Impact after the Change from Voluntary to Universal Oral Rotavirus Vaccination on Consecutive Emergency Department Visits for Acute Gastroenteritis among Children in Kobe City, Japan (2016–2022)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients, and Definition
2.2. Statistical Analysis
3. Results
3.1. Patient Demographics
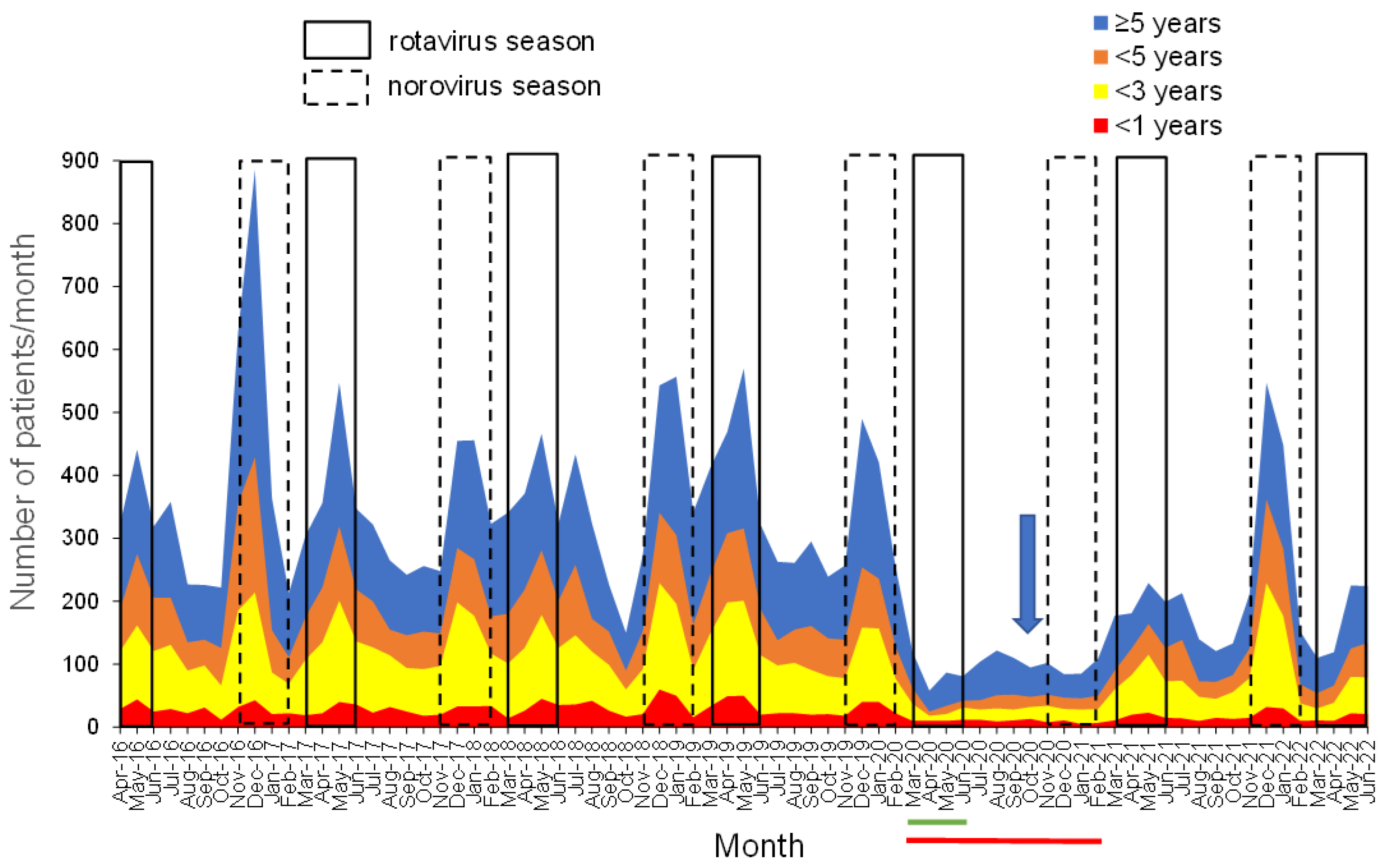
3.2. Monthly Changes in the Number of Patients with Gastroenteritis Stratified into <1 Year, 1 to <3 Years, 3 to <5 Years, and ≥5 Years
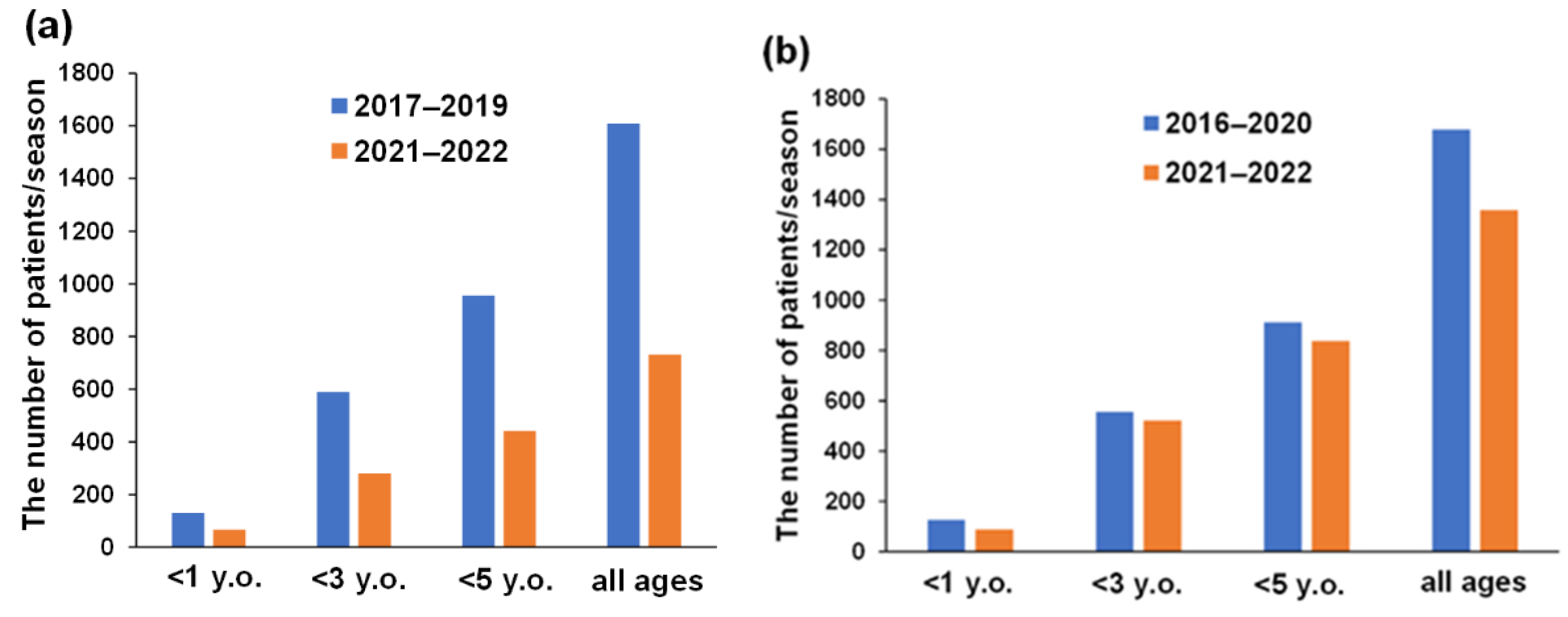
3.3. Annual Number of Patients with AGE during RV and NV Seasons and Their Vaccination History
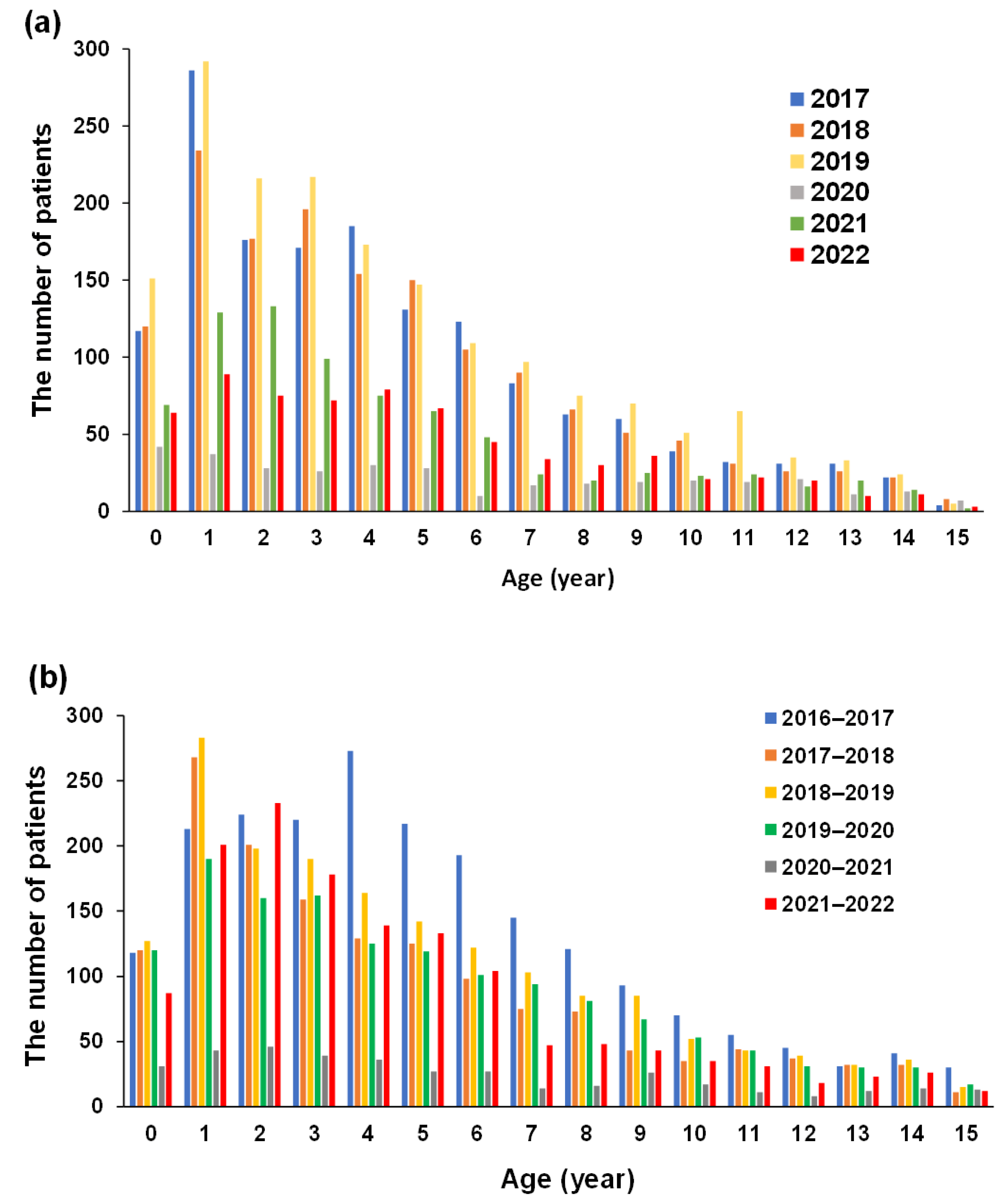
3.4. Changes in the Number of Patients with AGE According to Birth Year
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakagomi, T.; Nakagomi, O.; Takahashi, Y.; Enoki, M.; Suzuki, T.; Kilgore, P.E. Incidence and burden of rotavirus gastroenteritis in Japan as estimated from a prospective sentinel hospital study. J. Infect. Dis. 2005, 192, S106–S110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D.; World Health Organization—Coordinated Global Rotavirus Surveillance Network. Global, Regional, and National Estimates of Rotavirus Mortality in Children < 5 Years of Age, 2000–2013 . Clin. Infect. Dis. 2016, 62, S96–S105. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, T.; Akane, Y.; Honjo, S.; Kondo, K.; Kawasaki, Y. Rotavirus vaccination in Japan: Efficacy and safety of vaccines, changes in genotype, and surveillance efforts. J. Infect. Chemother. 2021, 27, 940–948. [Google Scholar] [CrossRef]
- Verberk, J.D.M.; van Dongen, J.A.P.; van de Kassteele, J.; Andrews, N.J.; van Gaalen, R.D.; Hahné, S.J.M.; Vennema, H.; Ramsay, M.; Braeckman, T.; Ladhani, S.; et al. Impact analysis of rotavirus vaccination in various geographic regions in Western Europe. Vaccine 2021, 39, 6671–6681. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Groome, M.J.; Page, N.A.; Bhagwandin, N.; Mwenda, J.M.; Steele, A.D. A decade of rotavirus vaccination in Africa-Saving lives and changing the face of diarrhoeal diseases: Report of the 12th African Rotavirus Symposium. Vaccine 2021, 39, 2319–2324. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Real-world effectiveness of rotavirus vaccines, 2006–2019: A literature review and meta-analysis. Lancet Glob. Health 2020, 8, e1195–e1202. [Google Scholar] [CrossRef]
