Codon Usage and Context Analysis of Genes Modulated during SARS-CoV-2 Infection and Dental Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Retrieval
2.2. Compositional Analysis
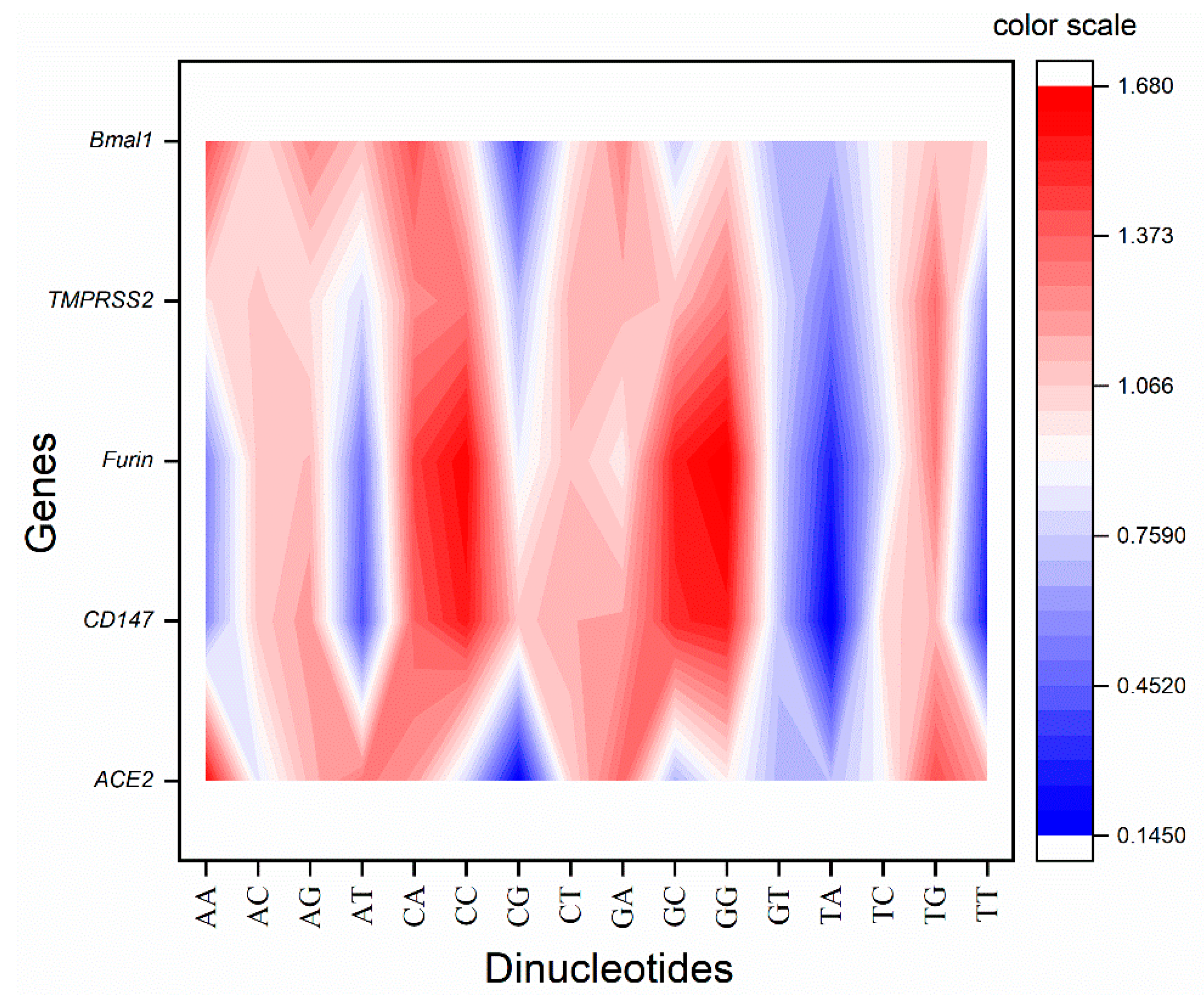
2.3. Dinucleotide Odds Ratio Analysis
2.4. Relative Synonymous Codon Usage (RSCU)
2.5. Rare Codon Analysis
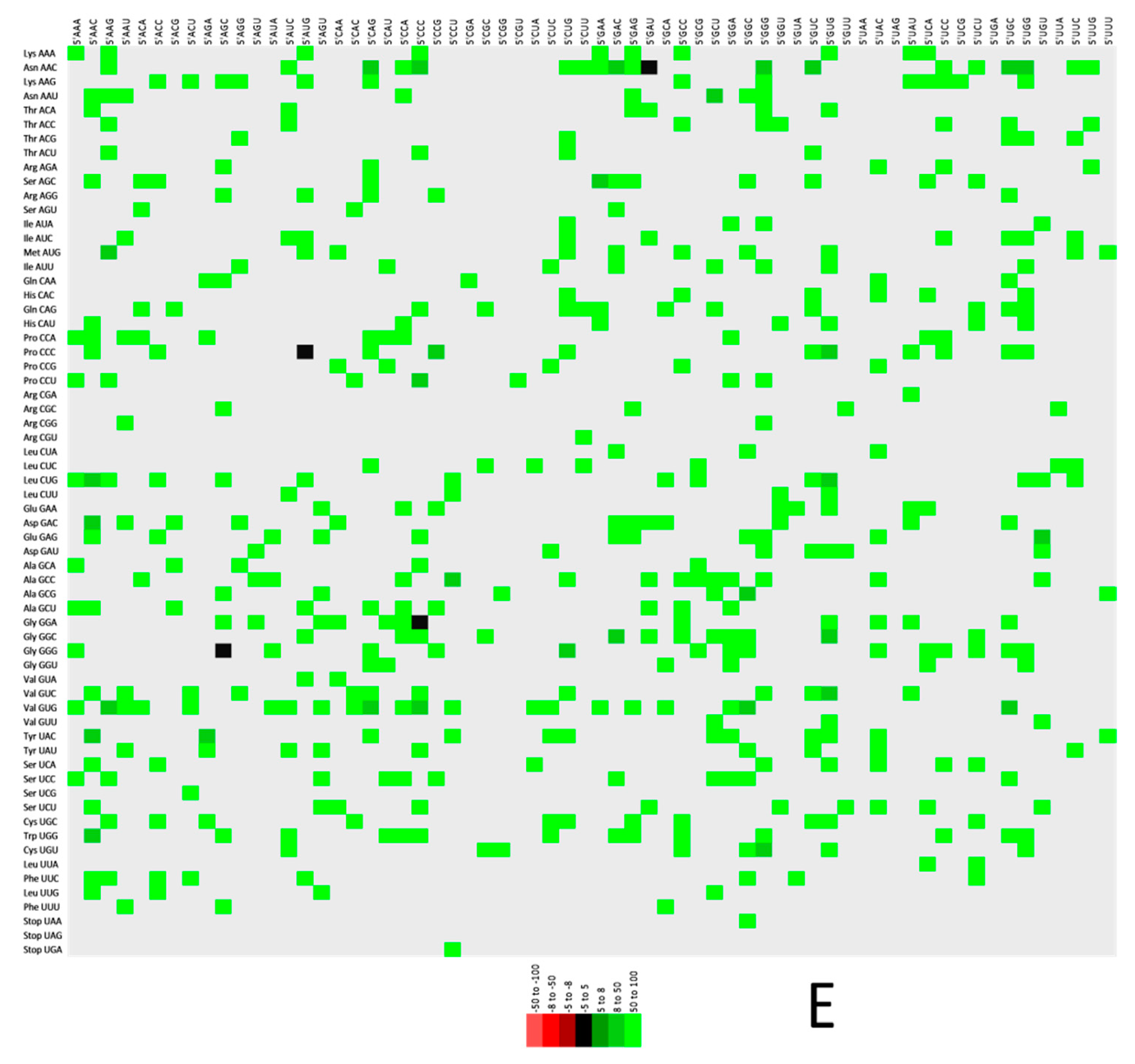
2.6. Codon Context Analysis
3. Results
3.1. Compositional Analysis
3.2. Odds Ratio Revealed Two Distinct Odds Ratio Pattern Sets
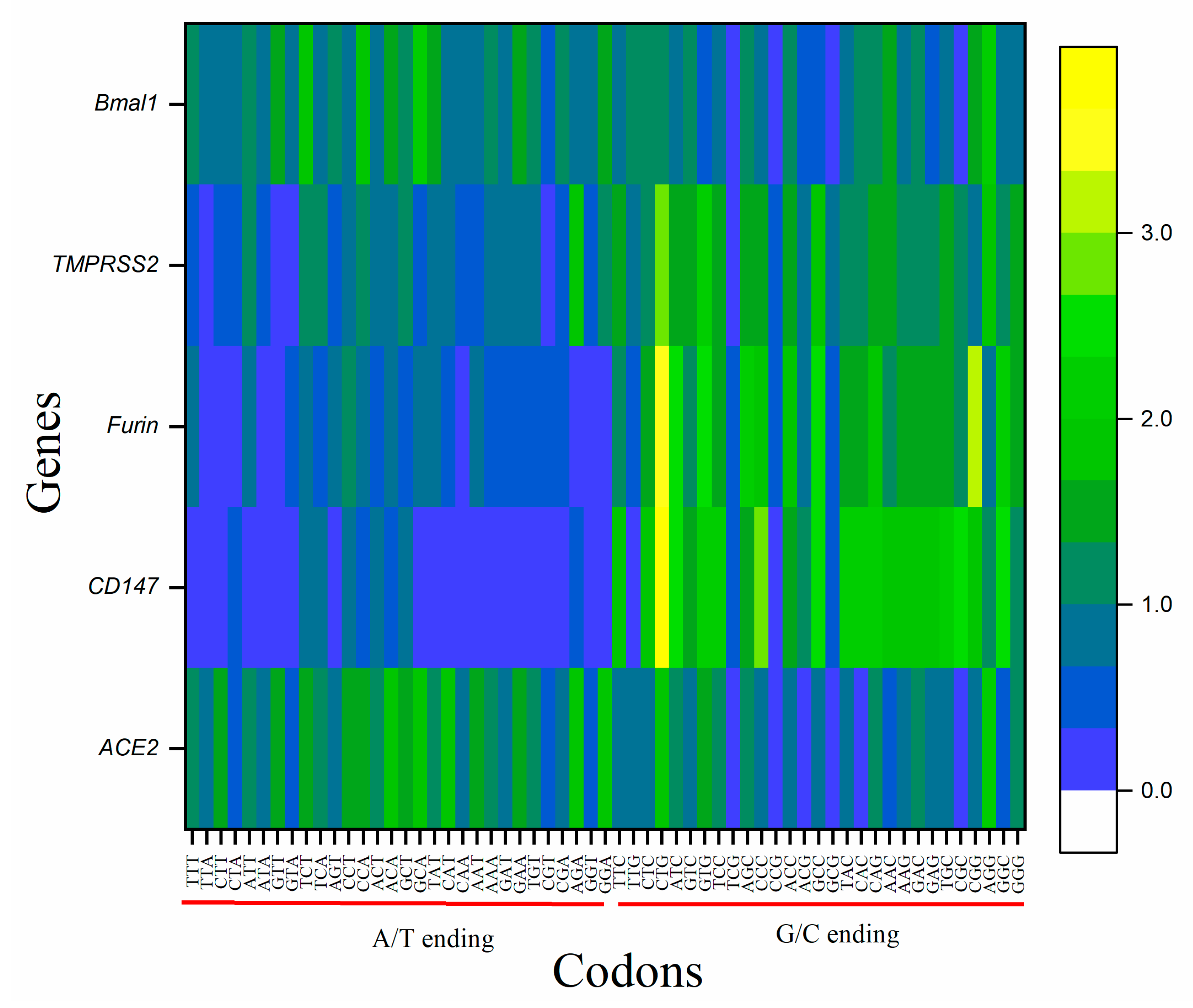
3.3. RSCU Analysis Revealed G/C Ending Codons Preference in CD147, FURIN, and TMPRSS2 Gene Set While A/T Ending Codons in ACE2 and BMAL1
3.4. Codons CGT, TCG and CTA Are Rarely Used in All the Genes
3.5. Codon Context Analysis Revealed Val (GTG) Initiated Codon Pair Abundance in CD147, FURIN, and TMPRSS2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020, 81, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, J.; Xu, Y.; Chen, X. Viral dynamics of SARS-CoV-2 in saliva from infected patients. J. Infect. 2020, 81, e48–e50. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 2019, 93, e01815–e01818. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Peng, R.; Wu, L.-A.; Wang, Q.; Qi, J.; Gao, G.F. Cell entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef]
- Qiao, J.; Li, W.; Bao, J.; Peng, Q.; Wen, D.; Wang, J.; Sun, B. The expression of SARS-CoV-2 receptor ACE2 and CD147, and protease TMPRSS2 in human and mouse brain cells and mouse brain tissues. Biochem. Biophys. Res. Commun. 2020, 533, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Dobrindt, K.; Hoagland, D.A.; Seah, C.; Kassim, B.; O’Shea, C.P.; Iskhakova, M.; Fernando, M.B.; Deans, P.J.M.; Powell, S.K.; Javidfar, B.; et al. Common genetic variation in humans impacts in vitro susceptibility to SARS-CoV-2 infection. Biorxiv 2021, 16, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Marruganti, C.; Sanz, M.; Aimetti, M.; Romandini, M. Periodontitis and COVID-19: Biological Mechanisms and Meta-analyses of Epidemiological Evidence. J. Dent. Res. 2022, 11, 1430–1440. [Google Scholar] [CrossRef]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case-control study. J. Clin. Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Sehirli, A.Ö.; Chukwunyere, U.; Aksoy, U.; Sayiner, S.; Abacioglu, N. The circadian clock gene BMAL1: Role in COVID-19 and periodontitis. Chronobiol. Int. 2021, 38, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Pan, S.; Zhang, L.; He, L.; Niu, Y. Paeonol attenuates ligation-induced periodontitis in rats by inhibiting osteoclastogenesis via regulating Nrf2/NF-κB/NFATc1 signaling pathway. Biochimie 2019, 156, 129–137. [Google Scholar] [CrossRef]
- Cai, W.; Marouf, N.; Said, K.N.; Tamimi, F. Nature of the Interplay Between Periodontal Diseases and COVID-19. Front. Dent. Med. 2021, 2, 735126. [Google Scholar] [CrossRef]
- Senapati, S.; Kumar, S.; Singh, A.K.; Banerjee, P.; Bhagavatula, S. Assessment of risk conferred by coding and regulatory variations of TMPRSS2 and CD26 in susceptibility to SARS-CoV-2 infection in human. J. Genet. 2020, 99, 53. [Google Scholar] [CrossRef]
