Metabolic Responses and Pathway Changes of Vero Cells under High-Vitamin B Medium
Abstract
1. Introduction
2. Results
2.1. Effect of B Vitamins on Vero Cell Proliferation
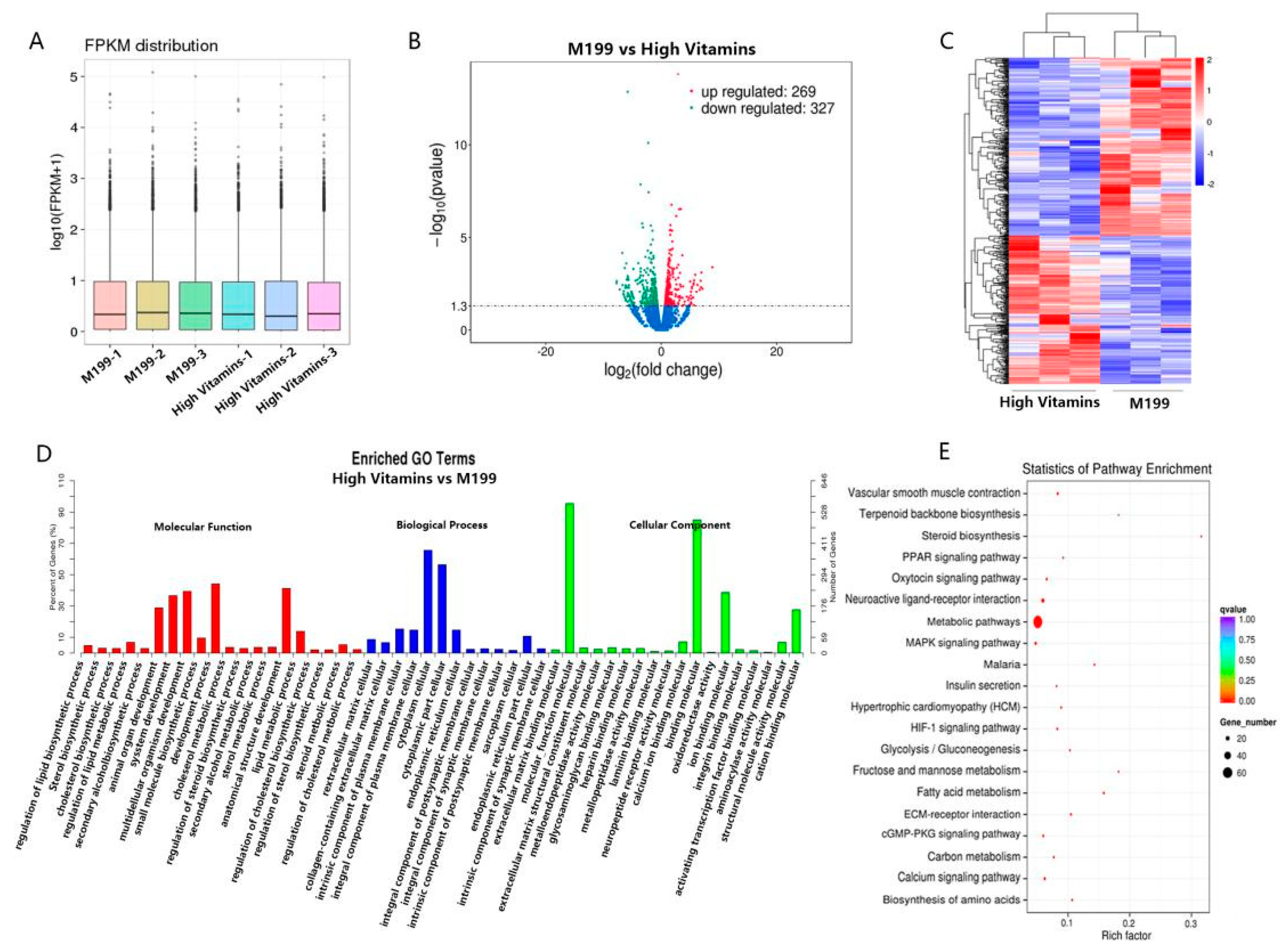
2.2. Transcriptomics Revealed Vitamin B Affected Metabolic Pathways in Vero Cells
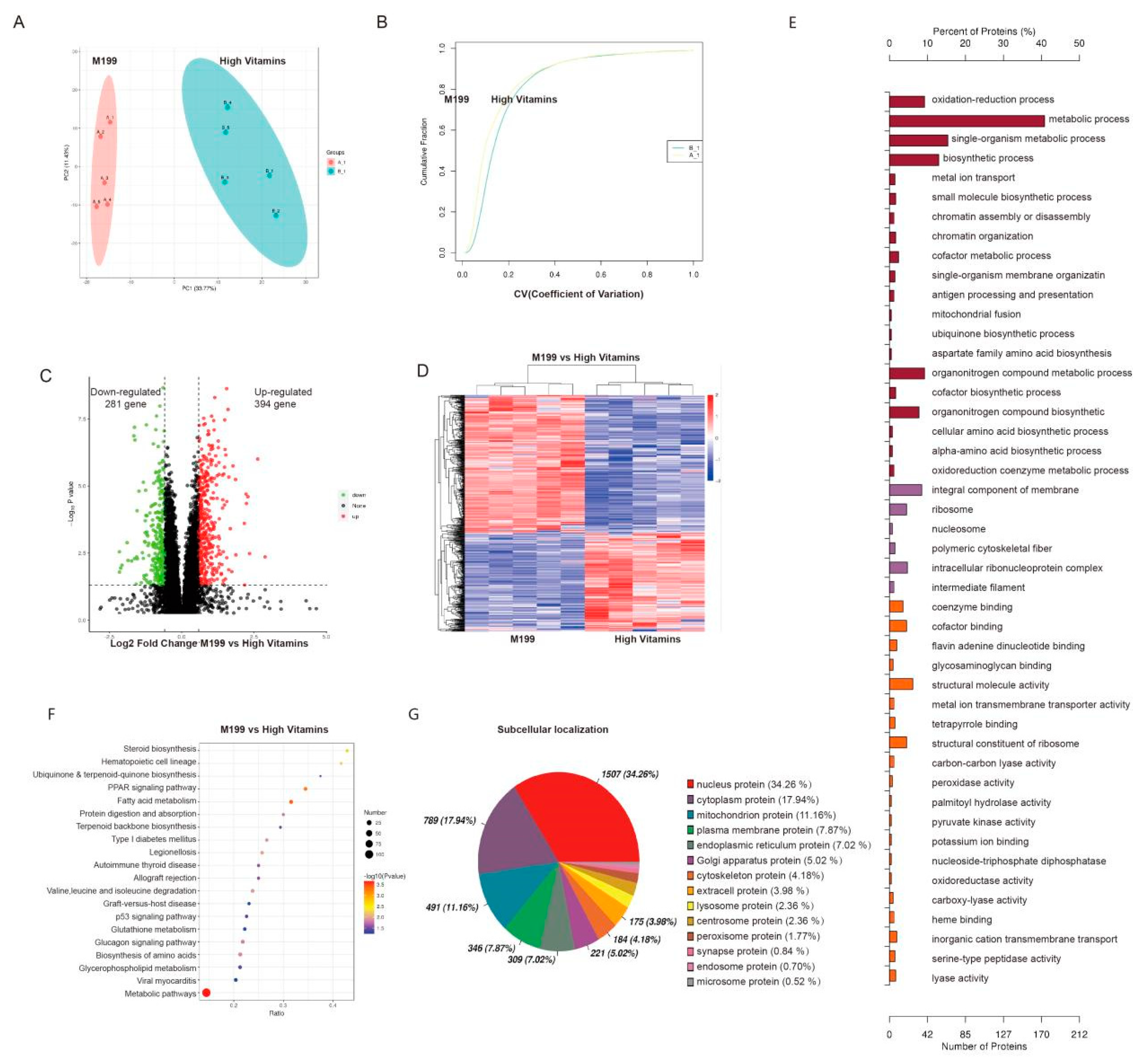
2.3. Proteomics Revealed that the Addition of Vitamin B Promoted the Proliferation of Vero Cells by Affecting Cell Metabolism
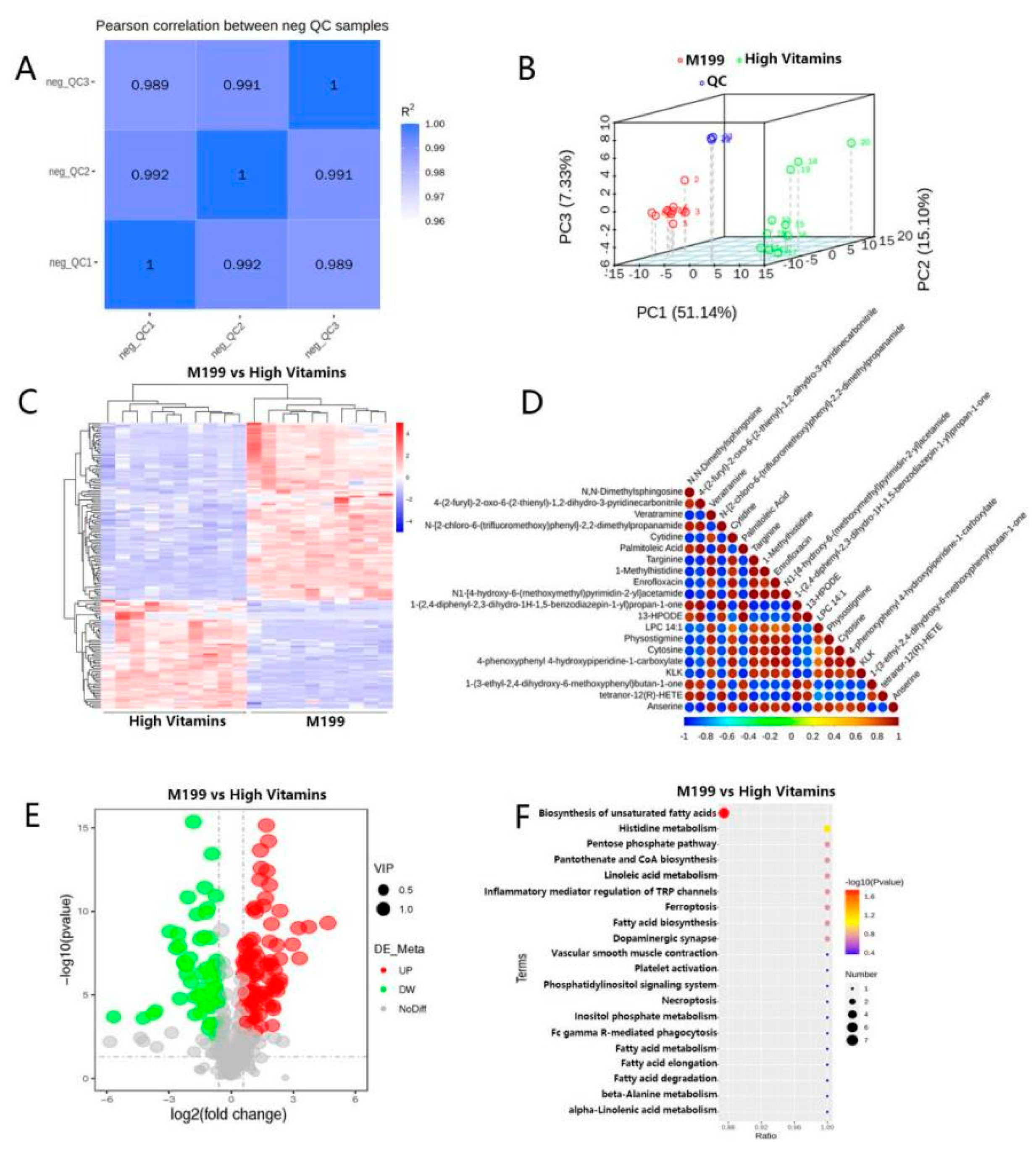
2.4. Metabolomics Results Surfaced that Vitamin B Affected Unsaturated Fatty Acid Metabolism
3. Discussion
4. Methods
4.1. Vero Cells and Culture
4.2. Cell Counting Kit (CCK)-8 Assay
4.3. Western Blotting
4.4. EdU Assay
4.5. RNA Preparation and RT-qPCR
4.6. Transcriptomics Analysis
4.7. Proteomic Analysis
4.8. Metabolomics Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montagnon, B.J.; Fanget, B.; Vincent-Falquet, J.C. Industrial-scale production of inactivated poliovirus vaccine prepared by culture of Vero cells on microcarrier. Rev. Infect. Dis. 2010, 6 (Suppl. 2), S341–S344. [Google Scholar] [CrossRef] [PubMed]
- Montomoli, E.; Khadang, B.; Piccirella, S.; Trombetta, C.; Mennitto, E.; Manini, I.; Stanzani, V.; Lapini, G. Cell culture-derived influenza vaccines from Vero cells: A new horizon for vaccine production. Expert Rev. Vaccines 2014, 11, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.N.; Mundt, W.; Kistner, O.; Howard, M.K. Vero cell platform in vaccine production: Moving towards cell culture-based viral vaccines. Expert Rev. Vaccines 2009, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Genzel, Y.; Dietzsch, C.; Rapp, E.; Schwarzer, J.; Reichl, U. MDCK and Vero cells for influenza virus vaccine production: A one-to-one comparison up to lab-scale bioreactor cultivation. Appl. Microbiol. Biotechnol. 2010, 88, 461–475. [Google Scholar] [CrossRef]