- Bergman, H.; Henschke, N.; Hungerford, D.; Pitan, F.; Ndwandwe, D.; Cunliffe, N.; Soares-Weiser, K. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2021, 11, CD008521. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Pérez-Schael, I.; Velázquez, F.R.; Abate, H.; Breuer, T.; Clemens, S.C.; Cheuvart, B.; Espinoza, F.; Gillard, P.; Innis, B.L.; et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 2006, 354, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Matson, D.O.; Dennehy, P.; Van Damme, P.; Santosham, M.; Rodriguez, Z.; Dallas, M.J.; Heyse, J.F.; Goveia, M.G.; Black, S.B.; et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 2006, 354, 23–33. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Rotavirus vaccines: An update. Wkly Epidemiol. Rec. 2009, 84, 533–540. [Google Scholar]
- Morioka, I.; Kamiyoshi, N.; Nishiyama, M.; Yamamura, T.; Minamikawa, S.; Iwatani, S.; Nagase, H.; Nozu, K.; Nishimura, N.; Taniguchi-Ikeda, M.; et al. Changes in the numbers of patients with acute gastroenteritis after voluntary introduction of the rota virus vaccine in a Japanese children’s primary emergency medical center. Environ. Health Prev. Med. 2017, 22, 15. [Google Scholar] [CrossRef]
- Oishi, T.; Taguchi, T.; Nakano, T.; Sudo, S.; Kuwajima, H.; Shibata RVGE Study Group. The occurrence of severe rotavirus gas troenteritis in children under 3 years of age before and after the introduction of rotavirus vaccine: A prospective observational study in three pediatric clinics in Shibata City, Niigata Prefecture, Japan. Jpn. J. Infect. Dis. 2014, 67, 304–306. [Google Scholar] [CrossRef]
- Oishi, T.; Tsukano, S.; Nakano, T.; Sudo, S.; Kuwajima, H. Impact of rotavirus vaccination in severe rotavirus gastroenteritis outpatient visits at three pediatric primary care clinics in Shibata city, Niigata Prefecture, Japan. Open J. Pediatr. 2014, 04, 291–299. [Google Scholar] [CrossRef]
