How MALDI-TOF Mass Spectrometry Technology Contributes to Microbial Infection Control in Healthcare Settings
Abstract
:1. Introduction
2. MALDI’s Historical Evolution
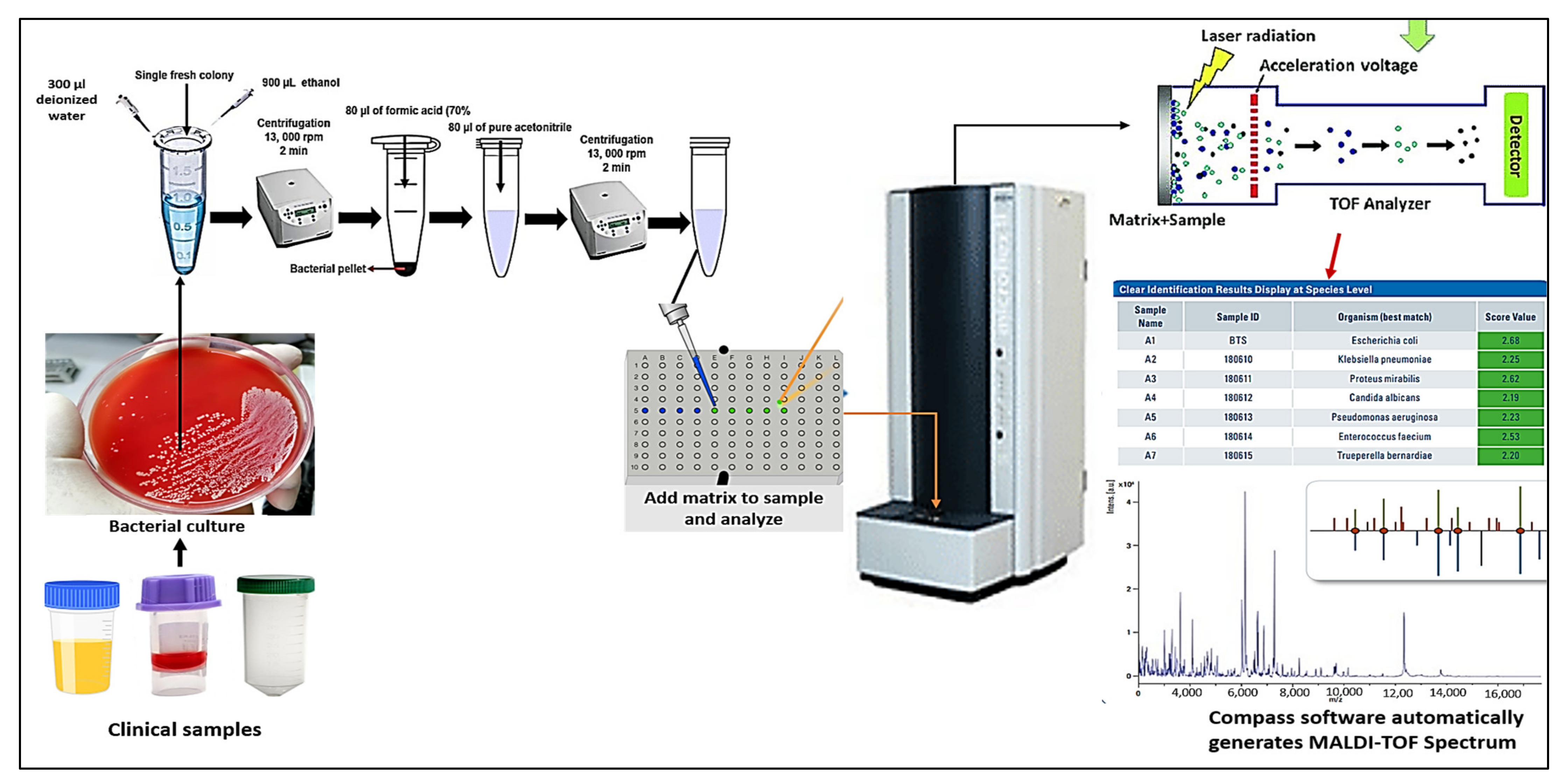
3. The Workflow of MALDI TOF Mass Spectrometry
4. Implications of MALDI-TOF MS for Microbial Recognition
4.1. Bacterial Identification
4.2. Yeasts and Filamentous Fungi Identification
4.3. Viral Identification
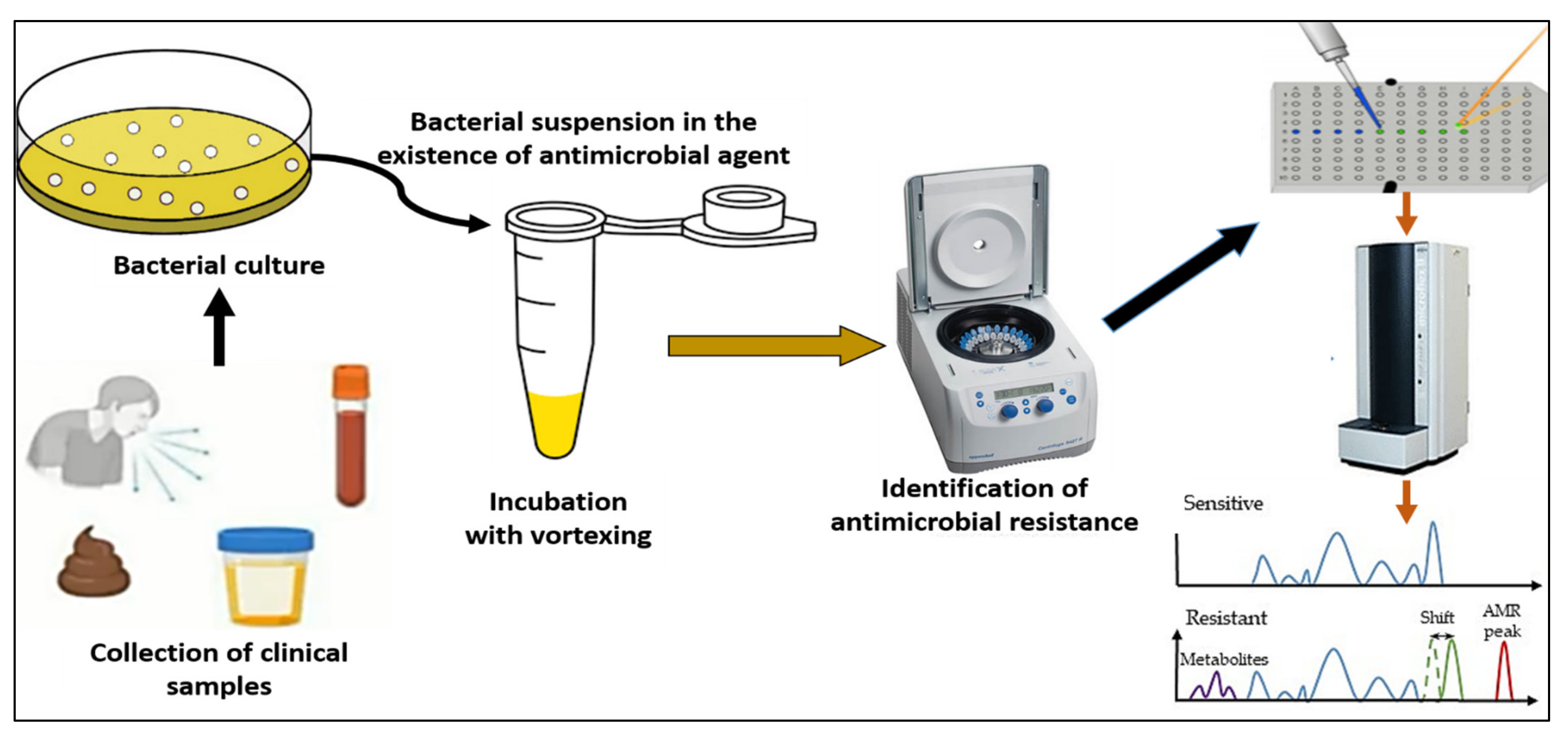
4.4. Detection of Antibiotic Resistance
4.5. The Advantages and Drawbacks of MALDI-TOF MS Technology
5. Outlooks for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Le, J.; Chen, Y.; Cai, Y.; Hong, Z.; Chai, Y. Recent advances in microfluidic devices for bacteria and fungus research. TrAC Trends Anal. Chem. 2019, 112, 175–195. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Moussa, I.M.; Dawoud, T.M.; Mubarak, A.S.; Al-Sarar, D.; Alsubki, R.A.; Alhaji, J.H.; Hamada, M.; Abalkhail, A. Acinetobacter baumannii as a community foodborne pathogen: Peptide mass fingerprinting analysis, genotypic of biofilm formation and phenotypic pattern of antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Rychert, J. Benefits and limitations of MALDI-TOF mass spectrometry for the identification of microorganisms. J. Infect. Epidemiol. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Hartley, M.G.; El-Maaytah, M.; McKenzie, C.; Greenman, J. The tongue microbiota of low odour and malodorous individuals. Microb. Ecol. Health Dis. 1996, 9, 215–223. [Google Scholar] [CrossRef]
- Bernardi, S.; Continenza, M.A.; Al-Ahmad, A.; Karygianni, L.; Follo, M.; Filippi, A.; Macchiarelli, G. Streptococcus spp. and Fusobacterium nucleatum in tongue dorsum biofilm from halitosis patients: A fluorescence in situ hybridization (FISH) and confocal laser scanning microscopy (CLSM) study. New Microbiol. 2019, 42, 108–113. [Google Scholar] [PubMed]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of adherent micro-organisms using different techniques. J. Med. Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tripodi, D.; Marzo, G.; Bernardi, S.; Continenza, M.A.; Piattelli, A.; Iaculli, F.; Mummolo, S. Microleakage of bacteria in different implant-abutment assemblies: An in vitro study. J. Appl. Biomater. Funct. Mater. 2015, 13, 174–180. [Google Scholar] [CrossRef]
- Bernardi, S.; Bianchi, S.; Botticelli, G.; Rastelli, E.; Tomei, A.; Palmerini, M.; Continenza, M.; Macchiarelli, G. Scanning electron microscopy and microbiological approaches for the evaluation of salivary microorganisms behaviour on anatase titanium surfaces: In vitro study. Morphologie 2018, 102, 1–6. [Google Scholar] [CrossRef]
