Seroprevalence of Measles Antibodies in a Highly MMR-Vaccinated Population
Abstract
:1. Introduction
2. Methods
2.1. Biobank Serum Samples
2.2. Measurement of Measles-Specific IgG
2.3. Statistical Analysis
3. Results
3.1. Demographics of Study Cohort
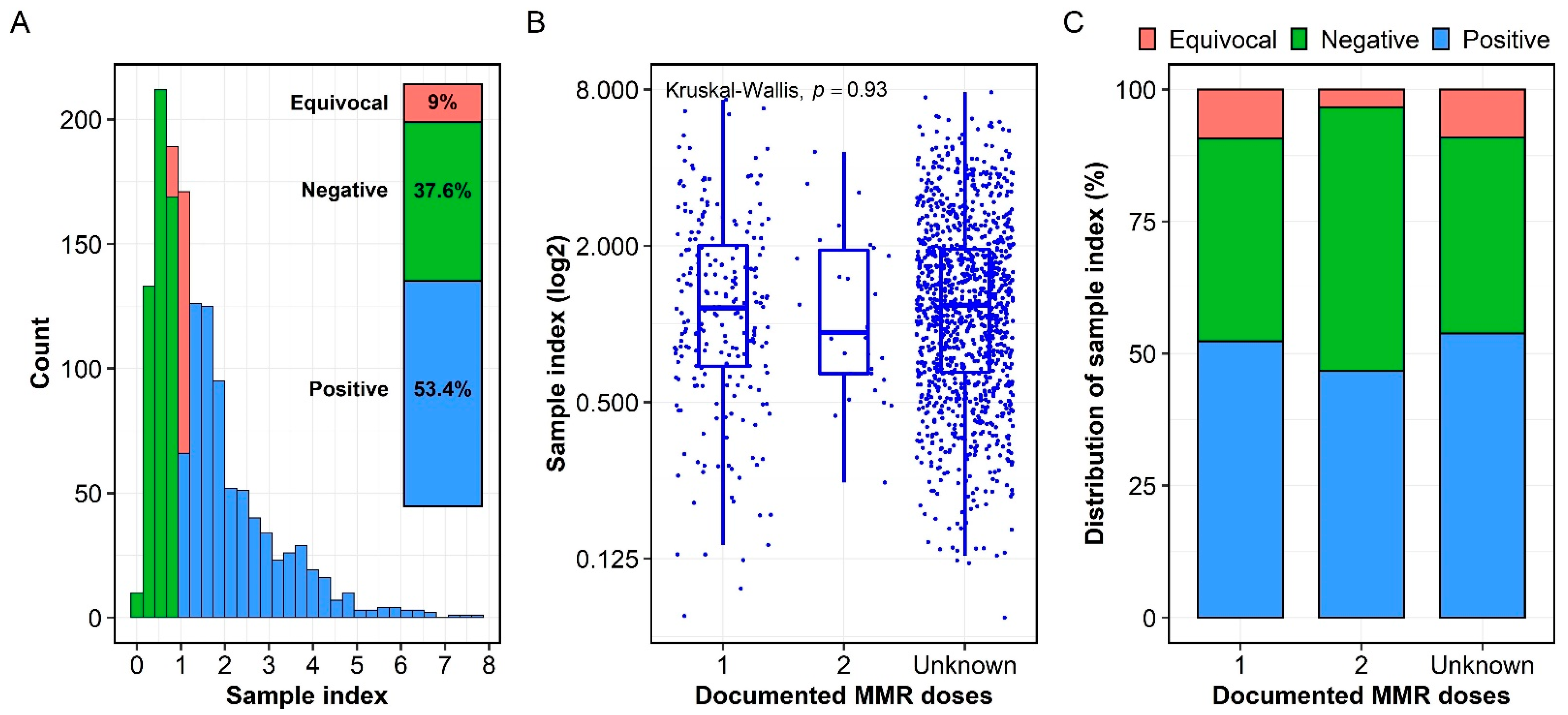
3.2. Seroprevalence of Measles-Specific IgG
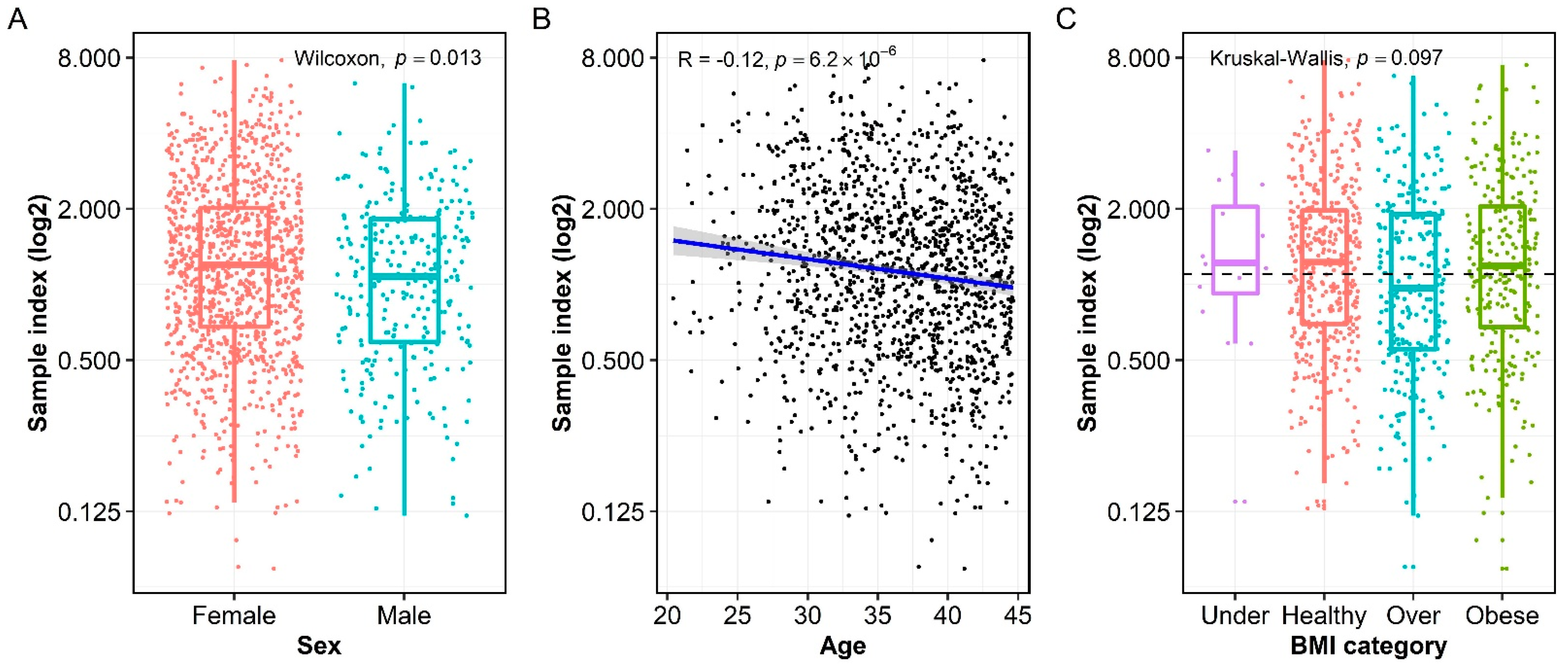
3.3. Association of Measles-Specific IgG with Age, Sex, and BMI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloch, A.B.; Orenstein, W.A.; Stetler, H.C.; Wassilak, S.G.; Amler, R.W.; Bart, K.J.; Kirby, C.D.; Hinman, A.R. Health impact of measles vaccination in the United States. Pediatrics 1985, 76, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.L.; Hinman, A.R. Summary and conclusions: Measles elimination meeting, 16–17 March 2000. J. Infect. Dis. 2004, 189 (Suppl. 1), S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Lee, A.D.; Clemmons, N.S.; Redd, S.B.; Poser, S.; Blog, D.; Zucker, J.R.; Leung, J.; Link-Gelles, R.; Pham, H.; et al. National Update on Measles Cases and Outbreaks—United States, January 1–October 1, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.G.; Ferrari, M.; Antoni, S.; Li, X.; Portnoy, A.; Lambert, B.; Hauryski, S.; Hatcher, C.; Nedelec, Y.; Patel, M.; et al. Progress Toward Regional Measles Elimination—Worldwide, 2000–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Durrheim, D.N.; Andrus, J.K.; Tabassum, S.; Bashour, H.; Githanga, D.; Pfaff, G. A dangerous measles future looms beyond the COVID-19 pandemic. Nat. Med. 2021, 27, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Gaythorpe, K.A.; Abbas, K.; Huber, J.; Karachaliou, A.; Thakkar, N.; Woodruff, K.; Li, X.; Echeverria-Londono, S.; Ferrari, M.; Jackson, M.L.; et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. Elife 2021, 10, e67023. [Google Scholar] [CrossRef]