- Boom, J.A.; Tate, J.E.; Sahni, L.C.; Rench, M.A.; Hull, J.J.; Gentsch, J.R.; Patel, M.M.; Baker, C.J.; Parashar, U.D. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010, 125, e199–e207. [Google Scholar] [CrossRef] [PubMed]
- Martinón-Torres, F.; Bouzón Alejandro, M.; Redondo Collazo, L.; Sánchez Lastres, J.M.; Pértega Díaz, S.; Seoane Pillado, M.T.; Martinón Sánchez, J.M. ROTACOST research team. Effectiveness of rotavirus vaccination in Spain. Hum. Vaccines 2011, 7, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Lopman, B.A.; Pitzer, V.E.; Sarkar, R.; Gladstone, B.; Patel, M.; Glasser, J.; Gambhir, M.; Atchison, C.; Grenfell, B.T.; Edmunds, W.J.; et al. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS ONE 2012, 7, e41720. [Google Scholar] [CrossRef]
- Lopman, B.A.; Curns, A.T.; Yen, C.; Parashar, U.D. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J. Infect. Dis. 2011, 204, 980–986. [Google Scholar] [CrossRef]
- Pollard, S.L.; Malpica-Llanos, T.; Friberg, I.K.; Fischer-Walker, C.; Ashraf, S.; Walker, N. Estimating the herd immunity effect of rotavirus vaccine. Vaccine 2015, 33, 3795–3800. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nakagomi, T.; Nakagomi, O. Cost-effectiveness analysis of a universal rotavirus immunization program in Japan. Jpn. J. Infect. Dis. 2011, 64, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Itzler, R.; O’Brien, M.A.; Yamabe, K.; Abe, M.; Dhankhar, P. Cost-effectiveness of a pentavalent rotavirus vaccine in Japan. J. Med. Econ. 2013, 16, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Matsuki, T.; Sato, K.; Mizuno, M.; Shibata, M.; Hasegawa, S.; Morita, M.; Iwasa, M.; Gopala, K.; Holl, K. Impact of rotavirus vaccination on the burden of acute gastroenteritis in Nagoya City, Japan. Vaccine 2018, 36, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Nozu, K.; Ishiko, S.; Nagase, H.; Ninchoji, T.; Nagano, C.; Takeda, H.; Unzaki, A.; Ishibashi, K.; Morioka, I.; et al. Epidemiological impact of universal varicella vaccination on consecutive emergency department visits for varicella and its economic impact among children in Kobe City, Japan. J. Infect. Chemother. 2022, 28, 35–40. [Google Scholar] [CrossRef]
