Nucleic Acid Vaccines against SARS-CoV-2
Abstract
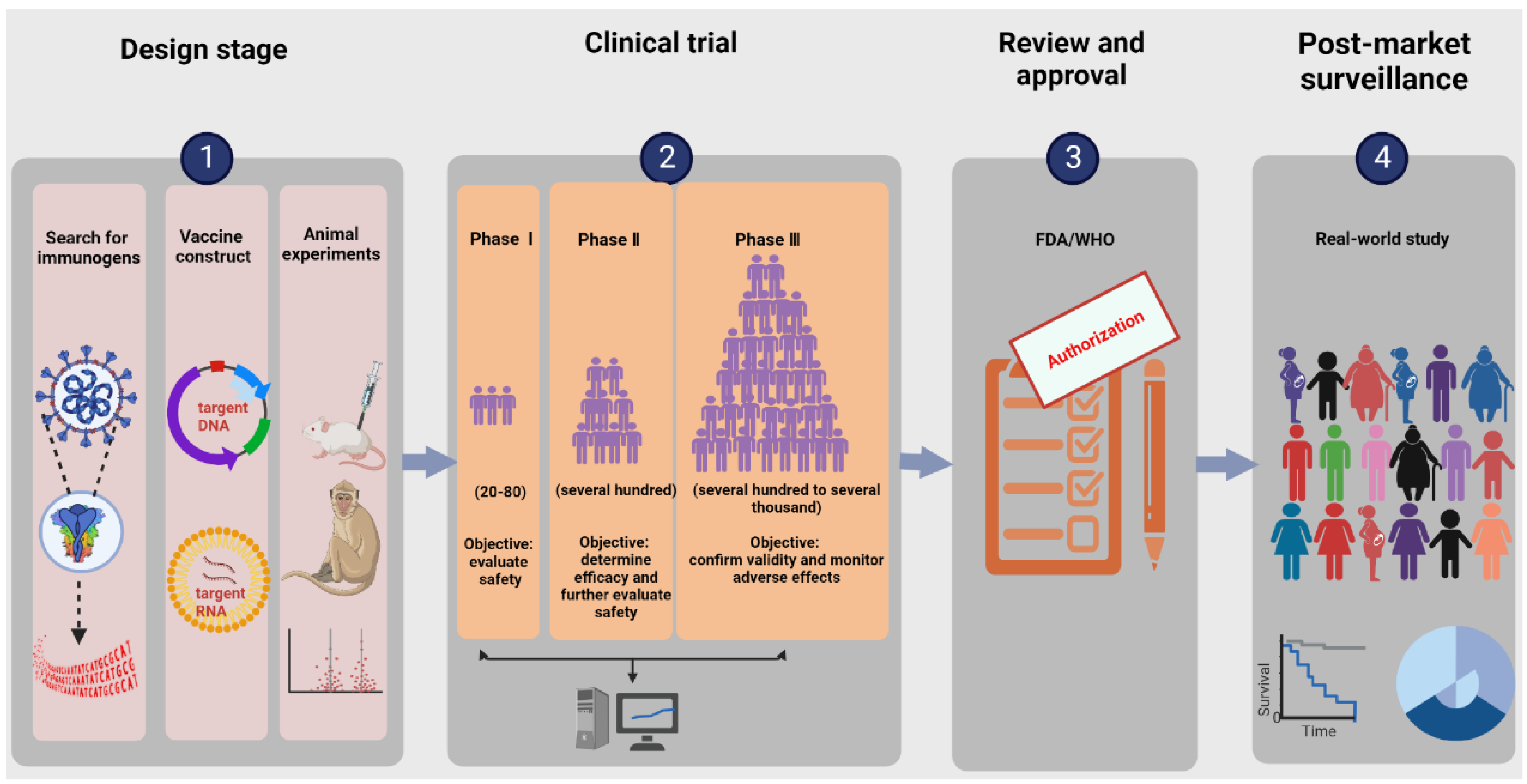
:1. Introduction
2. The Research and Development Process of Nucleic Acid Vaccines
2.1. Search for Immunogens
2.2. Designing Vaccine Constructs
2.3. Determining Toxicological Effects and Immune Effects in Animal Models
2.4. Clinical Trials
2.4.1. Dosage
2.4.2. Safety
2.4.3. Effectiveness and Immunogenicity
3. The Pros and Cons of Nucleic Acid Vaccines
3.1. Comparison of Nucleic Acid Vaccines and Traditional Vaccines
3.2. Comparison of DNA Vaccines and RNA Vaccines
4. Optimization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, X.; Ye, Q. Kidney involvement in COVID-19 and its treatments. J. Med. Virol. 2021, 93, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Ye, Q. Hepatic complications of COVID-19 and its treatment. J. Med. Virol. 2020, 92, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Lai, E.Y.; Luft, F.C.; Persson, P.B.; Mao, J. SARS-CoV-2 effects on the renin-angiotensin-aldosterone system, therapeutic implications. Acta Physiol. 2021, 231, e13608. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Lu, D.; Shang, S.; Fu, J.; Gong, F.; Shu, Q.; Mao, J. Crosstalk between coronavirus disease 2019 and cardiovascular disease and its treatment. ESC Heart. Fail. 2020, 7, 3464–3472. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Zhang, T.; Xu, J.; Shang, S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G245–G252. [Google Scholar] [CrossRef]
- Cutler, D.M.; Summers, L.H. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324, 1495–1496. [Google Scholar] [CrossRef]
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Mao, S.; Ye, Q. Assessment of global asymptomatic SARS-CoV-2 infection and management practices from China. Int. J. Biol. Sci. 2021, 17, 1119–1124. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J.; Fu, J.; Shang, S.; Shu, Q.; Zhang, T. Epidemiological analysis of COVID-19 and practical experience from China. J. Med. Virol. 