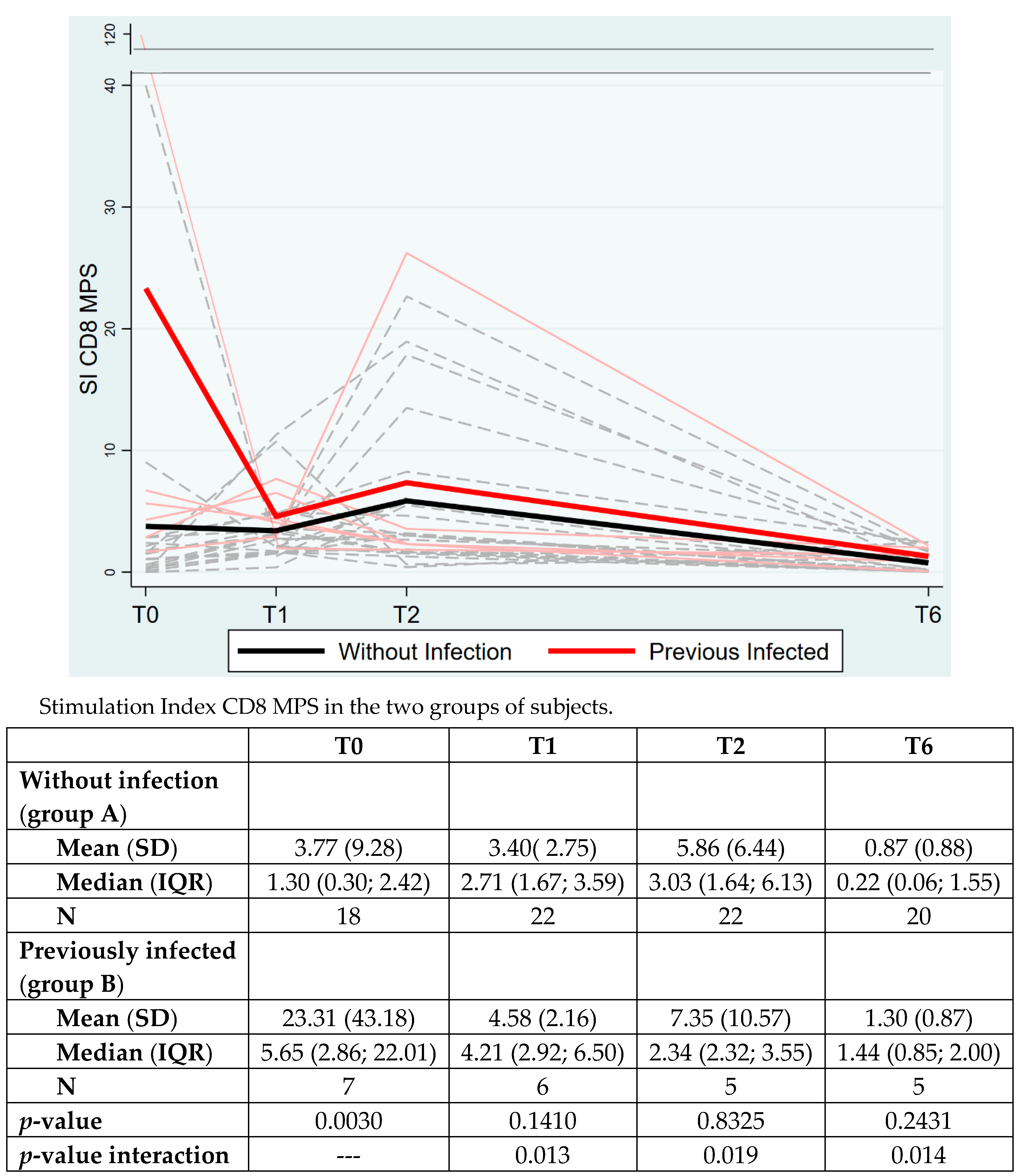
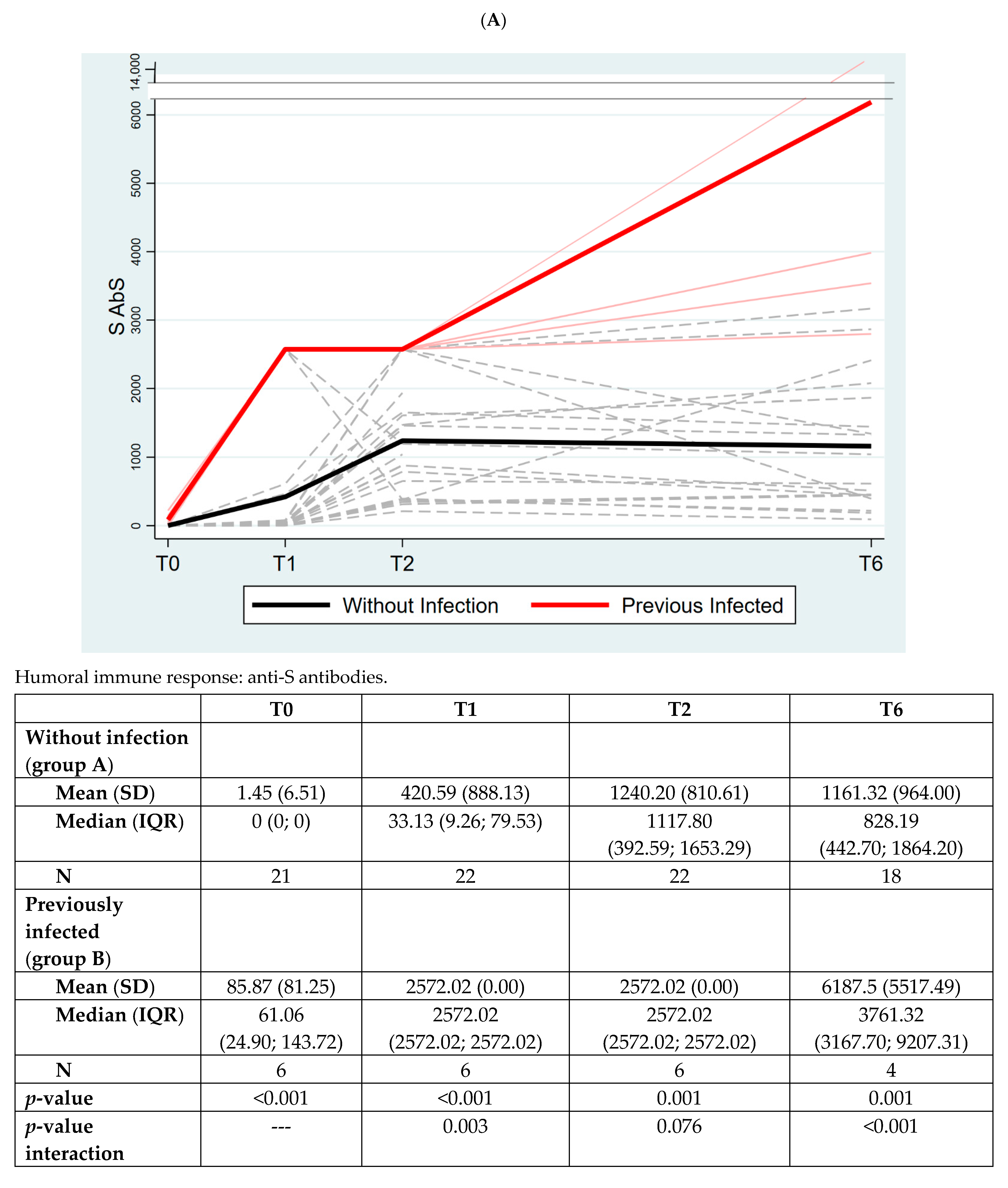
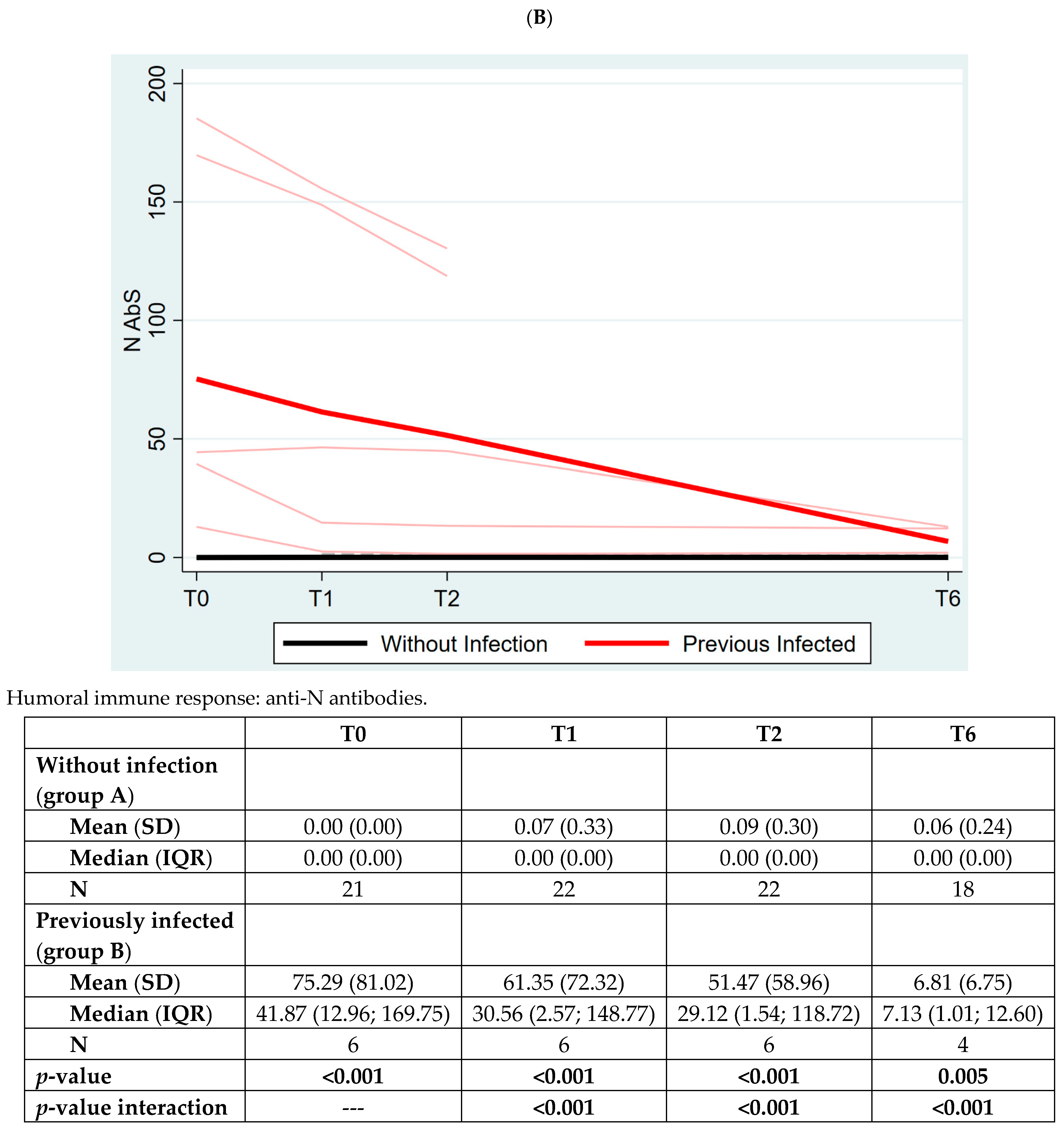
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCW | Healthcare workers |
| AIM | Activation-induced cell marker assay |
| MPs | Megapools |
| MPS | Megapools Spike |
| RT-PCR | Real-time reverse-transcriptase polymerase chain reaction |
| DMSO | Dimethyl sulfoxide |
| PBMC | Peripheral blood mononuclear cells |
| FCS | Fetal calf serum |
| DMSO | Dimethyl Sulfoxide |
| PHA | Phytohemagglutinin |
| mAbs | Monoclonal antibodies |
| APC | Allophycocianin |
| BV | Brilliant Violet |
| SI | Stimulation Index |
| RBD | Receptor binding domain |
| ECLIA | Electrochemiluminescence immunoassay |
| SD | Standard deviation |
References
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.E.; Cutrino-Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 30, eabh1282. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang, M.J.; Janowska, I.; Staniek, J.; et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Treibel, T.A.; Manisty, C.; Burton, M.; McKnight, Á.; Lambourne, J.; Augusto, J.B.; Couto- Parada, X.; Cutino-Moguel, T.; Noursadeghi, M.C.; Moon, J. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet 2020, 395, 1608–1610. [Google Scholar] [CrossRef]
- Augusto, J.B.; Menacho, K.; Andiapen, M.; Bowles, R.; Burton, M.; Welch, S.A.; Bhuva, A.N.; Seraphim, A.; Pade, C.; Joy, G.; et al. Healthcare Workers Bioresource: Study outline and baseline characteristics of a prospective healthcare worker cohort to study immune protection and pathogenesis in COVID-19. Wellcome Open Res. 2020, 5, 179. [Google Scholar] [CrossRef]