- Grenier, B.; Hamza, B.; Biron, G.; Xueref, C.; Viarme, F.; Roumiantzeff, M. Seroimmunity following vaccination in infants by an inactivated poliovirus vaccine prepared on Vero cells. Rev. Infect. Dis. 2010, 6 (Suppl. 2), S545–S547. [Google Scholar] [CrossRef]
- Ursache, R.V.; Thomassen, Y.E.; van Eikenhorst, G.; Verheijen, P.J.T.; Bakker, W.A.M. Mathematical model of adherent Vero cell growth and poliovirus production in animal component free medium. Bioprocess Biosyst. Eng. 2015, 38, 543–555. [Google Scholar] [CrossRef]
- Frazatti-Gallina, N.M.; Mourão-Fuches, R.M.; Paoli, R.L.; Silva, M.L.N.; Miyaki, C.; Valentini, E.J.G.; Raw, I.; Higashi, H.G. Vero-cell rabies vaccine produced using serum-free medium. Vaccine 2005, 23, 511–517. [Google Scholar] [CrossRef]
- Rourou, S.; van der Ark, A.; Majoul, S.; Trabelsi, K.; van der Velden, T.; Kallel, H. A novel animal-component-free medium for rabies virus production in Vero cells grown on Cytodex 1 microcarriers in a stirred bioreactor. Appl. Microbiol. Biotechnol. 2009, 85, 53–63. [Google Scholar] [CrossRef]
- Nahapetian, A.T.; Thomas, J.N.; Thilly, W.G. Optimization of environment for high density Vero cell culture: Effect of dissolved oxygen and nutrient supply on cell growth and changes in metabolites. J. Cell Sci. 1986, 81, 65–103. [Google Scholar] [CrossRef]
- Meydani, S.N.; Ribaya-Mercado, J.D.; Russell, R.M.; Sahyoun, N.; Morrow, F.D.; Gershoff, S.N. Vitamin B-6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am. J. Clin. Nutr. 1991, 53, 1275–1280. [Google Scholar] [CrossRef]