- Manna, P.M.L.; Costa, J.E.; Gomez, R.S. CD26 Immuno-Expression and Periodontal Disease Progression. J. Biomed. Biotechnol. 2001, 1, 91–94. [Google Scholar] [CrossRef]
- Bakthavatchalu, V.; Meka, A.; Mans, J.J.; Sathishkumar, S.; Lopez, M.C.; Bhattacharyya, I.; Boyce, B.F.; Baker, H.V.; Lamont, R.J.; Ebersole, J.L.; et al. Polymicrobial periodontal pathogen transcriptomes in calvarial bone and soft tissue. Mol. Oral Microbiol. 2011, 26, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Prelli Bozzo, C.; Nchioua, R.; Volcic, M.; Koepke, L.; Krüger, J.; Schütz, D.; Heller, S.; Stürzel, C.M.; Kmiec, D.; Conzelmann, C.; et al. IFITM proteins promote SARS-CoV-2 infection and are targets for virus inhibition in vitro. Nat. Commun. 2021, 12, 4584. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Bloch, E.M.; Pirenne, F.; Al-Riyami, A.Z.; Crowe, E.; Dau, L.; Land, K.; Townsend, M.; Jecko, T.; Rahimi-Levene, N.; et al. ABO blood group and COVID-19: A review on behalf of the ISBT COVID-19 Working Group. Vox Sang. 2021, 116, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Concolino, P.; Cecchetti, F.; D’Autilia, C.; Santonocito, C.; Di Stasio, E.; Zuppi, C.; Arcuri, C.; Deli, G.; Giardina, B.; Capoluongo, E.; et al. Association of periodontitis with GSTM1/GSTT1-null variants—A pilot study. Clin. Biochem. 2007, 40, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu Batur, L.; Hekim, N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 2021, 93, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Hingorjo, M.R.; Mumtaz, M.; Qureshi, M.A. The relationship of 1,25-dihydroxyvitamin D and Vitamin D binding protein in periodontitis. Pak. J. Med. Sci. 2019, 35, 847–851. [Google Scholar] [CrossRef]
- Abbas, M.; Verma, S.; Verma, S.; Siddiqui, S.; Khan, F.H.; Raza, S.T.; Siddiqi, Z.; Eba, A.; Mahdi, F. Association of GSTM1 and GSTT1 gene polymorphisms with COVID-19 susceptibility and its outcome. J. Med. Virol. 2021, 93, 5446–5451. [Google Scholar] [CrossRef]
- Hitti, F.L.; Yang, A.I.; Gonzalez-Alegre, P.; Baltuch, G.H. Human gene therapy approaches for the treatment of Parkinson’s disease: An overview of current and completed clinical trials. Park. Relat. Disord. 2019, 66, 16–24. [Google Scholar] [CrossRef]
- Muramatsu, S.; Fujimoto, K.; Kato, S.; Mizukami, H.; Asari, S.; Ikeguchi, K.; Kawakami, T.; Urabe, M.; Kume, A.; Sato, T.; et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol. Ther. 2010, 18, 1731–1735. [Google Scholar] [CrossRef]
- Watts, J.K.; Corey, D.R. Silencing disease genes in the laboratory and the clinic. J. Pathol. 2012, 226, 365–379. [Google Scholar] [CrossRef]
- Chakraborty, S.; Deb, B.; Barbhuiya, P.A.; Uddin, A. Analysis of codon usage patterns and influencing factors in Nipah virus. Virus Res. 2019, 263, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [PubMed]
