Effect of Vaccination Time Intervals on SARS-COV-2 Omicron Variant Strain Infection in Guangzhou: A Real-World Matched Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
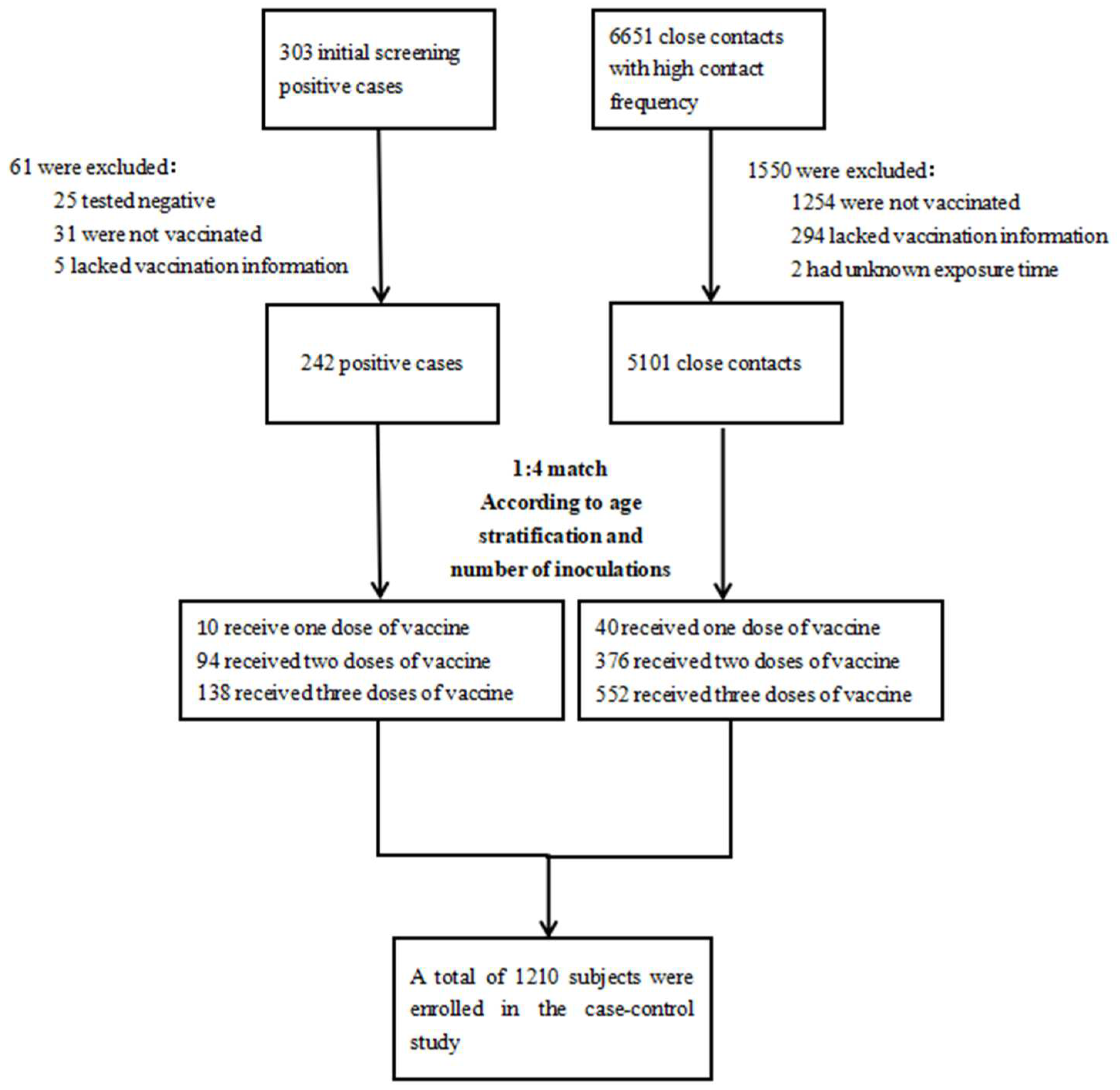
2.1. Study Design and Screening of Subjects
2.2. Definition of Cases and Close Contacts
2.3. Information Collection
2.4. Stratification Criteria for Vaccination Intervals
- 1.