- Duthie, S.J.; Beattie, J.H.; Gordon, M.-J.; Pirie, L.P.; Nicol, F.; Reid, M.D.; Duncan, G.J.; Cantlay, L.; Horgan, G.; McNeil, C.J. Nutritional B vitamin deficiency alters the expression of key proteins associated with vascular smooth muscle cell proliferation and migration in the aorta of atherosclerotic apolipoprotein E null mice. Genes Nutr. 2014, 10, 446. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem.-Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Hecker, A.; Kocamer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klüter, H. Human Alternatives to Fetal Bovine Serum for the Expansion of Mesenchymal Stromal Cells from Bone Marrow. Stem Cells 2009, 27, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Füllekrug, J.; Shevchenko, A.; Shevchenko, A.; Simons, K. Identification of glycosylated marker proteins of epithelial polarity in MDCK cells by homology driven proteomics. BMC Biochem. 2006, 7, 8. [Google Scholar] [CrossRef]
- Shen, C.F.; Guilbault, C.; Li, X.; Elahi, S.M.; Ansorge, S.; Kamen, A.; Gilbert, R. Development of suspension adapted Vero cell culture process technology for production of viral vaccines. Vaccine 2019, 37, 6996–7002. [Google Scholar] [CrossRef] [PubMed]
- Genzel, Y.; Rödig, J.; Rapp, E.; Reichl, U. Vaccine Production: Upstream Processing with Adherent or Suspension Cell Lines. Anim. Cell Biotechnol. 2013, 1104, 371–393. [Google Scholar]
- Akkermans, A.; Chapsal, J.M.; Coccia, E.M.; Depraetere, H.; Dierick, J.; Duangkhae, P.; Goel, S.; Halder, M.; Hendriksen, C.; Levis, R.; et al. Animal testing for vaccines. Implementing replacement, reduction and refinement: Challenges and priorities. Biologicals 2020, 68, 92–107. [Google Scholar] [CrossRef]
