Salivary Antibody Response of COVID-19 in Vaccinated and Unvaccinated Young Adult Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Elisa Procedure
2.2.1. ELISA for Detecting Total Antibody Number
2.2.2. ELISA for Detecting IgG Immunoglobulin
2.3. Statistical Analysis
3. Results
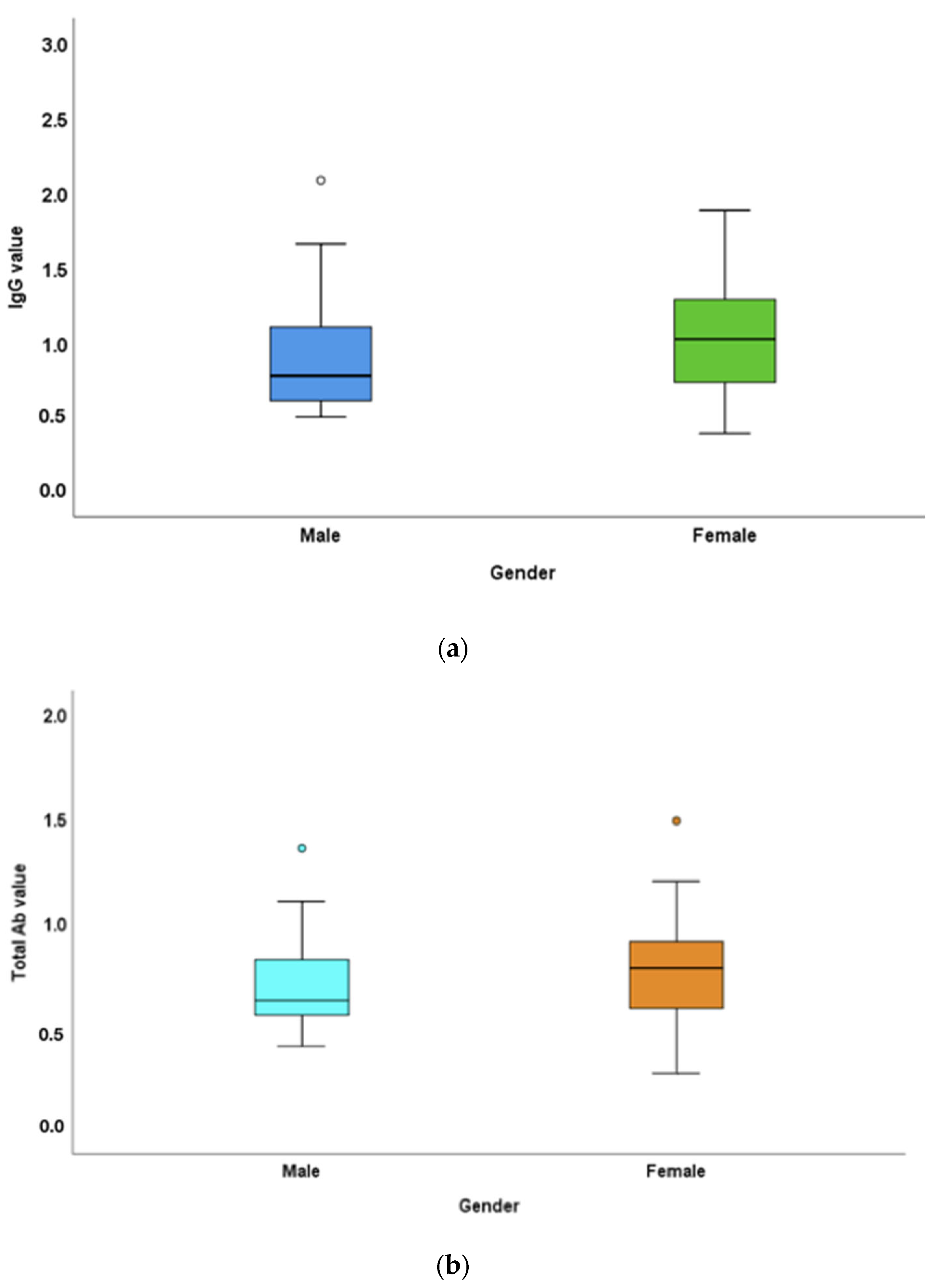
3.1. Antibody Titres in Males versus Females
3.2. Antibody Titres in Vaccinated versus Unvaccinated
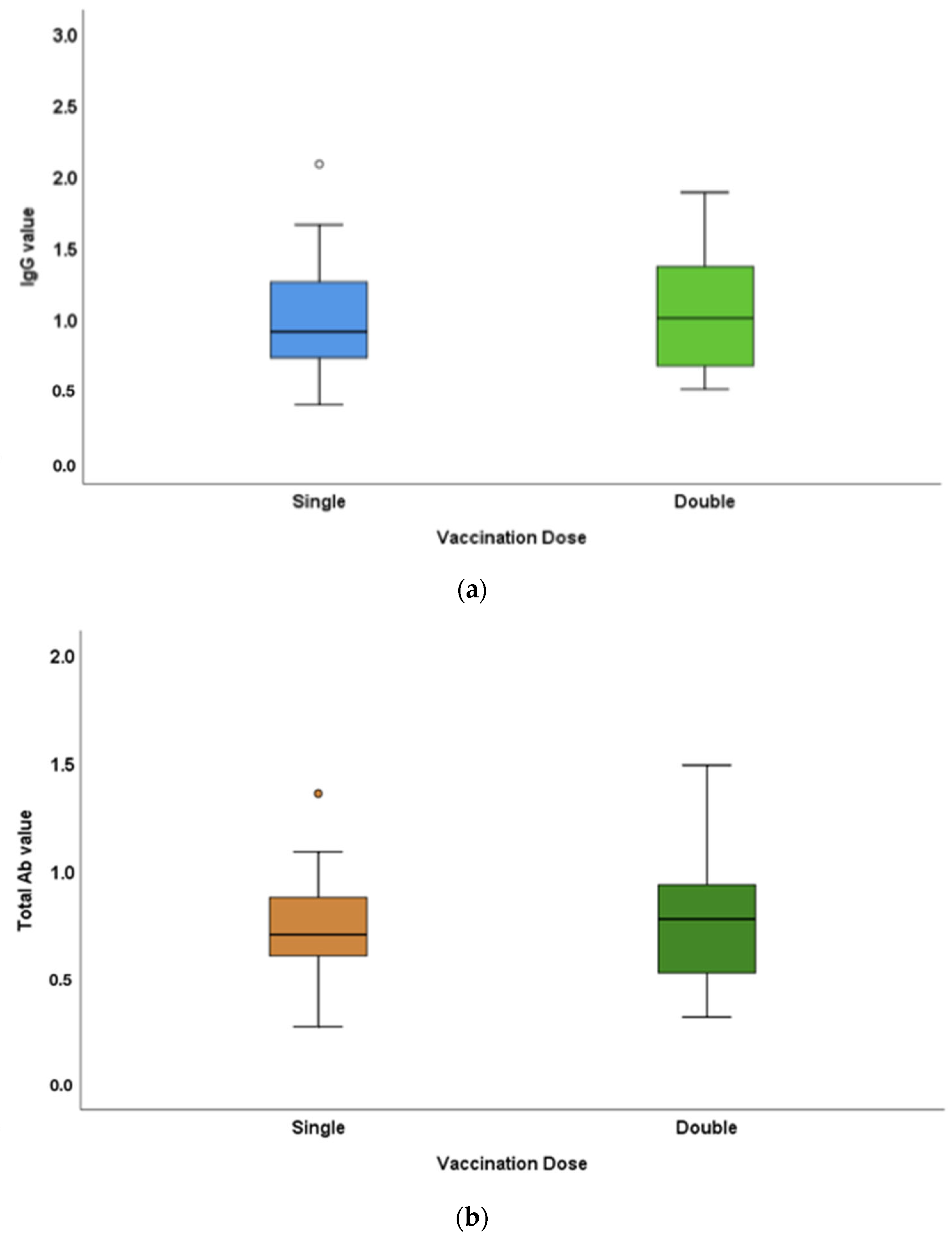
3.3. Antibody-Titre Difference between Single- and Double-Dose-Vaccinated
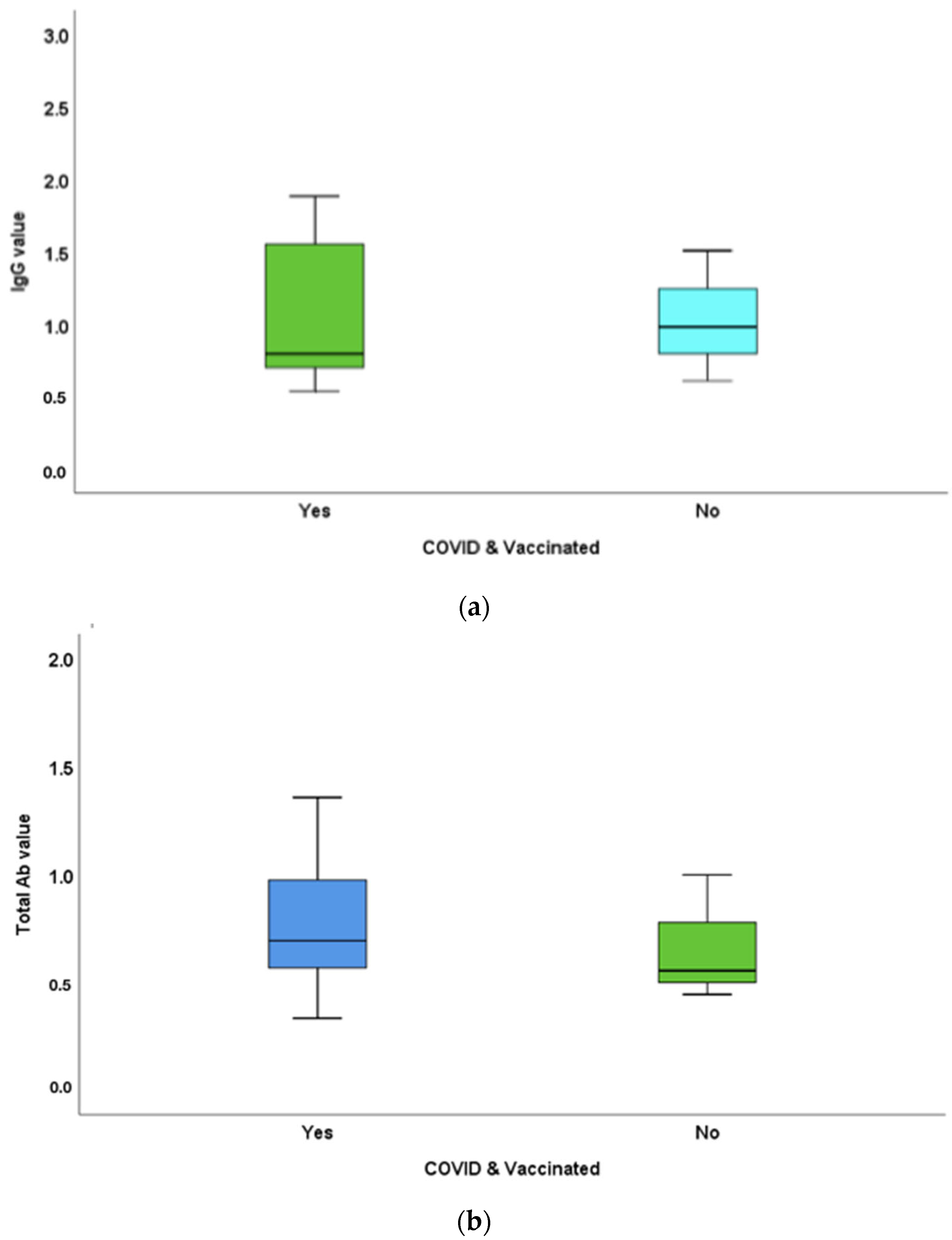
3.4. Antibody-Titre Difference between Vaccinated and Unvaccinated Individuals with COVID-19 History
4. Discussion
5. Conclusions
6. Limitations and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karlinsky, A.; Kobak, D. The World Mortality Dataset: Tracking excess mortality across countries during the COVID-19 pandemic. MedRxiv 2021. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19); StatPearls Publishing: StatPearls, FL, USA, 2022. [Google Scholar]
- Lopez, L.; Nguyen, T.; Weber, G.; Kleimola, K.; Bereda, M.; Liu, Y.; Accorsi, E.K.; Skates, S.J.; Santa Maria, J.P., Jr.; Smith, K.R.; et al. Seroprevalence of anti-SARS-CoV-2 IgG Antibodies in the Staff of a Public School System in the Midwestern United States. MedRxiv 2020. [Google Scholar] [CrossRef]