- TT1: The interval between the first dose of vaccine and the second dose. The second dose is generally recommended to be administered at the 21st day after the first dose. TT1 was thus classified as being greater than 30 days and less than 30 days.
- 2.
- TT2: The interval between the second dose of vaccine and the third dose. It is generally recommended to take the third dose after 180 days of the second dose, and studies have shown that the antibody level rises to the peak within one month after the third dose of vaccine, so TT2 is stratified by the 210 days.
- 3.
- TT3*: The interval between the last vaccination and infection for case group; the interval between the last injection and the first contact with confirmed cases for control group. TT3 was stratified by 60 days, 120 days, and 60 days for one-dose, two-dose, and three-dose vaccination, respectively.
- 4.
- TT4*: The interval between the first vaccination and infection for the case group; the interval between the first vaccination and the first contact of a confirmed case for the control group. The vaccination interval TT4 was stratified by 180 days for two-dose vaccination and 300 days for three-dose vaccination, respectively.
- *
- Stratification of the TT3 and TT4 intervals: After calculating the average of the TT3 and TT4 time intervals, the average of the time intervals and the planned vaccination time were considered to determine the current stratification criteria.
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.1.1. Demographic Characteristics of the Participants
3.1.2. Demographic Characteristics of Cases and Controls Vaccinated with Different Doses
3.2. Relationship between Time Interval and SARS-CoV-2 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Xue, Z.; Yan, L. Research status of new coronavirus variants and vaccines. Anhui J. Prev. Med. 2022, 28, 1–8+12. [Google Scholar]
- Thakur, V.; Ratho, R.K. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J. Med. Virol. 2022, 94, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, L.; Yin, X.; Li, C.; Hua, Z. Current Situation and New Ideas of SARS-CoV-2 Vaccine Research and Development. Pharm. Biotechnol. 2021, 28, 395–399. [Google Scholar]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.; Patiño, E.G.; De Oliveira Piorelli, R.; Conde, M.T.R.P.; Batista, A.P.; Zeng, G.; Xin, Q.; Kallas, E.G.; Flores, J.; Ockenhouse, C.F.; et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac—PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 853. [Google Scholar]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Shao, W.; Chen, X.; Zheng, C.; Liu, H.; Wang, G.; Zhang, B.; Li, Z.; Zhang, W. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: A literature review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 2383–2392. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Lu, L.; Mok, B.W.Y.; Chen, L.L.; Chan, J.M.C.; Tsang, O.T.Y.; Lam, B.H.S.; Chuang, V.W.M.; Chu, A.W.H.; Chan, W.M.; Ip, J.D.; et al. Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant by Sera from BNT162b2 or CoronaVac Vaccine Recipients. Clin. Infect. Dis. 2022, 75, e822–e826. [Google Scholar] [CrossRef]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; Santos de Oliviera, M.H.; Catahay, J.A.; Khan, S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef]
- Kertes, J.; Gez, S.B.; Saciuk, Y.; Supino-Rosin, L.; Stein, N.S.; Mizrahi-Reuveni, M.; Zohar, A.E. Effectiveness of mRNA BNT162b2 Vaccine 6 Months after Vaccination among Patients in Large Health Maintenance Organization, Israel. Emerg. Infect. Dis. 2022, 28, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Pollett, S.D.; Neerukonda, S.N.; Wang, W.; Wang, R.; Vassell, R.; Epsi, N.J.; Fries, A.C.; Agan, B.K.; Lindholm, D.A.; et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster-elicited serum but evades most convalescent serum and therapeutic antibodies. Sci. Transl. Med. 2022, 14, eabn8543. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.; Chen, M.; Bai, S.; Yuan, Q.; Wu, J. Immunogenicity of inactivated COVID-19 vaccines at different vaccination intervals. Hum. Vaccines Immunother. 2021, 17, 3310–3313. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.; Bruton, R.; Stephens, C.; Bentley, C.; Brown, K.; Amirthalingam, G.; Hallis, B.; Otter, A.; Zuo, J.; Moss, P. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 2022, 7, 14. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef]
- Del Rio, C.; Omer, S.B.; Malani, P.N. Winter of Omicron-The Evolving COVID-19 Pandemic. JAMA 2022, 327, 319–320. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, S.; Ansari, S.; Paweska, J.T.; Maurya, V.K.; Tripathi, A.K.; Abdel-Moneim, A.S. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J. Med. Virol. 2022, 94, 1738–1744. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Ellis, D.; Crawfold, K.H.D.; Dingens, A.S.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell 2020, 182, 1295–1310.e20. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wei, P.; Chen, Z.; Aviszus, K.; Yang, J.; Downing, W.; Jiang, C.; Liang, B.; Reynoso, L.; et al. The basis of a more contagious 501Y.V1 variant of SARS-COV-2. Cell Res. 2021, 31, 720–722. [Google Scholar] [CrossRef]