- Quax, T.E.F.; Claassens, N.J.; Söll, D.; van der Oost, J. Codon Bias as a Means to Fine-Tune Gene Expression. Mol. Cell 2015, 59, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, I.; Lajoie, M.J.; Gregg, C.J.; Hornung, G.; Church, G.M.; Pilpel, Y. Codon usage of highly expressed genes affects proteome-wide translation efficiency. Proc. Natl. Acad. Sci. USA 2018, 115, E4940–E4949. [Google Scholar] [CrossRef]
- Zhang, G.; Hubalewska, M.; Ignatova, Z. Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nat. Struct. Mol. Biol. 2009, 16, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, P.; Shah, P.; Rokas, A.; Liu, Y.; Johnson, C.H. Non-optimal codon usage is a mechanism to achieve circadian clock conditionality. Nature 2013, 495, 116–120. [Google Scholar] [CrossRef]
- Frenkel-Morgenstern, M.; Danon, T.; Christian, T.; Igarashi, T.; Cohen, L.; Hou, Y.-M.; Jensen, L.J. Genes adopt non-optimal codon usage to generate cell cycle-dependent oscillations in protein levels. Mol. Syst. Biol. 2012, 8, 572. [Google Scholar] [CrossRef]
- Di Giallonardo, F.; Schlub, T.E.; Shi, M.; Holmes, E.C. Dinucleotide Composition in Animal RNA Viruses Is Shaped More by Virus Family than by Host Species. J. Virol. 2017, 91, e02381-16. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Deng, H.; Gu, T.; Xu, J.; Ou, J.; Jiang, Z.; Jiao, Y.; Zou, T.; Wang, C. Characterization of the porcine epidemic diarrhea virus codon usage bias. Infect. Genet. Evol. 2014, 28, 95–100. [Google Scholar] [CrossRef]
- Clarke, T.F.; Clark, P.L. Rare codons cluster. PLoS ONE 2008, 3, e3412. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, X.; Buske, P.J.; Suzich, J.A.; Jin, H. Attenuate Newcastle disease virus by codon modification of the glycoproteins and phosphoprotein genes. Virology 2019, 528, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Moura, G.; Pinheiro, M.; Arrais, J.; Gomes, A.C.; Carreto, L.; Freitas, A.; Oliveira, J.L.; Santos, M.A.S. Large scale comparative codon-pair context analysis unveils general rules that fine-tune evolution of mRNA primary structure. PLoS ONE 2007, 2, e847. [Google Scholar] [CrossRef] [PubMed]
- Moura, G.; Pinheiro, M.; Silva, R.; Miranda, I.; Afreixo, V.; Dias, G.; Freitas, A.; Oliveira, J.L.; Santos, M.A. Comparative context analysis of codon pairs on an ORFeome scale. Genome Biol. 2005, 6, R28. [Google Scholar] [CrossRef]
- Presnyak, V.; Alhusaini, N.; Chen, Y.-H.; Martin, S.; Morris, N.; Kline, N.; Olson, S.; Weinberg, D.; Baker, K.E.; Graveley, B.R.; et al. Codon optimality is a major determinant of mRNA stability. Cell 2015, 160, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Li, W.H. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef]
- Kramer, E.B.; Farabaugh, P.J. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA 2007, 13, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Chaney, J.L.; Steele, A.; Carmichael, R.; Rodriguez, A.; Specht, A.T.; Ngo, K.; Li, J.; Emrich, S.; Clark, P.L. Widespread position-specific conservation of synonymous rare codons within coding sequences. PLoS Comput. Biol. 2017, 13, e1005531. [Google Scholar] [CrossRef]
- Gutman, G.A.; Hatfield, G.W. Nonrandom utilization of codon pairs in Escherichia coli. Proc. Natl. Acad. Sci. USA 1989, 86, 3699–3703. [Google Scholar] [CrossRef]