- Angel, Y.; Spitzer, A.; Hening, O.; Saiag, E.; Sprecher, E.; Padova, H.; Ben-Ami, R. Association Between Vaccination with BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA 2021, 325, 2457–2465. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; MAndelboim, M.; Gal, L.E.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Quaranta, A.; Napoli, C.; Diella, G.; De Giglio, O.; Caggiano, G.; Di Muzio, M.; Stefanizzi, P.; Orsi, G.B.; Liguori, G.; et al. How do Vaccinators Experience the Pandemic? Lifestyle Behaviors in a Sample of Italian Public Health Workers during the COVID-19 Era. Vaccines 2022, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Kulper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: Interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021, 26, 2100563. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Kulper-Schiek, W.; Reda, S.; Teeskova-Schwarzbach, M.; Koch, J.; Vygen-Bonnet, S.; Wichmann, O. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: Second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. 2021, 26, 2100920. [Google Scholar] [CrossRef] [PubMed]
- Higdon, M.M.; Wahl, B.; Jones, C.B.; Rosen, J.G.; Truelove, S.A.; Baidya, A.; Nande, A.A.; Shamaei Zadeh, P.A.; Walter, K.K.; Feikin, D.R.; et al. A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. OFID 2022, 9, ofac 138. [Google Scholar]
- Know, C.S.; Hasan, S.S. Real-world effectiveness of BNT162b2 mRNA vaccine: A meta-analysis of large observational studies. Inflammopharmacology 2021, 29, 1075–1090. [Google Scholar]
- Meggiolaro, A.; Schepisi, M.S.; Nikolaidis, G.; Mipatrini, D.; Siddu, A.; Rezza, G. Effectiveness of vaccination against symptomatic and asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- WHO. Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Carrasco Pro, S.; Sidney, J.; Paul, S.; Lindestam Arlehamn, C.; Weiskopf, D.; Peters, B.; Sette, A. Automatic Generation of Validated Specific Epitope Sets. J. Immunol. Res. 2015, 763461. [Google Scholar] [CrossRef] [Green Version]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Dan, J.M.; Lindestam Arlehamn, C.S.; Weiskopf, D.; da Silva Antunes, R.; Havenar-Daughton, C.; Reiss, S.M.; Brigger, M.; Bothwell, M.; Sette, A.; Crotty, S. A Cytokine-Independent Approach to Identify Antigen-Specific Human Germinal Center T Follicular Helper Cells and Rare Antigen-Specific CD4+ T Cells in Blood. J. Immunol. 2016, 197, 983–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, S.; Baxter, A.E.; Cirelli, K.M.; Dan, J.M.; Morou, A.; Daigneault, A.; Brassard, N.; Silvestri, G.; Routy, J.P.; Havenar-Daughton, C.; et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS ONE 2017, 12, e0186998. [Google Scholar] [CrossRef] [PubMed]
- Kohmer, N.; Westhaus, S.; Rühl, C.; Ciesek, S.; Rabenau, H.F. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J. Clin. Virol. 2020, 129, 104480. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Ostermann, P.N.; Walker, A.; Wienemann, T.; Marterns, A.; Adams, O.; Andree, M.; Hauka, S.; Lubke, N.; Keitel, V.; et al. Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1063–1071. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Tu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Piralla, A.; Zuo, F.; Du, L.; Cassaniti, I.; Wan, H.; Kumagai-Braesh, M.; Andrell, J.; Percivalle, E.; Sammartino, J.C.; et al. Immunity to SARS-CoV-2 up to 15 months after infection. iScience 2022, 25, 103743. [Google Scholar] [CrossRef]