- Meng, B.; Kemp, S.A.; Papa, G.; Datir, R.; Ferreira, I.A.T.M.; Marelli, S.; Harvey, W.T.; Lytras, S.; Mohamed, A.; Gallo, G.; et al. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep. 2021, 35, 109292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, W.; Shi, H.; Yan, X.; Dong, K.; You, Q.; Zhong, G.; Gong, H.; Chen, Z.; Jit, M.; et al. Who should be prioritized for COVID-19 vaccination in China? A descriptive study. BMC Med. 2021, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Souto Ferreira, L.; Canton, O.; Da Silva, R.L.P.; Poloni, S.; Sudbrack, V.; Borges, M.E.; Franco, C.; Darcie Marquitti, F.M.; de Moraes, J.C.; de Sousa Mascena Veras, M.A.; et al. Assessing the best time interval between doses in a two-dose vaccination regimen to reduce the number of deaths in an ongoing epidemic of SARS-CoV-2. PLoS Comput. Biol. 2022, 18, e1009978. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Nourbakhsh, S.; Sah, P.; Fitzpatrick, M.C.; Galvani, A.P. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021, 19, e3001211. [Google Scholar] [CrossRef]
- Català, M.; Li, X.; Prats, C.; Prieto-Alhambra, D. The impact of prioritisation and dosing intervals on the effects of COVID-19 vaccination in Europe: An agent-based cohort model. Sci. Rep. 2021, 11, 18812. [Google Scholar] [CrossRef]
- Silva, P.; Sagastizábal, C.; Nonato, L.G.; Struchiner, C.J.; Pereira, T. Optimized delay of the second COVID-19 vaccine dose reduces ICU admissions. Proc. Natl. Acad. Sci. USA 2021, 118, e2104640118. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gümüş, S.; Bektöre, B.; Bozkurt, H.; Gözalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2022, 94, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Olariu, T.R.; Ursoniu, S.; Marincu, I.; Lupu, M.A. Dynamics of Antibody Response to BNT162b2 mRNA COVID-19 Vaccine: A 7-Month Follow-Up Study. Medicina 2021, 57, 1330. [Google Scholar] [CrossRef]
- Kitro, A.; Sirikul, W.; Thongkum, W.; Soponpong, S.; Yasamut, U.; Kiratipaisarl, W.; Kosai, A.; Kasinrerk, W.; Tayapiwatana, C.; Srithanaviboonchai, K. Dynamic of anti-spike receptor binding domain (RBD) levels and short-term adverse events following a heterologous booster dose of BNT162b2 after two doses of CoronaVac in Thai health care workers. Vaccine 2022, 40, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Phua, S.K.; Liang, Y.L.; Oh, M.; Aw, T.C. SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore. Vaccines 2022, 10, 331. [Google Scholar] [CrossRef]
| Positive Cases for SARS-CoV-2 | Case Group and Control Group of the Case–Control Study | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 273) | Vaccinated (n = 242) | Unvaccinated (n = 31) | p-Value | Overall (n = 1210) | Case (n = 242) | Control (n = 968) | p-Value | |
| Age | 0.250 | 0.194 | ||||||
| Mean (IQR) | 33.24 (23.0, 43.0) | 33.81 (24.0, 43.0) | 28.77 (3.0, 40.0) | 34.96 (25.0, 44.0) | 33.81 (24.0, 43.0) | 35.24 (26.0,45.0) | ||
| Age group | 0.004 | 1.000 | ||||||
| Age < 18 | 32 (11.7%) | 22 (9.1%) | 10 (32.3%) | 110 (9.1%) | 22 (9.1%) | 88(9.1%) | ||
| 18 ≤ Age ≤ 40 | 161 (59.0%) | 147 (60.7%) | 14 (45.2%) | 735 (60.7%) | 147 (60.7%) | 588 (60.7%) | ||
| Age > 60 | 80 (29.3%) | 73 (30.2%) | 7 (22.5%) | 365 (30.2%) | 73 (30.2%) | 292 (30.2%) | ||