2020, 92, 755–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Han, X.; Ye, Q. The variants of SARS-CoV-2 and the challenges of vaccines. J. Med. Virol. 2022, 94, 1366–1372. [Google Scholar] [CrossRef]
- Meng, H.; Mao, J.; Ye, Q. Booster vaccination strategy: Necessity, immunization objectives, immunization strategy, and safety. J. Med. Virol. 2022, 94, 2369–2375. [Google Scholar] [CrossRef]
- Meng, H.; Mao, J.; Ye, Q. Strategies and safety considerations of booster vaccination in COVID-19. Bosn. J. Basic Med. Sci. 2022, 22, 366–373. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The global epidemic of SARS-CoV-2 variants and their mutational immune escape. J. Med. Virol. 2022, 94, 847–857. [Google Scholar] [CrossRef]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- World Health Organization. Background Document on the Inactivated Vaccine Sinovac-CoronaVac against COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BIBP-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the AZD1222 Vaccine against COVID-19 Developed by Oxford University and AstraZeneca. Available online: https://www.who.int/publications/i/item/background-document-on-the-azd1222-vaccine-against-covid-19-developed-by-oxford-university-and-astrazeneca (accessed on 10 January 2022).
- World Health Organization. Background Document on the Janssen Ad26.COV2. S (COVID-19) Vaccine. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad26.COV2.S-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA-1273 Vaccine (Moderna) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-the-mrna-1273-vaccine-(moderna)-against-covid-19 (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA Vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-mrna-vaccine-bnt162b2-(pfizer-biontech)-against-covid-19 (accessed on 10 January 2022).