- Orioles, M.; Galeotti, M.; Saccà, E.; Bulfoni, M.; Corazzin, M.; Bianchi, S.; Torge, D.; Macchiarelli, G.; Magi, G.E.; Schmidt, J.G. Effect of temperature on transfer of Midichloria-like organism and development of red mark syndrome in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 560, 738577. [Google Scholar] [CrossRef]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef]
- AlMasoud, N.; Muhamadali, H.; Chisanga, M.; AlRabiah, H.; Lima, C.A.; Goodacre, R. Discrimination of bacteria using whole organism fingerprinting: The utility of modern physicochemical techniques for bacterial typing. Analyst 2021, 146, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.A. Bacterial typing methods from past to present: A comprehensive overview. Gene Rep. 2022, 29, 101675. [Google Scholar] [CrossRef]
- Curry, A.; Appleton, H.; Dowsett, B. Application of transmission electron microscopy to the clinical study of viral and bacterial infections: Present and future. Micron 2006, 37, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Biel, S.S.; Gelderblom, H.R. Diagnostic electron microscopy is still a timely and rewarding method. J. Clin. Virol. 1999, 13, 105–119. [Google Scholar] [CrossRef]
- Tran, A.; Alby, K.; Kerr, A.; Jones, M.; Gilligan, P. Cost savings realized by implementation of routine microbiological identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2015, 53, 2473–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahi, P.; Prakash, O.; Shouche, Y. Matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry (MALDI-TOF MS) based microbial identifications: Challenges and scopes for microbial ecologists. Front. Microbiol. 2016, 7, 1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanno, D.; Saito, K.; Ohashi, K.; Toyokawa, M.; Yamadera, Y.; Shimura, H. Matrix-Assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometry with Time-of-Flight Peak Analysis for Rapid and Accurate Detection of Group B Streptococcus in Pregnant Women. Microbiol. Spectr. 2022, 10, e01732-21. [Google Scholar] [CrossRef]
- Welker, M.; Van Belkum, A.; Girard, V.; Charrier, J.-P.; Pincus, D. An update on the routine application of MALDI-TOF MS in clinical microbiology. Expert Rev. Proteom. 2019, 16, 695–710. [Google Scholar] [CrossRef]
- Oviaño, M.; Rodríguez-Sánchez, B. MALDI-TOF mass spectrometry in the 21st century clinical microbiology laboratory. Enferm. Infecc. Y Microbiol. Clin. 2021, 39, 192–200. [Google Scholar] [CrossRef]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T.; Matsuo, T. Protein and polymer analyses up to m/z 100,000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, H.; Ortega, E.O.; López, M.J.R.; Vera, A.R.; Hosseini, S. Characterization Techniques for Mass Spectrometry Analysis. In Material Characterization Techniques and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 47–69. [Google Scholar]