- Kim, S.Y.; Goldie, S.J.; Salomon, J.A. Cost-effectiveness of rotavirus vaccination in Vietnam. BMC Public Health 2009, 9, 29. [Google Scholar] [CrossRef]
- Bányai, K.; László, B.; Duque, J.; Steele, A.D.; Nelson, E.A.S.; Gentsch, J.R.; Parashar, U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012, 30, A122–A130. [Google Scholar] [CrossRef]
- Chissaque, A.; Bauhofer, A.F.L.; Cossa-Moiane, I.; Sitoe, E.; Munlela, B.; João, E.D.; Langa, J.S.; Chilaúle, J.J.; Boene, S.S.; Cassocera, M.; et al. Rotavirus A infection in pre- and post-vaccine period: Risk factors, genotypes distribution by vaccination status and age of children in Nampula Province, Northern Mozambique (2015–2019). PLoS ONE 2021, 16, e0255720. [Google Scholar] [CrossRef]
- Okitsu, S.; Khamrin, P.; Thongprachum, A.; Hikita, T.; Kumthip, K.; Pham, N.T.K.; Takanashi, S.; Hayakawa, S.; Maneekarn, N.; Ushijima, H. Diversity of human Sapovirus genotypes detected in Japanese pediatric patients with acute gastroenteritis, 2014–2017. J. Med. Virol. 2021, 93, 4865–4874. [Google Scholar] [CrossRef]
- Koo, H.L.; Neill, F.H.; Estes, M.K.; Munoz, F.M.; Cameron, A.; DuPont, H.L.; Atmar, R.L. Noroviruses: The Most common Pedi atric viral enteric pathogen at a Large University Hospital after introduction of rotavirus vaccination. J. Pediatric Infect. Dis. Soc. 2013, 2, 57–60. [Google Scholar] [CrossRef]
- Hemming, M.; Räsänen, S.; Huhti, L.; Paloniemi, M.; Salminen, M.; Vesikari, T. Major reduction of rotavirus, but not Norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur. J. Pediatr. 2013, 172, 739–746. [Google Scholar] [CrossRef]
- Payne, D.C.; Vinjé, J.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Weinberg, G.A.; Hall, C.B.; Chappell, J.; Bernstein, D.I.; Curns, A.T.; et al. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 2013, 368, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Bucardo, F.; Reyes, Y.; Svensson, L.; Nordgren, J. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS ONE 2014, 9, e98201. [Google Scholar] [CrossRef]