- Sherina, N.; Piralla, A.; Du Likun, W.H.; Kumagai-Braesch, M.; Andrell, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; Bergami, F.; et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med 2021, 2, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Harding, A.C.; Gilbert-Jaramillo, J.; Knight, M.L.; Longet, S.; Brown, A.; Adele, S.; Adland, E.; Brown, H.; Tipton, T.; et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat. Commun. 2021, 12, 5061. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.C.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Akondy, R.S.; Fitch, M.; Edupuganti, S.; Yang, S.; Kissick, H.T.; Li, K.W.; Youngblood, B.A.; Abdelsamed, H.A.; McGuire, D.J.; Cohen, K.W.; et al. Origin and differentiation of human memory CD8+ T cells after vaccination. Nature 2017, 552, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Ahmed, R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001, 2, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, P.; Zippelius, A.; Kurth, I.; Pittet, M.J.; Touvrey, C.; Iancu, E.M.; Corthesy, P.; Devevre, E.; Speiser, D.E.; Rufer, N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 2007, 178, 4112–4119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureshchandra, S.; Lewis, S.A.; Doratt, B.M.; Jankeel, A.; Coimbra Ibraim, I.; Messaoudi, I. Single-cell profiling of T and B cell repertoires following SARS-CoV-2 mRNA vaccine. JCI Insight. 2021, 6, e153201. [Google Scholar] [CrossRef]
- Kalimuddin, S.; Tham, C.Y.L.; Qui, M.; de Alwis, R.; Sim, J.X.Y.; Lim, J.M.E.; Tan, H.C.; Syenina, A.; Zhang, S.L.; Le Bert, N.; et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med 2021, 2, 682–688. [Google Scholar] [CrossRef]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer- Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pettekar, A.; Kuthury, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Lederer, K.; Castano, D.; Atria, G.D.; Oguin, T.H.; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 2020, 53, 1281–1295. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Philips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859. [Google Scholar] [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.-D.; Fatemi, R.; Parvez, S.A.; Zheng, C.; Hossain, G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]

| Overall N = 29 | Without Infection (Group A) N = 22 (76%) | Previously Infected (Group B) N = 7 (24%) | p-Value | |
|---|---|---|---|---|
| Female, N (%) | 18 (62.07) | 13 (59.09) | 5 (71.43) | 0.677 |
| Age, mean (SD) | 39.38 (12.92) | 39.91 (13.10) | 37.71 (13.20) | 0.970 |
| Comorbidities * | 2 (6.90) | 1 (4.55) | 1 (14.29) | 0.431 |
| Overall N = 29 | Without Infection (Group A) N = 22 | Previously Infected (Group B) N = 7 | p-Value | |
|---|---|---|---|---|
| CD4+ T cells | ||||
| T6_SI 4 MPS alpha | 4.91 (5.32) N = 24 | 5.09 (5.65) N = 20 | 4.00 (3.74) N = 4 | 0.862 |
| T6_SI 4 MPS beta | 6.11 (9.39) N = 25 | 7.00 (10.34) N = 20 | 2.55 (1.37) N = 5 | 0.371 |
| T6_SI 4 MPS gamma | 6.46 (9.30) N = 25 | 7.17 (10.26) N = 20 | 3.62 (2.42) N = 5 | 0.809 |
| T6_SI 4 MPS delta | 6.63 (11.84) N = 25 | 7.67 (13.06) N = 20 | 2.45 (1.80) N = 5 | 0.363 |
| T6_SI 4 MPS epsilon | 5.45 (6.25) N = 25 | 6.04 (6.85) N = 20 | 3.06 (1.77) N = 5 | 0.613 |
| CD8+ T cells | ||||
| T6_SI 8 MPS alpha | 2.03 (2.72) N = 24 | 2.16 (2.94) N = 20 | 1.39 (1.21) N = 4 | 0.477 |
| T6_SI 8 MPS beta | 1.56 (0.87) N = 25 | 1.66 (0.94) N = 20 | 1.18 (0.22) N = 5 | 0.530 |
| T6_SI 8 MPS gamma | 1.68 (0.82) N = 25 | 1.64 (0.80) N = 20 | 1.85 (0.98) N = 5 | 0.669 |
| T6_SI 8 MPS delta | 1.58(1.09) N = 25 | 1.51 (0.77) N = 20 | 1.86 (2.06) N = 5 | 0.669 |