- Dine, M.S.; Hutchins, S.S.; Thomas, A.; Williams, I.; Bellini, W.J.; Redd, S.C. Persistence of vaccine-induced antibody to measles 26–33 years after vaccination. J. Infect. Dis. 2004, 189, S123–S130. [Google Scholar]
- Ramsay, M.; Moffatt, D.; O’connor, M. Measles vaccine: A 27-year follow-up. Epidemiol. Infect. 1994, 112, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Schenk, J.; Abrams, S.; Theeten, H.; Van Damme, P.; Beutels, P.; Hens, N. Immunogenicity and persistence of trivalent measles, mumps, and rubella vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 286–295. [Google Scholar] [CrossRef]
- Bolotin, S.; Osman, S.; Hughes, S.L.; Ariyarajah, A.; Tricco, A.C.; Khan, S.; Li, L.; Johnson, C.; Friedman, L.; Gul, N.; et al. In Elimination Settings, Measles Antibodies Wane Following Vaccination but Not Following Infection—A Systematic Review and Meta-Analysis. J. Infect. Dis. 2022, 226, 1127–1139. [Google Scholar] [CrossRef]
- Crooke, S.N.; Haralambieva, I.H.; Grill, D.E.; Ovsyannikova, I.G.; Kennedy, R.B.; Poland, G.A. Seroprevalence and durability of rubella virus antibodies in a highly immunized population. Vaccine 2019, 37, 3876–3882. [Google Scholar] [CrossRef]
- Chen, R.T.; Markowitz, L.E.; Albrecht, P.; Stewart, J.A.; Mofenson, L.M.; Preblud, S.R.; Orenstein, W.A. Measles antibody: Reevaluation of protective titers. J. Infect. Dis. 1990, 162, 1036–1042. [Google Scholar] [CrossRef]
- Cohn, M.L.; Robinson, E.D.; Faerber, M.; Thomas, D.; Geyer, S.; Peters, S.; Martin, M.; Martin, A.; Sobel, D.; Jones, R. Measles vaccine failures: Lack of sustained measles-specific immunoglobulin G responses in revaccinated adolescents and young adults. Pediatr. Infect. Dis. J. 1994, 13, 34–38. [Google Scholar] [CrossRef]