- Groenke, N.; Trimpert, J.; Merz, S.; Conradie, A.M.; Wyler, E.; Zhang, H.; Hazapis, O.-G.; Rausch, S.; Landthaler, M.; Osterrieder, N.; et al. Mechanism of Virus Attenuation by Codon Pair Deoptimization. Cell Rep. 2020, 31, 107586. [Google Scholar] [CrossRef]
- Tulloch, F.; Atkinson, N.J.; Evans, D.J.; Ryan, M.D.; Simmonds, P. RNA virus attenuation by codon pair deoptimisation is an artefact of increases in CpG/UpA dinucleotide frequencies. Elife 2014, 3, e04531. [Google Scholar] [CrossRef]
- Le Nouën, C.; Luongo, C.L.; Yang, L.; Mueller, S.; Wimmer, E.; DiNapoli, J.M.; Collins, P.L.; Buchholz, U.J. Optimization of the Codon Pair Usage of Human Respiratory Syncytial Virus Paradoxically Resulted in Reduced Viral Replication In Vivo and Reduced Immunogenicity. J. Virol. 2020, 94, e01296-19. [Google Scholar] [CrossRef] [PubMed]
- Mauro, V.P.; Chappell, S.A. A critical analysis of codon optimization in human therapeutics. Trends Mol. Med. 2014, 20, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Wheelan, S.J.; Yarrington, R.M.; Boeke, J.D. GeneDesign: Rapid, automated design of multikilobase synthetic genes. Genome Res. 2006, 16, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, A.; Ness, J.E.; Gustafsson, C.; Minshull, J.; Govindarajan, S. Gene Designer: A synthetic biology tool for constructing artificial DNA segments. BMC Bioinform. 2006, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Punde, N.; Kooken, J.; Leary, D.; Legler, P.M.; Angov, E. Codon harmonization reduces amino acid misincorporation in bacterially expressed P. falciparum proteins and improves their immunogenicity. AMB Express 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Hatfield, G.W.; Roth, D.A. Optimizing scaleup yield for protein production: Computationally Optimized DNA Assembly (CODA) and Translation EngineeringTM. In Biotechnology Annual Review; El-Gewely, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 13, pp. 27–42. [Google Scholar]
- Jenkins, G.M.; Holmes, E.C. The extent of codon usage bias in human RNA viruses and its evolutionary origin. Virus Res. 2003, 92, 1–7. [Google Scholar] [CrossRef]
- Yang, X.; Luo, X.; Cai, X. Analysis of codon usage pattern in Taenia saginata based on a transcriptome dataset. Parasit Vectors 2014, 7, 527. [Google Scholar] [CrossRef]
- Mirsafian, H.; Mat Ripen, A.; Singh, A.; Teo, P.H.; Merican, A.F.; Mohamad, S.B. A comparative analysis of synonymous codon usage bias pattern in human albumin superfamily. Sci. World J. 2014, 2014, 639682. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Khan, M.R.I.; Wang, Y.; Ruan, Y.; Zhao, B.; Zhang, B.; Ma, X.; Zhang, K.; Zhao, X.; et al. An Engineered Rare Codon Device for Optimization of Metabolic Pathways. Sci. Rep. 2016, 6, 20608. [Google Scholar] [CrossRef]
- Yan, Q.; Philmus, B.; Hesse, C.; Kohen, M.; Chang, J.H.; Loper, J.E. The Rare Codon AGA Is Involved in Regulation of Pyoluteorin Biosynthesis in Pseudomonas protegens Pf-5. Front. Microbiol. 2016, 7, 497. [Google Scholar] [CrossRef]