- Gandhi, K.; Sharma, N.; Gautam, P.B.; Sharma, R.; Mann, B.; Pandey, V. Mass Spectroscopy. In Advanced Analytical Techniques in Dairy Chemistry; Springer: Berlin/Heidelberg, Germany, 2022; pp. 199–217. [Google Scholar]
- Patel, R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaide, F.; Amlerová, J.; Bou, G.; Ceyssens, P.; Coll, P.; Corcoran, D.; Fangous, M.-S.; González-Álvarez, I.; Gorton, R.; Greub, G.; et al. How to: Identify non-tuberculous Mycobacterium species using MALDI-TOF mass spectrometry. Clin. Microbiol. Infect. 2018, 24, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.H.; Cunningham, S.A.; Dailey, A.L.; Gustafson, D.R.; Patel, R. Identification of anaerobic bacteria by Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry with on-plate formic acid preparation. J. Clin. Microbiol. 2013, 51, 782–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassagne, C.; Ranque, S.; Normand, A.-C.; Fourquet, P.; Thiebault, S.; Planard, C.; Hendrickx, M.; Piarroux, R. Mould routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS ONE 2011, 6, e28425. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.-Y.; Chiang-Ni, C.; Teng, S.-H. Current status of MALDI-TOF mass spectrometry in clinical microbiology. J. Food Drug Anal. 2019, 27, 404–414. [Google Scholar] [CrossRef]
- Elbehiry, A.; Aldubaib, M.; Al Rugaie, O.; Marzouk, E.; Abaalkhail, M.; Moussa, I.; El-Husseiny, M.H.; Abalkhail, A.; Rawway, M. Proteomics-based screening and antibiotic resistance assessment of clinical and sub-clinical Brucella species: An evolution of brucellosis infection control. PLoS ONE 2022, 17, e0262551. [Google Scholar] [CrossRef]
- Yang, S.; Jin, Y.; Zhao, G.; Liu, J.; Zhou, X.; Yang, J.; Wang, J.; Cui, Y.; Hu, X.; Li, Y. Improvement of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for identification of clinically important Candida species. Clin. Lab. 2014, 60, 37–46. [Google Scholar] [CrossRef]
- Florio, W.; Baldeschi, L.; Rizzato, C.; Tavanti, A.; Ghelardi, E.; Lupetti, A. Detection of antibiotic-resistance by MALDI-TOF mass spectrometry: An expanding area. Front. Cell. Infect. Microbiol. 2020, 10, 572909. [Google Scholar] [CrossRef]
- Florio, W.; Tavanti, A.; Ghelardi, E.; Lupetti, A. MALDI-TOF MS applications to the detection of antifungal resistance: State of the art and future perspectives. Front. Microbiol. 2018, 9, 2577. [Google Scholar] [CrossRef]
- Wolk, D.M.; Clark, A.E. Matrix-assisted laser desorption time of flight mass spectrometry. Clin. Lab. Med. 2018, 38, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karas, M.; Bachmann, D.; Hillenkamp, F. Influence of the wavelength in high-irradiance ultraviolet laser desorption mass spectrometry of organic molecules. Anal. Chem. 1985, 57, 2935–2939. [Google Scholar] [CrossRef]