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Bennet, B.M.; Wolf, J.; Laureano, R.; Sellers, R.S. Review of Current Vaccine Development Strategies to Prevent Coronavirus Disease 2019 (COVID-19). Toxicol. Pathol. 2020, 48, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. India’s DNA COVID vaccine is a world first–more are coming. Nature 2021, 597, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, G.; Pradhapsingh, B.; Singh Pawar, C.; Khan, H.A.; Alrokayan, S.H.; Prasad, N.R. SARS-CoV-2: Pathogenesis, Molecular Targets and Experimental Models. Front. Pharmacol. 2021, 12, 638334. [Google Scholar] [CrossRef] [PubMed]
- Prates, E.T.; Garvin, M.R.; Pavicic, M.; Jones, P.; Shah, M.; Demerdash, O.; Amos, B.K.; Geiger, A.; Jacobson, D. Potential Pathogenicity Determinants Identified from Structural Proteomics of SARS-CoV and SARS-CoV-2. Mol. Biol. Evol. 2021, 38, 702–715. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, K.; Li, Y.; Lu, Q.; Wang, Q.; Zhang, Y.; Wang, L.; Liao, H.; Zheng, A.; et al. The molecular basis for SARS-CoV-2 binding to dog ACE2. Nat. Commun. 2021, 12, 4195. [Google Scholar] [CrossRef]
- Fan, X.; Cao, D.; Kong, L.; Zhang, X. Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein. Nat. Commun. 2020, 11, 3618. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Kirchdoerfer, R.N.; Wang, N.; Pallesen, J.; Wrapp, D.; Turner, H.L.; Cottrell, C.A.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 2018, 8, 15701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef] [Green Version]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.A. DNA vaccines: A review. J. Intern. Med. 2003, 253, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Geiben-Lynn, R.; Greenland, J.R.; Frimpong-Boateng, K.; van Rooijen, N.; Hovav, A.H.; Letvin, N.L. CD4 + T lymphocytes mediate in vivo clearance of plasmid DNA vaccine antigen expression and potentiate CD8 + T-cell immune responses. Blood 2008, 112, 4585–4590. [Google Scholar] [CrossRef] [Green Version]
- Hotez, P.J.; Corry, D.B.; Strych, U.; Bottazzi, M.E. COVID-19 vaccines: Neutralizing antibodies and the alum advantage. Nat. Rev. Immunol. 2020, 20, 399–400. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef]
- Cold Spring Harbor Laboratory. BNT162b Vaccines are Immunogenic and Protect Non-Human Primates against SARS-CoV-2; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2020. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Larkin, J.; Swanton, C. Academically led clinical trials: Challenges and opportunities. Ann. Oncol. 2015, 26, 2010–2011. [Google Scholar] [CrossRef] [PubMed]
- Makoni, M. Africa’s need for more COVID-19 clinical trials. Lancet 2021, 397, 2037. [Google Scholar] [CrossRef]
- Bill and Melinda Gates Foundation. Clinical Trials. Available online: https://docs.gatesfoundation.org/documents/clinical_trials.pdf (accessed on 10 January 2022).
- U.S. Food and Drug Administration. What Are the Different Types of Clinical Research? Available online: https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research (accessed on 10 January 2022).
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen-Quick, A.; Andrade, V.M.; Morrow, M.P.; Kraynyak, K.; Agnes, J.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine 2021, 31, 100689. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Norquist, J.M.; Khawaja, S.S.; Kurian, C.; Mast, T.C.; Liaw, K.L.; Robertson, M.N.; Evans, B.; Gutsch, D.; Saddier, P. Adaptation of a previously validated vaccination report card for use in adult vaccine clinical trials to align with the 2007 FDA Toxicity Grading Scale Guidance. Hum. Vaccin. Immunother. 2012, 8, 1208–1212. [Google Scholar] [CrossRef] [Green Version]