- Chen, G.T.; Inouye, M. Role of the AGA/AGG codons, the rarest codons in global gene expression in Escherichia coli. Genes Dev. 1994, 8, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.R.; Papamichail, D.; Skiena, S.; Futcher, B.; Wimmer, E.; Mueller, S. Virus attenuation by genome-scale changes in codon pair bias. Science 2008, 320, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Martrus, G.; Nevot, M.; Andres, C.; Clotet, B.; Martinez, M.A. Changes in codon-pair bias of human immunodeficiency virus type 1 have profound effects on virus replication in cell culture. Retrovirology 2013, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Le Nouën, C.; Brock, L.G.; Luongo, C.; McCarty, T.; Yang, L.; Mehedi, M.; Wimmer, E.; Mueller, S.; Collins, P.L.; Buchholz, U.J.; et al. Attenuation of human respiratory syncytial virus by genome-scale codon-pair deoptimization. Proc. Natl. Acad. Sci. USA 2014, 111, 13169–13174. [Google Scholar] [CrossRef]
- Ni, Y.-Y.; Zhao, Z.; Opriessnig, T.; Subramaniam, S.; Zhou, L.; Cao, D.; Cao, Q.; Yang, H.; Meng, X.-J. Computer-aided codon-pairs deoptimization of the major envelope GP5 gene attenuates porcine reproductive and respiratory syndrome virus. Virology 2014, 450, 132–139. [Google Scholar] [CrossRef]
- Saha, J.; Bhattacharjee, S.; Pal Sarkar, M.; Saha, B.K.; Basak, H.K.; Adhikary, S.; Roy, V.; Mandal, P.; Chatterjee, A.; Pal, A. A comparative genomics-based study of positive strand RNA viruses emphasizing on SARS-CoV-2 utilizing dinucleotide signature, codon usage and codon context analyses. Gene Rep. 2021, 23, 101055. [Google Scholar] [CrossRef]
- Tats, A.; Tenson, T.; Remm, M. Preferred and avoided codon pairs in three domains of life. BMC Genom. 2008, 9, 463. [Google Scholar] [CrossRef]
- Broadbent, A.J.; Santos, C.P.; Anafu, A.; Wimmer, E.; Mueller, S.; Subbarao, K. Evaluation of the attenuation, immunogenicity, and efficacy of a live virus vaccine generated by codon-pair bias de-optimization of the 2009 pandemic H1N1 influenza virus, in ferrets. Vaccine 2016, 34, 563–570. [Google Scholar] [CrossRef]
- Eschke, K.; Trimpert, J.; Osterrieder, N.; Kunec, D. Attenuation of a very virulent Marek’s disease herpesvirus (MDV) by codon pair bias deoptimization. PLoS Pathog. 2018, 14, e1006857. [Google Scholar] [CrossRef]
- Mueller, S.; Coleman, J.R.; Papamichail, D.; Ward, C.B.; Nimnual, A.; Futcher, B.; Skiena, S.; Wimmer, E. Live attenuated influenza virus vaccines by computer-aided rational design. Nat. Biotechnol. 2010, 28, 723–726. [Google Scholar] [CrossRef]
- Fu, J.; Sha, B.E.; Thomas, L.L. HIV-1-infected peripheral blood mononuclear cells enhance neutrophil survival and HLA-DR expression via increased production of GM-CSF: Implications for HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2011, 56, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Margetts, P.J.; Anthony, D.C.; Pitossi, F.; Gauldie, J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Investig. 2001, 107, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Sendra, L.; Herrero, M.J.; Aliño, S.F. Translational Advances of Hydrofection by Hydrodynamic Injection. Genes 2018, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Forum on Regenerative Medicine. Exploring Novel Clinical Trial Designs for Gene-Based Therapies: Proceedings of a Workshop; Beachy, S.H., Alper, J., Hackmann, M., Addie, S., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2020; ISBN 978-0-309-67298-6. [Google Scholar]