- Nakamura, N.; Kobayashi, S.; Minagawa, H.; Matsushita, T.; Sugiura, W.; Iwatani, Y. Molecular epidemiology of enteric viruses in patients with acute gastroenteritis in Aichi Prefecture, Japan. J. Med. Virol. 2016, 88, 1180–1186. [Google Scholar] [CrossRef]
- Khamrin, P.; Thongprachum, A.; Okitsu, S.; Hayakawa, S.; Maneekarn, N.; Ushijima, H. Multiple astrovirus MLB1, MLB2, VA2 clades, and classic human astrovirus in children with acute gastroenteritis in Japan. J. Med. Virol. 2016, 88, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, L.; Tollefson, D.; Kharono, B.; Drain, K.P. Prevalence of rotavirus among older children and adults with diarrhea: A systematic review and meta-analysis. Vaccine 2021, 39, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Kyo, K.; Takano, C.; Kasuga, Y.; Ogawa, E.; Ishige, M.; Pham, N.T.K.; Okitsu, S.; Ushijima, H.; Urakami, T.; Fuchigami, T.; et al. Severe rotavirus gastroenteritis in children older than 5 years after vaccine introduction. J. Infect. Chemother. 2021, 27, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Phelan, A.L.; Katz, R.; Gostin, L.O. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. Jama 2020, 323, 709–710. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nozu, K.; Ishiko, S.; Kondo, A.; Ninchoji, T.; Nagano, C.; Takeda, H.; Unzaki, A.; Ishibashi, K.; Morioka, I.; et al. Impact of the state of emergency during the COVID-19 pandemic in 2020 on asthma exacerbations among children in Kobe City, Japan. Int. J. Environ. Res. Public Health 2021, 18, 11407. [Google Scholar] [CrossRef]
- Sawakami, T.; Karako, K.; Song, P. Behavioral changes adopted to constrain COVID-19 in Japan: What are the implications for seasonal influenza prevention and control? Glob. Health Med. 2021, 3, 125–128. [Google Scholar] [CrossRef]