- Cain, T.C.; Lubman, D.M.; Weber, W.J., Jr. Differentiation of bacteria using protein profiles from matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1994, 8, 1026–1030. [Google Scholar] [CrossRef]
- Holland, R.; Wilkes, J.; Rafii, F.; Sutherland, J.; Persons, C.; Voorhees, K.; Lay, J., Jr. Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorption/ionization with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 1227–1232. [Google Scholar] [CrossRef]
- Claydon, M.A.; Davey, S.N.; Edwards-Jones, V.; Gordon, D.B. The rapid identification of intact microorganisms using mass spectrometry. Nat. Biotechnol. 1996, 14, 1584–1586. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nakayama, T. MALDI-Based Mass Spectrometry in Clinical Testing: Focus on Bacterial Identification. Appl. Sci. 2022, 12, 2814. [Google Scholar] [CrossRef]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.-E.; Rolain, J.M.; Raoult, D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef]
- Carroll, K.C.; Patel, R. Systems for identification of bacteria and fungi. In Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2015; pp. 29–43. [Google Scholar]
- Vrioni, G.; Tsiamis, C.; Oikonomidis, G.; Theodoridou, K.; Kapsimali, V.; Tsakris, A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: Current achievements and future perspectives. Ann. Transl. Med. 2018, 6, 240. [Google Scholar] [CrossRef]
- Angeletti, S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J. Microbiol. Methods 2017, 138, 20–29. [Google Scholar] [CrossRef]
- Nomura, F. Proteome-based bacterial identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS): A revolutionary shift in clinical diagnostic microbiology. Biochim. Et Biophys. Acta-Proteins Proteom. 2015, 1854, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; De Carolis, E.; Vella, A.; Sanguinetti, M. MALDI-TOF mass spectrometry in the clinical mycology laboratory: Identification of fungi and beyond. Expert Rev. Proteom. 2013, 10, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, A.; Durussel, C.; Bille, J.; Greub, G.; Prod’Hom, G. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 2010, 48, 1549–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastin, B.; Bird, P.; Benzinger, M.J.; Crowley, E.; Agin, J.; Goins, D.; Sohier, D.; Timke, M.; Shi, G.; Kostrzewa, M. Confirmation and identification of Salmonella spp., Cronobacter spp., and other Gram-negative organisms by the Bruker MALDI biotyper method: Collaborative study, first action 2017.09. J. AOAC Int. 2018, 101, 1593–1609. [Google Scholar] [CrossRef]
- Martiny, D.; Busson, L.; Wybo, I.; El Haj, R.A.; Dediste, A.; Vandenberg, O. Comparison of the Microflex LT and Vitek MS systems for routine identification of bacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2012, 50, 1313–1325. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Passet, V.; Rakotondrasoa, A.; Brisse, S. Identification of Klebsiella pneumoniae, Klebsiella quasipneumoniae, Klebsiella variicola and related phylogroups by MALDI-TOF mass spectrometry. Front. Microbiol. 2018, 9, 3000. [Google Scholar] [CrossRef] [Green Version]