- McNeil, M.M.; DeStefano, F. Vaccine-associated hypersensitivity. J. Allergy Clin. Immunol. 2018, 141, 463–472. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients with Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196–1201. [Google Scholar] [CrossRef]
- Mansour, J.; Short, R.G.; Bhalla, S.; Woodard, P.K.; Verma, A.; Robinson, X.; Raptis, D.A. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: A report of two cases. Clin. Imaging 2021, 78, 247–249. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Husby, A.; Hansen, J.V.; Fosbøl, E.; Thiesson, E.M.; Madsen, M.; Thomsen, R.W.; Sørensen, H.T.; Andersen, M.; Wohlfahrt, J.; Gislason, G.; et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: Population based cohort study. BMJ 2021, 375, e068665. [Google Scholar] [CrossRef]
- Aikawa, T.; Ogino, J.; Kita, Y.; Funayama, N. Myocardial microthrombi after COVID-19 mRNA vaccination. Eur. Heart. J. 2021, 42, 4501. [Google Scholar] [CrossRef]
- Ghincea, A.; Ryu, C.; Herzog, E.L. An Acute Exacerbation of Idiopathic Pulmonary Fibrosis after BNT162b2 mRNA COVID-19 Vaccination: A Case Report. Chest 2022, 161, e71–e73. [Google Scholar] [CrossRef]
- Bartsch, S.M.; O’Shea, K.J.; Ferguson, M.C.; Bottazzi, M.E.; Wedlock, P.T.; Strych, U.; McKinnell, J.A.; Siegmund, S.S.; Cox, S.N.; Hotez, P.J.; et al. Vaccine Efficacy Needed for a COVID-19 Coronavirus Vaccine to Prevent or Stop an Epidemic as the Sole Intervention. Am. J. Prev. Med. 2020, 59, 493–503. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Gilboa, T.; Ogata, A.F.; Maley, A.M.; Cohen, L.; Busch, E.L.; Lazarovits, R.; Mao, C.P.; Cai, Y.; Zhang, J.; et al. Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat. Biomed. Eng. 2020, 4, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Houlihan, C.F.; Vora, N.; Byrne, T.; Lewer, D.; Kelly, G.; Heaney, J.; Gandhi, S.; Spyer, M.J.; Beale, R.; Cherepanov, P.; et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet 2020, 396, e6–e7. [Google Scholar] [CrossRef]
- Wang, X.; Mosmann, T. In vivo priming of CD4 T cells that produce interleukin (IL)-2 but not IL-4 or interferon (IFN)-gamma, and can subsequently differentiate into IL-4- or IFN-gamma-secreting cells. J. Exp. Med. 2001, 194, 1069–1080. [Google Scholar] [CrossRef]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Puranik, A.; Lenehan, P.J.; Silvert, E.; Niesen, M.J.M.; Corchado-Garcia, J.; O’Horo, J.C.; Virk, A.; Swift, M.D.; Halamka, J.; Badley, A.D.; et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv 2021. [Google Scholar] [CrossRef]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; AlMukdad, S.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat. Med. 2021, 27, 2136–2143. [Google Scholar] [CrossRef]
- Kaplonek, P.; Cizmeci, D.; Fischinger, S.; Collier, A.R.; Suscovich, T.; Linde, C.; Broge, T.; Mann, C.; Amanat, F.; Dayal, D.; et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci. Transl. Med. 2022, 14, eabm2311. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Yankauckas, M.A.; Morrow, J.E.; Parker, S.E.; Abai, A.; Rhodes, G.H.; Dwarki, V.J.; Gromkowski, S.H. Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing NP gene. DNA Cell Biol. 1993, 12, 771–776. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Stone, C.A., Jr.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 1731–1733.e3. [Google Scholar] [CrossRef]
- Howe, H.S.; Leung, B.P.L. Anti-Cytokine Autoantibodies in Systemic Lupus Erythematosus. Cells 2019, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Comi, G.; Bar-Or, A.; Lassmann, H.; Uccelli, A.; Hartung, H.P.; Montalban, X.; Sørensen, P.S.; Hohlfeld, R.; Hauser, S.L. Role of B Cells in Multiple Sclerosis and Related Disorders. Ann. Neurol. 2021, 89, 13–23. [Google Scholar] [CrossRef]