- Peng, Z. Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005, 16, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Mirizio, G.G.; Barin, G.R.; de Andrade, R.V.; Nimer, N.F.S.; La Sala, L. Clock Genes, Inflammation and the Immune System-Implications for Diabetes, Obesity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9743. [Google Scholar] [CrossRef]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral. Sci. 2020, 12, 2. [Google Scholar] [CrossRef]
- Van Leuven, J.T.; Ederer, M.M.; Burleigh, K.; Scott, L.; Hughes, R.A.; Codrea, V.; Ellington, A.D.; Wichman, H.A.; Miller, C.R. ΦX174 Attenuation by Whole-Genome Codon Deoptimization. Genome Biol. Evol. 2021, 13, evaa214. [Google Scholar] [CrossRef]





| CODONS | Single Letter Amino Acid | ACE2 | CD147 | FURIN | TMPRSS2 | BMAL1 |
|---|---|---|---|---|---|---|
| TTT | F | 1.104 | 0.061 | 0.763 | 0.545 | 1.178 |
| TTC | 0.896 | 1.939 | 1.237 | 1.455 | 0.822 | |
| TTA | L | 0.743 | 0.000 | 0.095 | 0.230 | 0.965 |
| TTG | 0.778 | 0.136 | 0.555 | 0.700 | 1.233 | |
| CTT | 1.474 | 0.136 | 0.095 | 0.584 | 0.778 | |
| CTC | 0.691 | 1.683 | 1.457 | 1.106 | 1.205 | |
| CTA | 0.534 | 0.349 | 0.286 | 0.406 | 0.730 | |
| CTG | 1.780 | 3.697 | 3.511 | 2.975 | 1.090 | |
| ATT | I | 1.158 | 0.258 | 0.607 | 1.119 | 1.267 |
| ATC | 1.178 | 2.585 | 2.355 | 1.344 | 0.793 | |
| ATA | 0.664 | 0.158 | 0.037 | 0.537 | 0.940 | |
| GTT | V | 1.474 | 0.000 | 0.072 | 0.243 | 1.482 |
| GTC | 0.637 | 1.586 | 1.065 | 1.356 | 1.084 | |
| GTA | 0.473 | 0.104 | 0.435 | 0.209 | 0.833 | |
| GTG | 1.416 | 2.310 | 2.428 | 2.192 | 0.600 | |
| TCT | S | 1.577 | 0.712 | 0.613 | 1.326 | 1.790 |
| TCC | 1.287 | 2.294 | 1.496 | 1.500 | 0.837 | |
| TCA | 1.229 | 0.712 | 0.457 | 1.235 | 0.814 | |
| TCG | 0.000 | 0.475 | 0.547 | 0.132 | 0.182 | |
| AGT | 0.726 | 0.298 | 0.800 | 0.398 | 1.241 | |
| AGC | 1.182 | 1.507 | 2.087 | 1.409 | 1.136 | |
| CCT | P | 1.500 | 0.724 | 1.154 | 0.629 | 0.942 |
| CCC | 0.972 | 2.692 | 1.608 | 1.517 | 0.928 | |
| CCA | 1.528 | 0.362 | 0.683 | 1.262 | 1.975 | |
| CCG | 0.000 | 0.222 | 0.555 | 0.592 | 0.156 | |
| ACT | T | 1.162 | 0.890 | 0.515 | 0.790 | 0.959 |
| ACC | 0.826 | 1.371 | 1.731 | 1.579 | 1.107 | |
| ACA | 1.804 | 0.448 | 0.933 | 0.948 | 1.579 | |
| ACG | 0.208 | 1.291 | 0.820 | 0.683 | 0.355 | |
| GCT | A | 1.503 | 0.648 | 0.364 | 1.011 | 1.143 |
| GCC | 0.839 | 2.536 | 2.405 | 1.789 | 0.511 | |
| GCA | 1.658 | 0.260 | 0.751 | 0.580 | 2.228 | |
| GCG | 0.000 | 0.555 | 0.480 | 0.620 | 0.119 | |
| TAT | Y | 1.233 | 0.000 | 0.635 | 0.772 | 1.362 |
| TAC | 0.768 | 2.000 | 1.365 | 1.228 | 0.638 | |
| CAT | H | 1.755 | 0.000 | 0.421 | 0.903 | 0.699 |
| CAC | 0.245 | 2.000 | 1.579 | 1.097 | 1.301 | |
| CAA | Q | 0.816 | 0.000 | 0.230 | 0.545 | 0.754 |