| T6_SI 8 MPS epsilon | 1.76 (0.92) N = 25 | 1.80 (0.90) N = 20 | 1.58 (1.06) N = 5 | 0.621 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dentone, C.; Fenoglio, D.; Ponzano, M.; Cerchiaro, M.; Altosole, T.; Franciotta, D.; Portunato, F.; Mikulska, M.; Taramasso, L.; Magnasco, L.; et al. Healthcare Worker Study Cohort to Determine the Level and Durability of Cellular and Humoral Immune Responses after Two Doses of SARS-CoV-2 Vaccination. Vaccines 2022, 10, 1784. https://doi.org/10.3390/vaccines10111784
Dentone C, Fenoglio D, Ponzano M, Cerchiaro M, Altosole T, Franciotta D, Portunato F, Mikulska M, Taramasso L, Magnasco L, et al. Healthcare Worker Study Cohort to Determine the Level and Durability of Cellular and Humoral Immune Responses after Two Doses of SARS-CoV-2 Vaccination. Vaccines. 2022; 10(11):1784. https://doi.org/10.3390/vaccines10111784
Chicago/Turabian StyleDentone, Chiara, Daniela Fenoglio, Marta Ponzano, Matteo Cerchiaro, Tiziana Altosole, Diego Franciotta, Federica Portunato, Malgorzata Mikulska, Lucia Taramasso, Laura Magnasco, and et al. 2022. "Healthcare Worker Study Cohort to Determine the Level and Durability of Cellular and Humoral Immune Responses after Two Doses of SARS-CoV-2 Vaccination" Vaccines 10, no. 11: 1784. https://doi.org/10.3390/vaccines10111784
APA StyleDentone, C., Fenoglio, D., Ponzano, M., Cerchiaro, M., Altosole, T., Franciotta, D., Portunato, F., Mikulska, M., Taramasso, L., Magnasco, L., Uras, C., Magne, F., Ferrera, F., Scavone, G., Signori, A., Vena, A., Visconti, V., Filaci, G., Sette, A., ... Bassetti, M. (2022). Healthcare Worker Study Cohort to Determine the Level and Durability of Cellular and Humoral Immune Responses after Two Doses of SARS-CoV-2 Vaccination. Vaccines, 10(11), 1784. https://doi.org/10.3390/vaccines10111784