- Alfego, D.; Sullivan, A.; Poirier, B.; Williams, J.; Grover, A.; Gillim, L.; Adcock, D.; Letovsky, S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. eClinicalMedicine 2021, 36, 100902. [Google Scholar] [CrossRef] [PubMed]
- Loubiere, S.; Monfardini, E.; Allaria, C.; Mosnier, M.; Allibert, A.; Ninove, L.; Bosetti, T.; Farnarier, C.; Hamouda, I.; Auquier, P.; et al. Seroprevalence of SARS-CoV-2 antibodies among homeless people living rough, in shelters and squats: A large population-based study in France. PLoS ONE 2021, 16, e0255498. [Google Scholar]
- Inbaraj, L.R.; George, C.E.; Chandrasingh, S. Seroprevalence of COVID-19 infection in a rural district of South India: A population-based seroepidemiological study. PLoS ONE 2021, 16, e0249247. [Google Scholar] [CrossRef]
- Pisanic, N.; Randad, P.R.; Kruczynski, K.; Manabe, Y.C.; Thomas, D.L.; Pekosz, A.; Klein, S.L.; Betenbaugh, M.J.; Clarke, W.A.; Laeyendecker, O.; et al. COVID-19 Serology at Population Scale: SARS-CoV-2-Specific Antibody Responses in Saliva. J. Clin. Microbiol. 2020, 59, e02204-20. [Google Scholar] [CrossRef]
- Fernandes, L.L.; Pacheco, V.B.; Borges, L.; Athwal, H.K.; de Paula Eduardo, F.; Bezinelli, L.; Correa, L.; Jimenez, M.; Dame-Teixeira, N.; Lombaert, I.M.A.; et al. Saliva in the Diagnosis of COVID-19: A Review and New Research Directions. J. Dent. Res. 2020, 99, 1435–1443. [Google Scholar] [CrossRef]
- Czumbel, L.M.; Kiss, S.; Farkas, N.; Mandel, I.; Hegyi, A.; Nagy, Á.; Lohinai, Z.; Szakács, Z.; Hegyi, P.; Steward, M.C.; et al. Saliva as a Candidate for COVID-19 Diagnostic Testing: A Meta-Analysis. Front. Med. 2020, 7, 465. [Google Scholar]