| CAG | 1.185 | 2.000 | 1.770 | 1.455 | 1.246 | |
| AAT | N | 1.412 | 0.310 | 0.721 | 0.571 | 0.638 |
| AAC | 0.589 | 1.690 | 1.279 | 1.429 | 1.362 | |
| AAA | K | 1.148 | 0.222 | 0.409 | 0.905 | 1.236 |
| AAG | 0.852 | 1.778 | 1.591 | 1.095 | 0.764 | |
| GAT | D | 0.956 | 0.283 | 0.597 | 0.687 | 0.941 |
| GAC | 1.044 | 1.717 | 1.403 | 1.313 | 1.059 | |
| GAA | E | 1.360 | 0.232 | 0.423 | 0.767 | 1.487 |
| GAG | 0.640 | 1.768 | 1.577 | 1.233 | 0.513 | |
| TGT | C | 1.083 | 0.000 | 0.485 | 0.656 | 1.157 |
| TGC | 0.917 | 2.000 | 1.515 | 1.344 | 0.843 | |
| CGT | R | 0.441 | 0.000 | 0.375 | 0.125 | 0.350 |
| CGC | 0.175 | 2.551 | 1.186 | 1.246 | 0.114 | |
| CGA | 0.804 | 0.000 | 0.507 | 0.376 | 1.220 | |
| CGG | 0.615 | 1.777 | 3.174 | 0.752 | 1.343 | |
| AGA | 1.942 | 0.487 | 0.080 | 1.622 | 0.709 | |
| AGG | 2.024 | 1.185 | 0.678 | 1.880 | 2.263 | |
| GGT | G | 0.436 | 0.043 | 0.315 | 0.356 | 0.782 |
| GGC | 0.568 | 2.629 | 2.087 | 1.164 | 0.932 | |
| GGA | 1.738 | 0.303 | 0.244 | 1.015 | 1.369 | |
| GGG | 1.258 | 1.025 | 1.354 | 1.464 | 0.917 |
| ACE2 (4249 Codons) | CD147 (1666 Codons) | FURIN (5425 Codons) | TMPRSS2 (1522 Codons) | BMAL1 (30,873 Codons) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. No. | Codon Pair | Codon Numbers | Normalized Codon Number | Codon Pair | Codon Numbers | Normalized Codon Number | Codon Pair | Codon Numbers | Normalized Codon Number | Codon Pair | Codon Numbers | Normalized Codon Number | Codon Pair | Codon Numbers | Normalized Codon Number |
| 1 | CAA-GAA | 22 | 5.18 | GUG-CUG | 20 | 12.0 | GUG-GCC | 41 | 7.56 | GUG-UGC | 9 | 5.91 | GAA-UAU | 196 | 6.35 |
| 2 | AAU-GAA | 21 | 4.94 | GAG-GAC | 18 | 10.8 | GCC-AAC | 28 | 5.16 | CUG-CAG | 9 | 5.91 | AAA-GAU | 165 | 5.34 |
| 3 | AAA-AAU | 20 | 4.71 | GGC-UCC | 16 | 9.60 | CUG-GGC | 28 | 5.16 | AAC-CCC | 9 | 5.91 | GAU-GAA | 148 | 4.79 |
| 4 | UAU-GAA | 19 | 4.47 | CUG-AAG | 14 | 8.40 | GGC-GAG | 27 | 4.98 | AAC-AAU | 9 | 5.91 | AUA-GAU | 148 | 4.79 |
| 5 | GAA-AAU | 19 | 4.47 | UCA-GAG | 12 | 7.20 | AAU-GAC | 27 | 4.98 | UUG-AAC | 6 | 5.91 | GAA-AUC | 146 | 4.73 |
| 6 | CUG-UUC | 19 | 4.47 | GUG-AAG | 12 | 7.20 | GGG-CUG | 26 | 4.79 | UUC-AUG | 6 | 5.91 | AGC-AUG | 146 | 4.73 |
| 7 | GUU-GGG | 18 | 4.24 | GCG-CUG | 12 | 7.20 | GCC-CCC | 26 | 4.79 | UGU-GCC | 6 | 5.91 | GCA-GAU | 130 | 4.21 |
| 8 | GAG-AUG | 17 | 4.00 | GCC-CUC | 12 | 7.20 | GAG-GUG | 26 | 4.79 | UGG-AUU | 6 | 5.91 | AUG-GAC | 130 | 4.21 |
| 9 | GGA-UUC | 16 | 3.77 | CUG-GUC | 12 | 7.20 | CGG-CUG | 26 | 4.79 | UGC-AUC | 6 | 5.91 | AUG-AUU | 115 | 3.72 |
| 10 | GAA-GAC | 16 | 3.77 | CCC-GGC | 12 | 7.20 | CAG-CAG | 26 | 4.79 | UCC-GGG | 6 | 5.91 | AUG-AAC | 110 | 3.56 |
| 11 | CAG-AAA | 16 | 3.77 | CCC-GAG | 12 | 7.20 | GUG-GAG | 25 | 4.61 | UCC-AAC | 6 | 5.91 | GAA-UUG | 101 | 3.27 |
| 12 | AUG-GCA | 16 | 3.77 | AUC-AUC | 12 | 7.20 | CUG-CCC | 25 | 4.61 | UAU-GAC | 6 | 5.91 | UUU-GUC | 100 | 3.24 |
| 13 | AAA-CCA | 16 | 3.77 | ACU-GAC | 12 | 7.20 | UGG-GCC | 21 | 3.87 | UAC-GGG | 6 | 5.91 | UUG-UUU | 100 | 3.24 |