- Rosen, J.B.; Rota, J.S.; Hickman, C.J.; Sowers, S.B.; Mercader, S.; Rota, P.A.; Bellini, W.J.; Huang, A.J.; Doll, M.K.; Zucker, J.R. Outbreak of measles among persons with prior evidence of immunity, New York City, 2011. Clin. Infect. Dis. 2014, 58, 1205–1210. [Google Scholar] [CrossRef]
- McLean, H.Q.; Fiebelkorn, A.P.; Temte, J.L.; Wallace, G.S. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 2013, 62, 1–34. [Google Scholar]
- Seagle, E.E.; Bednarczyk, R.A.; Hill, T.; Fiebelkorn, A.P.; Hickman, C.J.; Icenogle, J.P.; Belongia, E.A.; McLean, H.Q. Measles, mumps, and rubella antibody patterns of persistence and rate of decline following the second dose of the MMR vaccine. Vaccine 2018, 36, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Guerra, F.M.; Crowcroft, N.S.; Friedman, L.; Deeks, S.L.; Halperin, S.A.; Severini, A.; Hatchette, T.F.; Bolotin, S. Waning of measles maternal antibody in infants in measles elimination settings—A systematic literature review. Vaccine 2018, 36, 1248–1255. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Bolotin, S.; Severini, A.; Hatchette, T.; McLachlan, E.; Savage, R.; Hughes, S.L.; Wang, J.; Deeks, S.L.; Wilson, S.; Brisson, M.; et al. Assessment of population immunity to measles in Ontario, Canada: A Canadian Immunization Research Network (CIRN) study. Hum. Vaccin. Immunother. 2019, 15, 2856–2864. [Google Scholar] [CrossRef] [Green Version]
- Blaizot, S.; Herzog, S.A.; Abrams, S.; Theeten, H.; Litzroth, A.; Hens, N. Sample size calculation for estimating key epidemiological parameters using serological data and mathematical modelling. BMC Med. Res. Methodol. 2019, 19, 51. [Google Scholar] [CrossRef] [Green Version]