- Alby, K.; Gilligan, P.H.; Miller, M.B. Comparison of matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry platforms for the identification of Gram-negative rods from patients with cystic fibrosis. J. Clin. Microbiol. 2013, 51, 3852–3854. [Google Scholar] [CrossRef] [Green Version]
- Couturier, M.R.; Mehinovic, E.; Croft, A.C.; Fisher, M.A. Identification of HACEK clinical isolates by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2011, 49, 1104–1106. [Google Scholar] [CrossRef] [Green Version]
- Hong, E.; Bakhalek, Y.; Taha, M.-K. Identification of Neisseria meningitidis by MALDI-TOF MS may not be reliable. Clin. Microbiol. Infect. 2019, 25, 717–722. [Google Scholar] [CrossRef]
- Oviaño, M.; Gómara, M.; Barba, M.J.; Revillo, M.J.; Barbeyto, L.P.; Bou, G. Towards the early detection of β-lactamase-producing Enterobacteriaceae by MALDI-TOF MS analysis. J. Antimicrob. Chemother. 2017, 72, 2259–2262. [Google Scholar] [CrossRef]
- Rodrıguez-Sánchez, B.; Marın, M.; Sánchez-Carrillo, C.; Cercenado, E.; Ruiz, A.; Rodrıguez-Créixems, M.; Bouza, E. Improvement of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of difficult-toidentify bacteria and its impact in the workflow of a clinical microbiology laboratory. Diagn. Microbiol. Infect. Dis. 2014, 79, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Cercenado, E.; Sánchez-Carrillo, C.; Ruiz, A.; González, Á.G.; Rodríguez-Sánchez, B.; Bouza, E. Accurate differentiation of Streptococcus pneumoniae from other species within the Streptococcus mitis group by peak analysis using MALDI-TOF MS. Front. Microbiol. 2017, 8, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harju, I.; Lange, C.; Kostrzewa, M.; Maier, T.; Rantakokko-Jalava, K.; Haanperä, M. Improved differentiation of Streptococcus pneumoniae and other S. mitis group streptococci by MALDI Biotyper using an improved MALDI Biotyper database content and a novel result interpretation algorithm. J. Clin. Microbiol. 2017, 55, 914–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Sánchez, B.; Alcalá, L.; Marín, M.; Ruiz, A.; Alonso, E.; Bouza, E. Evaluation of MALDI-TOF MS (matrix-assisted laser desorption-ionization time-of-flight mass spectrometry) for routine identification of anaerobic bacteria. Anaerobe 2016, 42, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Veloo, A.; Jean-Pierre, H.; Justesen, U.; Morris, T.; Urban, E.; Wybo, I.; Kostrzewa, M.; Friedrich, A.; Shah, H.; Nagy, E. Validation of a for anaerobic bacteria optimized MALDI-TOF MS biotyper database: The ENRIA project. Anaerobe 2018, 54, 224–230. [Google Scholar] [CrossRef]