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; Twisk, J.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.; Habibuw, M.R.; Dijkmans, B.A. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 535–537. [Google Scholar] [CrossRef] [Green Version]
- Bronner, I.M.; van der Meulen, M.F.; de Visser, M.; Kalmijn, S.; van Venrooij, W.J.; Voskuyl, A.E.; Dinant, H.J.; Linssen, W.H.; Wokke, J.H.; Hoogendijk, J.E. Long-term outcome in polymyositis and dermatomyositis. Ann. Rheum. Dis. 2006, 65, 1456–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.L.; Chen, J.C.; Yeh, C.T.; Chang, M.Y.; Liang, C.K.; Chiu, C.T.; Lin, D.Y.; Liaw, Y.F. Gene gun bombardment with DNA-coated gold particles is a potential alternative to hydrodynamics-based transfection for delivering genes into superficial hepatocytes. Hum. Gene Ther. 2008, 19, 391–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, R.D.; Tekari, A.; Frauchiger, D.A.; Krismer, A.; Benneker, L.M.; Gantenbein, B. Efficient Nonviral Transfection of Primary Intervertebral Disc Cells by Electroporation for Tissue Engineering Application. Tissue Eng. Part C Methods 2017, 23, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far from Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]

| Vaccine Name | Technology | Developer/Company | Immunization Protocol | Immunity | Effectiveness | Current Status |
|---|---|---|---|---|---|---|
| ZyCoV-D | DNA vaccine | Zydus Cadila (Ahmedabad, India) | 3 doses (2.0 mg/dose), 4 weeks apart | humoral and cellular immunity | 66.6% | approved by India |
| INO-4800 | DNA vaccine | Inovio (Plymouth Meeting, PA, USA) | 2 doses (2.0 mg/dose), 4 weeks apart | humoral and cellular immunity | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| AG0302-COVID19 | DNA vaccine | AnGes (Osaka, Japan) | 2 doses (2.0 mg/dose), 2/4 weeks apart | unpublished results | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| GX-19N | DNA vaccine | Genexine (Seoul, Korea) | unpublished results | unpublished results | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| BNT162b2 | mRNA vaccine | Pfizer (New York, NY, USA)/BioNTech (Mainz, Germany) | 2 doses (30 μg/0.3 mL/dose), 3 weeks apart | humoral and cellular immunity | 95.0% | approved by WHO |
| mRNA-1273 | mRNA vaccine | Moderna (Cambridge, MA, USA) | 2 doses (100 μg/0.5 mL/dose), 28 days apart | humoral and cellular immunity | 94.1% | approved by WHO |
| ARCoV | mRNA vaccine | WALVAX (Yunnan, China)/ABOGEN (Suzhou, China) | unpublished results | humoral and cellular immunity | unpublished results | phase Ⅱ/Ⅲ clinical trials |
| Vaccine Name | Common Local Adverse Reactions | Common Systemic Adverse Reactions | Serious Adverse Events |
|---|---|---|---|
| ZyCoV-D | pain, redness, swelling, and itching | headache, fever, muscle pain, and fatigue | cerebrovascular stroke, cardiorespiratory arrest with septicaemia, and alcoholic liver disease |
| INO-4800 | pain and erythema | nausea | none currently reported |
| BNT162b2 | pain, redness, and swelling | fatigue, headache, chills, and muscle pain | hypersensitivity reaction, paroxysmal ventricular arrhythmia, and death |
| mRNA-1273 | pain, erythema, swelling, and lymphadenopathy | fatigue, headache, myalgia, and arthralgia | hypersensitivity reactions, Bell’s palsy, and death |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ye, Q. Nucleic Acid Vaccines against SARS-CoV-2. Vaccines 2022, 10, 1849. https://doi.org/10.3390/vaccines10111849
Liu Y, Ye Q. Nucleic Acid Vaccines against SARS-CoV-2. Vaccines. 2022; 10(11):1849. https://doi.org/10.3390/vaccines10111849
Chicago/Turabian StyleLiu, Ying, and Qing Ye. 2022. "Nucleic Acid Vaccines against SARS-CoV-2" Vaccines 10, no. 11: 1849. https://doi.org/10.3390/vaccines10111849
APA StyleLiu, Y., & Ye, Q. (2022). Nucleic Acid Vaccines against SARS-CoV-2. Vaccines, 10(11), 1849. https://doi.org/10.3390/vaccines10111849