| Gender | 0.303 | 0.708 | ||||||
| Male | 126 (46.2%) | 109 (45.0%) | 17 (54.8%) | 558 (46.1%) | 109 (45.0%) | 449 (46.4%) | ||
| Female | 147 (53.8%) | 133 (55.0%) | 14 (45.2%) | 652 (53.9%) | 133 (55.0%) | 519 (53.6%) | ||
| 1 Dose of a Vaccine | 2 Doses of a Vaccine | 3 Doses of a Vaccine | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case (n = 10) | Control (n = 40) | p-Value | Case (n = 94) | Control (n = 376) | p-Value | Case (n = 138) | Control (n = 552) | p-Value | |
| Age | |||||||||
| Mean (IQR) | 21.50 (18.8, 24.3) | 27.23 (25.0, 31.0) | 0.051 | 27.44 (18.0, 36.0) | 28.76 (18.0, 36.0) | 0.457 | 39.04 (28.0, 48.3) | 40.22 (29.0, 49.8) | 0.356 |
| Gender | |||||||||
| Male | 6 (60%) | 16 (40.0%) | 0.433 | 43 (45.7%) | 190 (50.5%) | 0.406 | 60 (43.5%) | 243 (44.0%) | 0.908 |
| Female | 4 (40%) | 24 (60.0%) | 51 (54.3%) | 186 (49.5%) | 78 (56.5%) | 309 (56.0%) | |||
| Time interval | |||||||||
| TT1(IQR) | - | - | - | 33.02 (22.0, 33.0) | 36.87 (23.3, 36.0) | 0.315 | 30.02 (23.0, 32.0) | 30.93 (24.0, 34.0) | 0.337 |
| TT2(IQR) | - | - | - | - | - | - | 199.78 (185.8, 212.0) | 201.97 (187.0, 213.0) | 0.480 |
| TT3(IQR) | 216.30 (213.5, 258.8) | 168.53 (34.9, 267.0) | 0.126 | 218.05 (179.0, 259.0) | 217.58 (190.0, 267.5) | 0.952 | 79.58 (42.0, 107.0) | 93.39 (53.3, 121.0) | 0.001 |
| TT4(IQR) | - | - | - | 251.07 (227.5, 281.8) | 254.45 (235.5, 304.0) | 0.644 | 309.38 (274.0, 327.0) | 326.28 (284.0, 355.8) | <0.001 |
| Case | Control | p-Value | One dose OR (95% CI) | |
|---|---|---|---|---|
| The whole population | ||||
| TT3 ≤ 60 days | 1 (10.0%) | 14 (35.0%) | Reference | - |
| TT3 > 60 days | 9 (90.0%) | 29 (65.0%) | 0.153 | 4.85 (0.56, 42.26) |
| Case | Control | p-Value | Two Doses OR (95% CI) | |
|---|---|---|---|---|
| The whole population | ||||
| TT1 ≤ 30 days | 61 (64.9%) | 212(56.4%) | Reference | - |
| TT1 > 30 days | 33 (35.1%) | 164 (43.6%) | 0.136 | 0.70 (0.44, 1.12) |
| TT3 ≤ 120 days | 8 (8.5%) | 46 (12.2%) | Reference | - |
| TT3 > 120 days | 86 (91.5%) | 330 (87.8%) | 0.314 | 1.50 (0.68, 3.29) |
| TT4 ≤ 180 days | 18 (19.1%) | 85 (22.6%) | Reference | - |
| TT4 > 180 days | 76 (80.9%) | 291 (77.4%) | 0.469 | 1.23 (0.67, 2.18) |
| Age < 18 | ||||
| TT1 ≤ 30 days | 15 (71.4%) | 62 (73.8%) | Reference | - |
| TT1 > 30 days | 6 (28.6%) | 22 (26.2%) | 0.825 | 1.13 (0.39, 3.27) |
| TT3 ≤ 120 days | 3 (14.3%) | 29 (34.5%) | Reference | - |
| TT3 > 120 days | 18 (85.7%) | 55 (65.5%) | 0.083 | 3.16 (0.86, 11.64) |
| TT4 ≤ 180 days | 15 (71.4%) | 70 (83.3%) | Reference | - |
| TT4 > 180 days | 6 (28.6%) | 14(16.7 %) | 0.220 | 2.00 (0.66, 6.05) |
| 18 ≤ Age ≤ 40 | ||||
| TT1 ≤ 30 days | 36 (62.1%) | 108(46.6%) | Reference | - |
| TT1 > 30 days | 22 (37.9%) | 124 (53.4%) | 0.036 | 0.53 (0.30, 0.96) |