- Robinne, S.; Saad, J.; Morsli, M.; Hamidou, Z.H.; Tazerart, F.; Drancourt, M.; Baron, S.A. Rapid Identification of Mycobacterium tuberculosis Complex Using Mass Spectrometry: A Proof of Concept. Front. Microbiol. 2022, 13, 753969. [Google Scholar] [CrossRef]
- O’Connor, J.; Corcoran, G.; O’Reilly, B.; O’Mahony, J.; Lucey, B. Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF MS) for Investigation of Mycobacterium tuberculosis Complex Outbreaks: A Type Dream? J. Clin. Microbiol. 2020, 58, e02077-19. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Nagy, E.; Schröttner, P.; Pranada, A.B. How MALDI-TOF mass spectrometry can aid the diagnosis of hard-to-identify pathogenic bacteria—the rare and the unknown. Expert Rev. Mol. Diagn. 2019, 19, 667–682. [Google Scholar] [CrossRef]
- Lau, A.F. Matrix-assisted laser desorption ionization time-of-flight for fungal identification. Clin. Lab. Med. 2021, 41, 267–283. [Google Scholar] [CrossRef]
- Zvezdanova, M.; Escribano, P.; Ruiz, A.; Martínez-Jiménez, M.; Peláez, T.; Collazos, A.; Guinea, J.; Bouza, E.; Rodríguez-Sánchez, B. Increased species-assignment of filamentous fungi using MALDI-TOF MS coupled with a simplified sample processing and an in-house library. Med. Mycol. 2019, 57, 63–70. [Google Scholar] [CrossRef]
- Normand, A.; Becker, P.; Gabriel, F.; Cassagne, C.; Accoceberry, I.; Gari-Toussaint, M.; Hasseine, L.; De Geyter, D.; Pierard, D.; Surmont, I. Validation of a new web application for identification of fungi by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2017, 55, 2661–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguinetti, M.; Posteraro, B. Identification of molds by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2017, 55, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R. A moldy application of MALDI: MALDI-ToF mass spectrometry for fungal identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.-F.; Hou, X.; Xiao, M.; Zhang, L.; Cheng, J.-W.; Zhou, M.-L.; Huang, J.-J.; Zhang, J.-J.; Xu, Y.-C.; Hsueh, P.-R. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) analysis for the identification of pathogenic microorganisms: A review. Microorganisms 2021, 9, 1536. [Google Scholar] [CrossRef]
- Sjoholm, M.I.; Dillner, J.; Carlson, J. Multiplex detection of human herpesviruses from archival specimens by using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2008, 46, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Guan, Q.; Huan, Y.; Liu, Z.; Qi, J.; Ge, S. Development of high-throughput genotyping method of all 18 HR HPV based on the MALDI-TOF MS platform and compared with the Roche Cobas 4800 HPV assay using clinical specimens. BMC Cancer 2019, 19, 825. [Google Scholar] [CrossRef] [Green Version]