- Green, M.S.; Shohat, T.; Lerman, Y.; Cohen, D.; Slepon, R.; Duvdevani, P.; Varsano, N.; Dagan, R.; Mendelson, E. Sex differences in the humoral antibody response to live measles vaccine in young adults. Int. J. Epidemiol. 1994, 23, 1078–1081. [Google Scholar] [CrossRef]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Pan, C.H.; Adams, R.J.; Laube, B.L.; Griffin, D.E. Vaccine-induced measles virus-specific T cells do not prevent infection or disease but facilitate subsequent clearance of viral RNA. mBio 2014, 5, e01047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlow, M.A.; Marin, M.; Moore, K.; Patel, M. CDC Guidance for Use of a Third Dose of MMR Vaccine During Mumps Outbreaks. J. Public Health Manag. Pract. 2020, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.Q.; Chen, J.; Monroe, J.M.; Ratishvili, T.; Warner, N.D.; Grill, D.E.; Haralambieva, I.H.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. The influence of sex, BMI, and age on cellular and humoral immune responses against measles after a 3rd dose of MMR vaccine. J. Infect. Dis. 2022, jiac351. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quach, H.Q.; Ovsyannikova, I.G.; Grill, D.E.; Warner, N.D.; Poland, G.A.; Kennedy, R.B. Seroprevalence of Measles Antibodies in a Highly MMR-Vaccinated Population. Vaccines 2022, 10, 1859. https://doi.org/10.3390/vaccines10111859
Quach HQ, Ovsyannikova IG, Grill DE, Warner ND, Poland GA, Kennedy RB. Seroprevalence of Measles Antibodies in a Highly MMR-Vaccinated Population. Vaccines. 2022; 10(11):1859. https://doi.org/10.3390/vaccines10111859
Chicago/Turabian StyleQuach, Huy Quang, Inna G. Ovsyannikova, Diane E. Grill, Nathaniel D. Warner, Gregory A. Poland, and Richard B. Kennedy. 2022. "Seroprevalence of Measles Antibodies in a Highly MMR-Vaccinated Population" Vaccines 10, no. 11: 1859. https://doi.org/10.3390/vaccines10111859