- Kiesslich, S.; Kamen, A.A. Vero cell upstream bioprocess development for the production of viral vectors and vaccines. Biotechnol. Adv. 2020, 44, 107608. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020, 27, 225–228. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; Macdonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2021, 28, 202–221. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development:milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022, 7, 32. [Google Scholar]
- Panella, S.; Muoio, F.; Jossen, V.; Harder, Y.; Eibl-Schindler, R.; Tallone, T. Chemically Defined Xeno- and Serum-Free Cell Culture Medium to Grow Human Adipose Stem Cells. Cells 2021, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Guinan, J.; Lopez, B.S. Generating Bovine Monocyte-Derived Dendritic Cells for Experimental and Clinical Applications Using Commercially Available Serum-Free Medium. Front. Immunol. 2020, 11, 591185. [Google Scholar] [CrossRef] [PubMed]
- Badenes, S.M.; Fernandes, T.G.; Cordeiro, C.S.M.; Boucher, S.; Kuninger, D.; Vemuri, M.C.; Diogo, M.M.; Cabral, J.M.S. Defined Essential 8™ Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS ONE 2016, 11, e0151264. [Google Scholar]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Ho, Y.; Lu, H.; Ng, S. Applications and analysis of hydrolysates in animal cell culture. Bioresour Bioprocess. 2021, 8, 93. [Google Scholar]
- Urrutia, M.; Blein-Nicolas, M.; Prigent, S.; Bernillon, S.; Deborde, C.; Balliau, T.; Maucourt, M.; Jacob, D.; Ballias, P.; Bénard, C.; et al. Maize metabolome and proteome responses to controlled cold stress partly mimic early-sowing effects in the field and differ from those of Arabidopsis. Plant Cell Environ. 2021, 44, 1504–1521. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Barbazuk, W.B.; Sandford, M.; May, G.; Song, Z.; Zhou, W.; Nikolau, B.J.; Herman, E.M. Silencing of soybean seed storage proteins results in a rebalanced protein composition preserving seed protein content without major collateral changes in the metabolome and transcriptome. Plant Physiol. 2011, 156, 330–345. [Google Scholar] [CrossRef]