| 14 | GAC-CAG | 15 | 3.53 | ACG-GCC | 12 | 7.20 | GGC-UAC | 21 | 3.87 | UAC-CAA | 6 | 5.91 | GUC-UCA | 100 | 3.24 |
| 15 | GAA-GAG | 15 | 3.53 | AAC-GGC | 12 | 7.20 | GGC-CGG | 21 | 3.87 | GUG-UAC | 6 | 5.91 | GAU-AAA | 100 | 3.24 |
| 16 | GCA-UAU | 14 | 3.29 | UUC-GUG | 10 | 6.00 | GGC-ACC | 21 | 3.87 | GUC-GAU | 6 | 5.91 | ACA-GAA | 100 | 3.24 |
| 17 | CUU-GGA | 14 | 3.29 | UCC-GAC | 10 | 6.00 | GAG-GCC | 21 | 3.87 | GGG-GCC | 6 | 5.91 | AAC-UAC | 100 | 3.24 |
| 18 | AUG-AAU | 14 | 3.29 | UCC-AAG | 10 | 6.00 | GAG-CCC | 21 | 3.87 | GGA-UAC | 6 | 5.91 | GUU-UUA | 99 | 3.21 |
| 19 | AAA-GCA | 14 | 3.29 | GGC-CAG | 10 | 6.00 | CUG-GCC | 21 | 3.87 | GCG-CUG | 6 | 5.91 | GCA-GCA | 99 | 3.21 |
| 20 | UUU-CUG | 12 | 2.82 | GGC-ACC | 10 | 6.00 | CUC-ACC | 21 | 3.87 | GCC-UGC | 6 | 5.91 | GCA-AUG | 99 | 3.21 |
| 21 | UUU-CAA | 12 | 2.82 | GCC-GGC | 10 | 6.00 | CGG-GAC | 21 | 3.87 | GCC-GGC | 6 | 5.91 | GAA-GCA | 99 | 3.21 |
| 22 | UUG-AAA | 12 | 2.82 | GAC-GAC | 10 | 6.00 | CGG-AAG | 21 | 3.87 | GAC-UGG | 6 | 5.91 | CUA-UCA | 99 | 3.21 |
| 23 | UUC-CUG | 12 | 2.82 | GAC-CAG | 10 | 6.00 | CAG-GGC | 21 | 3.87 | GAC-UCC | 6 | 5.91 | CCC-UCU | 99 | 3.21 |
| 24 | UUC-CAU | 12 | 2.82 | CUG-GGC | 10 | 6.00 | CAC-AUC | 21 | 3.87 | GAA-AAC | 6 | 5.91 | CAG-CUC | 99 | 3.21 |
| 25 | UGU-GAC | 12 | 2.82 | AAU-GAC | 10 | 6.00 | ACC-CUG | 21 | 3.87 | GAA-AAA | 6 | 5.91 | CAA-GGA | 99 | 3.21 |
| 26 | UGG-AUG | 12 | 2.82 | UAC-GAG | 8 | 4.80 | AAC-CAC | 21 | 3.87 | CUG-AAC | 6 | 5.91 | AUG-GCU | 99 | 3.21 |
| 27 | GGG-GAA | 12 | 2.82 | GUC-UUC | 8 | 4.80 | GCC-UUC | 20 | 3.69 | CCU-CUG | 6 | 5.91 | AGG-AUG | 99 | 3.21 |
| 28 | GCU-AAU | 12 | 2.82 | GUC-CUG | 8 | 4.80 | CAG-AAG | 20 | 3.69 | CCC-ACU | 6 | 5.91 | AGG-AUA | 99 | 3.21 |
| 29 | GAA-GCU | 12 | 2.82 | GUC-CGC | 8 | 4.80 | CAC-CUG | 20 | 3.69 | CAG-UAC | 6 | 5.91 | ACU-GUU | 99 | 3.21 |
| 30 | GAA-ACA | 12 | 2.82 | GGG-CAG | 8 | 4.80 | GUC-UUC | 19 | 3.50 | CAG-CCC | 6 | 5.91 | AAU-GAU | 99 | 3.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandia, R.; Pandey, M.K.; Khan, A.A.; Rzhepakovsky, I.V.; Gurjar, P.; Karobari, M.I. Codon Usage and Context Analysis of Genes Modulated during SARS-CoV-2 Infection and Dental Inflammation. Vaccines 2022, 10, 1874. https://doi.org/10.3390/vaccines10111874
Khandia R, Pandey MK, Khan AA, Rzhepakovsky IV, Gurjar P, Karobari MI. Codon Usage and Context Analysis of Genes Modulated during SARS-CoV-2 Infection and Dental Inflammation. Vaccines. 2022; 10(11):1874. https://doi.org/10.3390/vaccines10111874
Chicago/Turabian StyleKhandia, Rekha, Megha Katare Pandey, Azmat Ali Khan, Igor Vladimirovich Rzhepakovsky, Pankaj Gurjar, and Mohmed Isaqali Karobari. 2022. "Codon Usage and Context Analysis of Genes Modulated during SARS-CoV-2 Infection and Dental Inflammation" Vaccines 10, no. 11: 1874. https://doi.org/10.3390/vaccines10111874
APA StyleKhandia, R., Pandey, M. K., Khan, A. A., Rzhepakovsky, I. V., Gurjar, P., & Karobari, M. I. (2022). Codon Usage and Context Analysis of Genes Modulated during SARS-CoV-2 Infection and Dental Inflammation. Vaccines, 10(11), 1874. https://doi.org/10.3390/vaccines10111874