- MacMullan, M.A.; Ibrayeva, A.; Trettner, K.; Deming, L.; Das, S.; Tran, F.; Moreno, J.R.; Casian, J.G.; Chellamuthu, P.; Kraft, J.; et al. ELISA detection of SARS-CoV-2 antibodies in saliva. Sci. Rep. 2020, 10, 20818. [Google Scholar] [CrossRef]
- Das, S.; Kar, S.S.; Samanta, S.; Banerjee, J.; Giri, B.; Dash, S.K. Immunogenic and reactogenic efficacy of Covaxin and Covishield: A comparative review. Immunol. Res. 2022, 70, 289–315. [Google Scholar] [CrossRef]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020, 81, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Randad, P.R.; Pisanic, N.; Kruczynski, K.; Manabe, Y.C.; Thomas, D.; Pekosz, A.; Klein, S.L.; Heaney, C.D. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. MedRxiv 2020. [Google Scholar] [CrossRef]
- Keuning, M.W.; Grobben, M.; de Groen, A.-E.C.; Berman-de Jong, E.P.; Bijlsma, M.W.; Cohen, S.; Felderhof, M.; Pajkrt, D. Saliva SARS-CoV-2 Antibody Prevalence in Children. Microbiol. Spectr. 2021, 9, e0073121. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J. Biol. Regul. Homeost. Agents 2020, 34, 339–343. [Google Scholar]
- Falahi, S.; Kenarkoohi, A. Sex and gender differences in the outcome of patients with COVID-19. J. Med. Virol. 2021, 93, 151–152. [Google Scholar] [CrossRef]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef]