| TT3 ≤ 120 days | 5 (8.6%) | 13 (5.6%) | Reference | - |
| TT3 > 120 days | 53 (91.4%) | 219 (94.4%) | 0.398 | 0.63 (0.22, 1.84) |
| TT4 ≤ 180 days | 3 (5.2%) | 12 (5.2%) | Reference | - |
| TT4 > 180 days | 55 (94.8%) | 220 (94.8%) | 1.000 | 1.00 (0.27, 3.67) |
| Age > 40 | ||||
| TT1 ≤ 30 days | 10 (66.7%) | 42 (70.0%) | Reference | - |
| TT1 > 30 days | 5 (33.3%) | 18 (30.0%) | 0.802 | 1.17 (0.35, 3.90) |
| TT3 ≤ 120 days | 0 (0.0%) | 4 (6.7%) | - | - |
| TT3 > 120 days | 15 (100.0%) | 56 (93.3%) | - | - |
| TT4 ≤ 180 days | 0 (0.0%) | 3 (5.0%) | - | - |
| TT4 > 180 days | 15 (100.0%) | 57 (95.0%) | - | - |
| Male | ||||
| TT1 ≤ 30 days | 25 (58.1%) | 96 (50.5%) | Reference | - |
| TT1 > 30 days | 18 (41.9%) | 94 (49.5%) | 0.368 | 0.74 (0.38, 1.44) |
| TT3 ≤ 120 days | 4 (9.3%) | 21 (11.1%) | Reference | - |
| TT3 > 120 days | 39 (90.7%) | 169 (88.9%) | 0.738 | 1.21 (0.39, 3.73) |
| TT4 ≤ 180 days | 8 (18.6%) | 39 (20.5%) | Reference | - |
| TT4 > 180 days | 35 (81.4%) | 151 (81.5%) | 0.777 | 1.13 (0.49, 2.63) |
| Female | ||||
| TT1 ≤ 30 days | 36 (70.6%) | 116 (62.4%) | Reference | - |
| TT1 > 30 days | 15 (29.4%) | 70 (37.6%) | 0.280 | 0.69 (0.35, 1.35) |
| TT3 ≤ 120 days | 4 (7.8%) | 25 (13.4%) | Reference | - |
| TT3 > 120 days | 47 (92.2%) | 161 (86.6%) | 0.286 | 1.83 (0.61, 5.51) |
| TT4 ≤ 180 days | 10 (19.6%) | 46 (24.7%) | Reference | - |
| TT4 > 180 days | 41 (80.4%) | 140 (75.3%) | 0.447 | 1.35 (0.63, 2.90) |
| Case | Control | p-Value | Three Doses OR (95% CI) | |
|---|---|---|---|---|
| The whole population | ||||
| TT1 ≤ 30 days | 92 (66.7%) | 386 (61.1%) | Reference | - |
| TT1 > 30 days | 46 (33.3%) | 246 (38.9%) | 0.221 | 0.79 (0.53, 1.16) |
| TT2 ≤ 210 days | 100 (72.5%) | 458 (72.5%) | Reference | - |
| TT2 > 210 days | 38 (27.5%) | 174 (27.5%) | 0.999 | 1.00 (0.67, 1.51) |
| TT3 ≤ 60 days | 48 (34.8%) | 182 (28.8%) | Reference | - |
| TT3 > 60 days | 90 (65.2%) | 450 (71.2%) | 0.165 | 0.79 (0.51, 1.12) |
| TT4 ≤ 300 days | 48(34.8%) | 167 (26.4%) | Reference | - |
| TT4 > 300 days | 90 (65.2%) | 465 (73.6%) | 0.048 | 0.67 (0.46, 0.99) |
| 18 ≤ Age ≤ 40 | ||||
| TT1 ≤30 days | 48 (60.0%) | 233 (58.3%) | Reference | - |
| TT1 > 30 days | 32 (40.0%) | 167 (41.8%) | 0.772 | 0.93 (0.57, 1.52) |
| TT2 ≤ 210 days | 52 (65.0%) | 285 (71.3%) | Reference | - |
| TT2 > 210 days | 28 (35.0%) | 115 (28.8%) | 0.266 | 1.33 (0.80, 2.22) |
| TT3 ≤ 60 days | 27 (33.8%) | 104 (26.0%) | Reference | - |
| TT3 > 60 days | 53 (66.3%) | 296 (74.0%) | 0.157 | 0.69 (0.41, 1.15) |
| TT4 ≤ 300 days | 21 (26.3%) | 85 (21.3%) | Reference | - |
| TT4 > 300 days | 59 (73.8%) | 315 (78.8%) | 0.326 | 0.76 (0.44, 1.32) |
| Age > 40 | ||||
| TT1 ≤ 30 days | 44 (75.9%) | 153 (65.9%) | Reference | - |
| TT1 > 30 days | 14 (24.1%) | 79 (34.1%) | 0.150 | 0.62 (0.32, 1.19) |