- Vo, T.H.; Okasha, O.; Al-Hello, H.; Polkowska, A.; Räsänen, S.; Bojang, M.; Nuorti, J.P.; Jalava, K. An outbreak of Norovirus infections among lunch customers at a restaurant, Tampere, Finland, 2015. Food Environ. Virol. 2016, 8, 174–179. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, H.; Nozu, K.; Ishiko, S.; Kondo, A.; Yamamoto, N.; Tamura, A.; Aoto, Y.; Unzaki, A.; Ishibashi, K.; Morioka, I.; et al. Multivariate analysis of the impact of weather and air pollution on emergency department visits for unprovoked seizure among children: A retrospective clinical observational study. Epilepsy Behav. 2021, 125, 108434. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Nozu, K.; Ishiko, S.; Nagase, H.; Ninchoji, T.; Nagano, C.; Takeda, H.; Unzaki, A.; Ishibashi, K.; Morioka, I.; et al. Multivariate analysis of the impact of weather and air pollution on emergency department visits for night-time headaches among children: Retrospective, clinical observational study. BMJ Open 2021, 11, e046520. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||||||
| Year | Total Number of Visits | All Ages | <1 Year | Rotavirus Vaccination (+) | 1–<3 Years | Rotavirus Vaccination (+) | 3–<5 Years | Rotavirus Vaccination (+) |
| Number of Patients | Number of Patients | Number of Patients | Number of Patients | |||||
| 2016 # | 20,490 | 1084 | 98 (9.0%) | 52 | 306 (28.2%) | 117 | 265 (24.4%) | 67 |
| (53.1%) | (38.2%) | (25.3%) | ||||||
| 2017 | 28,674 | 1554 | 117 (7.5%) | 68 | 462 (29.7%) | 184 | 356 (22.9%) | 120 |
| (58.1%) | (39.8%) | (33.7%) | ||||||
| 2018 | 27,643 | 1502 | 120 (8.0%) | 67 | 411 (27.4%) | 179 | 350 (23.3%) | 169 |
| (55.8%) | (43.6%) | (48.3%) | ||||||
| 2019 | 27,066 | 1772 | 152 (8.6%) | 88 | 510 (28.8%) | 252 | 391 (22.1%) | 174 |
| (57.9%) | (49.4%) | (44.5%) | ||||||
| 2020 | 13,274 | 346 | 42 (12.1%) | 20 | 65 (18.8%) | 44 | 56 | 18 |
| (47.6%) | (67.7%) | (16.2%) | (32.1%) | |||||
| 2021 | 13,291 | 786 | 69 (8.8%) | 45 | 262 (33.3%) | 152 | 174 (22.1%) | 87 |
| (65.2%) | (58.0%) | (50.0%) | ||||||
| 2022 $ | 6630 | 678 | 64 (9.4%) | 37 | 164 (24.2%) | 104 | 151 (22.3%) | 78 |
| (57.8%) | (63.4%) | (51.7%) | ||||||
| (B) | ||||||||
| Year | All Ages | <1 Year | 1–<3 Years | 3–<5 years | ||||
| Number of Patients | Number of Patients | Number of Patients | Number of Patients | |||||
| 2016–2017 | 2089 | 118 (5.6%) | 437 (20.9%) | 494 (23.6%) | ||||
| 2017–2018 | 1482 | 120 (8.1%) | 469 (31.6%) | 288 (19.4%) | ||||