- Grzelak, L.; Velay, A.; Madec, Y.; Gallais, F.; Staropoli, I.; Schmidt-Mutter, C.; Wendling, M.-J.; Meyer, N.; Planchais, C.; Rey, D.; et al. Sex differences in the decline of neutralizing antibodies to SARS-CoV-2. MedRxiv 2020. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health-Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Wherry, E.J.; et al. mRNA Vaccination Induces Durable Immune Memory to SARS-CoV-2 with Continued Evolution to Variants of Concern. bioRxiv 2021. [Google Scholar] [CrossRef]
- Dolgin, E. COVID vaccine immunity is waning—How much does that matter? Nature 2021, 597, 606–607. [Google Scholar] [CrossRef]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020, 5, eabe5511. [Google Scholar] [CrossRef] [PubMed]
- Alkharaan, H.; Bayati, S.; Hellström, C.; Aleman, S.; Olsson, A.; Lindahl, K.; Bogdanovic, G.; Healy, K.; Tsilingaridis, G.; De Palma, P.; et al. Persisting Salivary IgG Against SARS-CoV-2 at 9 Months after Mild COVID-19: A Complementary Approach to Population Surveys. J. Infect. Dis. 2021, 224, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Dulovic, A.; Junker, D.; Ruetalo, N.; Kaiser, P.D.; Pinilla, Y.T.; Heinzel, C.; Haering, J.; Traenkle, B.; Wagner, T.R.; et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat. Commun. 2021, 12, 3109. [Google Scholar] [CrossRef] [PubMed]