| TT2 ≤ 210 days | 48 (82.8%) | 173 (74.6%) | Reference | - |
| TT2 > 210 days | 10 (17.2%) | 59 (25.4%) | 0.193 | 0.61 (0.29, 1.28) |
| TT3 ≤ 60 days | 21 (36.2%) | 78 (33.6%) | Reference | - |
| TT3 > 60 days | 37 (63.8%) | 154 (66.4%) | 0.710 | 0.89 (0.50, 1.63) |
| TT4 ≤ 300 days | 27 (46.6%) | 82 (35.3%) | Reference | - |
| TT4 > 300 days | 31 (53.4%) | 150 (64.7%) | 0.117 | 0.62 (0.35, 1.12) |
| Male | ||||
| TT1 ≤ 30 days | 38 (63.3%) | 164 (55.6%) | Reference | - |
| TT1 > 30 days | 22 (36.7%) | 131 (44.4%) | 0.271 | 0.73 (0.41, 1.29) |
| TT2 ≤ 210 days | 48 (80.0%) | 216 (73.2%) | Reference | - |
| TT2 > 210 days | 12 (20.0%) | 79 (26.8%) | 0.275 | 0.68 (0.35, 1.35) |
| TT3 ≤ 60 days | 19 (31.7%) | 79 (26.8%) | Reference | - |
| TT3 > 60 days | 41 (68.3%) | 216 (73.2%) | 0.441 | 0.80 (0.43, 1.44) |
| TT4 ≤ 300 days | 21 (35.0%) | 77 (26.1%) | Reference | - |
| TT4 > 300 days | 39 (65.0%) | 218 (73.9%) | 0.162 | 0.66 (0.36, 1.18) |
| Female | ||||
| TT1 ≤ 30 days | 54 (69.2%) | 222 (65.9%) | Reference | - |
| TT1 > 30 days | 24 (30.8%) | 115 (34.1%) | 0.572 | 0.86 (0.51, 1.50) |
| TT2 ≤ 210 days | 52 (66.7%) | 242 (71.8%) | Reference | - |
| TT2 > 210 days | 26 (33.3%) | 95 (28.2%) | 0.368 | 1.27 (0.75, 2.16) |
| TT3 ≤ 60 days | 29 (37.2%) | 103 (30.6%) | Reference | - |
| TT3 > 60 days | 49 (62.8%) | 234 (69.4%) | 0.259 | 0.74 (0.45, 1.24) |
| TT4 ≤ 300 days | 27 (34.6%) | 90 (26.7%) | Reference | - |
| TT4 > 300 days | 51 (65.4%) | 247 (73.3%) | 0.163 | 0.69 (0.41, 1.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Guo, T.; Zhong, J.; Fang, C.; Xiong, H.; Hu, Z.; Zhu, Y.; Tan, J.; Liu, S.; Jing, Q.; et al. Effect of Vaccination Time Intervals on SARS-COV-2 Omicron Variant Strain Infection in Guangzhou: A Real-World Matched Case–Control Study. Vaccines 2022, 10, 1855. https://doi.org/10.3390/vaccines10111855
Li Y, Guo T, Zhong J, Fang C, Xiong H, Hu Z, Zhu Y, Tan J, Liu S, Jing Q, et al. Effect of Vaccination Time Intervals on SARS-COV-2 Omicron Variant Strain Infection in Guangzhou: A Real-World Matched Case–Control Study. Vaccines. 2022; 10(11):1855. https://doi.org/10.3390/vaccines10111855
Chicago/Turabian StyleLi, Yufen, Tong Guo, Jiayi Zhong, Chuanjun Fang, Husheng Xiong, Zengyun Hu, Yajuan Zhu, Jinlin Tan, Shuang Liu, Qinlong Jing, and et al. 2022. "Effect of Vaccination Time Intervals on SARS-COV-2 Omicron Variant Strain Infection in Guangzhou: A Real-World Matched Case–Control Study" Vaccines 10, no. 11: 1855. https://doi.org/10.3390/vaccines10111855
APA StyleLi, Y., Guo, T., Zhong, J., Fang, C., Xiong, H., Hu, Z., Zhu, Y., Tan, J., Liu, S., Jing, Q., & Zhang, D. (2022). Effect of Vaccination Time Intervals on SARS-COV-2 Omicron Variant Strain Infection in Guangzhou: A Real-World Matched Case–Control Study. Vaccines, 10(11), 1855. https://doi.org/10.3390/vaccines10111855