- Kim, M.; Vogtmann, E.; Ahlquist, D.A.; Devens, M.E.; Kisiel, J.B.; Taylor, W.R.; White, B.A.; Hale, V.L.; Sung, J.; Chia, N.; et al. Fecal Metabolomic Signatures in Colorectal Adenoma Patients Are Associated with Gut Microbiota and Early Events of Colorectal Cancer Pathogenesis. mBio 2020, 11, e03186-19. [Google Scholar] [CrossRef]
- Verdon, J.; Labanowski, J.; Sahr, T.; Ferreira, T.; Lacombe, C.; Buchrieser, C.; Berjeaud, J.-M.; Héchard, Y. Fatty acid composition modulates sensitivity of Legionella pneumophila to warnericin RK, an antimicrobial peptide. Biochim. Biophys. Acta BBA-Biomembr. 2011, 1808, 1146–1153. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Yan, J.; Yang, Z.; Zhao, Y.; Wang, H.; Yang, X. Metabolic Responses and Pathway Changes of Vero Cells under High-Vitamin B Medium. Vaccines 2022, 10, 1787. https://doi.org/10.3390/vaccines10111787
Yu S, Yan J, Yang Z, Zhao Y, Wang H, Yang X. Metabolic Responses and Pathway Changes of Vero Cells under High-Vitamin B Medium. Vaccines. 2022; 10(11):1787. https://doi.org/10.3390/vaccines10111787
Chicago/Turabian StyleYu, Shouzhi, Junyu Yan, Zhaona Yang, Yuxiu Zhao, Hui Wang, and Xiaoming Yang. 2022. "Metabolic Responses and Pathway Changes of Vero Cells under High-Vitamin B Medium" Vaccines 10, no. 11: 1787. https://doi.org/10.3390/vaccines10111787
APA StyleYu, S., Yan, J., Yang, Z., Zhao, Y., Wang, H., & Yang, X. (2022). Metabolic Responses and Pathway Changes of Vero Cells under High-Vitamin B Medium. Vaccines, 10(11), 1787. https://doi.org/10.3390/vaccines10111787