| 2018–2019 | 1716 | 147 (8.6%) | 461 (26.9%) | 354 (20.6%) | ||||
| 2019–2020 | 1423 | 120 (8.4%) | 350 (24.6%) | 287 (20.2%) | ||||
| 2020–2021 | 380 | 31 (8.2%) | 89 (23.4%) | 75 (19.7%) | ||||
| 2021–2022 | 1358 | 87 (6.4%) | 434 (32.0%) | 317 (23.3%) | ||||
| Years | Annual Population < 16 |
|---|---|
| 2016 | 207,458 |
| 2017 | 205,085 |
| 2018 | 204,599 |
| 2019 | 201,499 |
| 2020 | 198,494 |
| 2021 | 195,115 |
| 2022 | 191,674 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, H.; Nozu, K.; Hanafusa, H.; Nambu, Y.; Kido, T.; Kondo, A.; Tamura, A.; Awano, H.; Morioka, I.; Nagase, H.; et al. Impact after the Change from Voluntary to Universal Oral Rotavirus Vaccination on Consecutive Emergency Department Visits for Acute Gastroenteritis among Children in Kobe City, Japan (2016–2022). Vaccines 2022, 10, 1831. https://doi.org/10.3390/vaccines10111831
Yamaguchi H, Nozu K, Hanafusa H, Nambu Y, Kido T, Kondo A, Tamura A, Awano H, Morioka I, Nagase H, et al. Impact after the Change from Voluntary to Universal Oral Rotavirus Vaccination on Consecutive Emergency Department Visits for Acute Gastroenteritis among Children in Kobe City, Japan (2016–2022). Vaccines. 2022; 10(11):1831. https://doi.org/10.3390/vaccines10111831
Chicago/Turabian StyleYamaguchi, Hiroshi, Kandai Nozu, Hiroaki Hanafusa, Yoshinori Nambu, Takumi Kido, Atsushi Kondo, Akihiro Tamura, Hiroyuki Awano, Ichiro Morioka, Hiroaki Nagase, and et al. 2022. "Impact after the Change from Voluntary to Universal Oral Rotavirus Vaccination on Consecutive Emergency Department Visits for Acute Gastroenteritis among Children in Kobe City, Japan (2016–2022)" Vaccines 10, no. 11: 1831. https://doi.org/10.3390/vaccines10111831
APA StyleYamaguchi, H., Nozu, K., Hanafusa, H., Nambu, Y., Kido, T., Kondo, A., Tamura, A., Awano, H., Morioka, I., Nagase, H., & Ishida, A. (2022). Impact after the Change from Voluntary to Universal Oral Rotavirus Vaccination on Consecutive Emergency Department Visits for Acute Gastroenteritis among Children in Kobe City, Japan (2016–2022). Vaccines, 10(11), 1831. https://doi.org/10.3390/vaccines10111831