- Lapić, I.; Šegulja, D.; Rogić, D. Assessment of salivary antibody response to BNT162b2 mRNA COVID-19 vaccination. J. Med. Virol. 2021, 93, 5257–5259. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Omicron likely to weaken COVID vaccine protection. Nature 2021, 600, 367–368. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundar, S.; Ramadoss, R.; Shanmugham, R.; Anandapadmanabhan, L.T.; Paneerselvam, S.; Ramani, P.; Batul, R.; Karobari, M.I. Salivary Antibody Response of COVID-19 in Vaccinated and Unvaccinated Young Adult Populations. Vaccines 2022, 10, 1819. https://doi.org/10.3390/vaccines10111819
Sundar S, Ramadoss R, Shanmugham R, Anandapadmanabhan LT, Paneerselvam S, Ramani P, Batul R, Karobari MI. Salivary Antibody Response of COVID-19 in Vaccinated and Unvaccinated Young Adult Populations. Vaccines. 2022; 10(11):1819. https://doi.org/10.3390/vaccines10111819
Chicago/Turabian StyleSundar, Sandhya, Ramya Ramadoss, Rajeshkumar Shanmugham, Lakshmi Trivandrum Anandapadmanabhan, Suganya Paneerselvam, Pratibha Ramani, Rumesa Batul, and Mohmed Isaqali Karobari. 2022. "Salivary Antibody Response of COVID-19 in Vaccinated and Unvaccinated Young Adult Populations" Vaccines 10, no. 11: 1819. https://doi.org/10.3390/vaccines10111819
APA StyleSundar, S., Ramadoss, R., Shanmugham, R., Anandapadmanabhan, L. T., Paneerselvam, S., Ramani, P., Batul, R., & Karobari, M. I. (2022). Salivary Antibody Response of COVID-19 in Vaccinated and Unvaccinated Young Adult Populations. Vaccines, 10(11), 1819. https://doi.org/10.3390/vaccines10111819