- Xiu, L.; Zhang, C.; Wu, Z.; Peng, J. Establishment and application of a universal coronavirus screening method using MALDI-TOF mass spectrometry. Front. Microbiol. 2017, 8, 1510. [Google Scholar] [CrossRef] [Green Version]
- Yoshinari, T.; Hayashi, K.; Hirose, S.; Ohya, K.; Ohnishi, T.; Watanabe, M.; Taharaguchi, S.; Mekata, H.; Taniguchi, T.; Maeda, T. Matrix-Assisted Laser Desorption and Ionization Time-of-Flight Mass Spectrometry Analysis for the Direct Detection of SARS-CoV-2 in Nasopharyngeal Swabs. Anal. Chem. 2022, 94, 4218–4226. [Google Scholar] [CrossRef]
- Thomas, J.J.; Falk, B.; Fenselau, C.; Jackman, J.; Ezzell, J. Viral characterization by direct analysis of capsid proteins. Anal. Chem. 1998, 70, 3863–3867. [Google Scholar] [CrossRef]
- Calderaro, A.; Arcangeletti, M.C.; Rodighiero, I.; Buttrini, M.; Montecchini, S.; Simone, R.V.; Medici, M.C.; Chezzi, C.; De Conto, F. Identification of different respiratory viruses, after a cell culture step, by matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). Sci. Rep. 2016, 6, 36082. [Google Scholar] [CrossRef] [Green Version]
- Krokhin, O.; Li, Y.; Andonov, A.; Feldmann, H.; Flick, R.; Jones, S.; Stroeher, U.; Bastien, N.; Dasuri, K.V.; Cheng, K. Mass Spectrometric Characterization of Proteins from the SARS Virus: A Preliminary Report* S. Mol. Cell. Proteom. 2003, 2, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.K.; Howard, T.; Walsh, R.; Pepper, J.; Loegering, J.; Phinney, B.; Salemi, M.R.; Rashidi, H.H. Novel application of automated machine learning with MALDI-TOF-MS for rapid high-throughput screening of COVID-19: A proof of concept. Sci. Rep. 2021, 11, 8219. [Google Scholar] [CrossRef] [PubMed]
- Lazari, L.C.; Zerbinati, R.M.; Rosa-Fernandes, L.; Santiago, V.F.; Rosa, K.F.; Angeli, C.B.; Schwab, G.; Palmieri, M.; Sarmento, D.J.; Marinho, C.R. MALDI-TOF mass spectrometry of saliva samples as a prognostic tool for COVID-19. J. Oral Microbiol. 2022, 14, 2043651. [Google Scholar] [CrossRef] [PubMed]
- Camarasa, C.G.; Cobo, F. Application of MALDI-TOF mass spectrometry in clinical virology. In The Use of Mass Spectrometry Technology (MALDI-TOF) in Clinical Microbiology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 167–180. [Google Scholar]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Idelevich, E.; Sparbier, K.; Kostrzewa, M.; Becker, K. Rapid detection of antibiotic resistance by MALDI-TOF mass spectrometry using a novel direct-on-target microdroplet growth assay. Clin. Microbiol. Infect. 2018, 24, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Kerremans, J.; Verboom, P.; Stijnen, T.; Roijen, L.H.-V.; Goessens, W.; Verbrugh, H.; Vos, M. Rapid identification and antimicrobial susceptibility testing reduce antibiotic use and accelerate pathogen-directed antibiotic use. J. Antimicrob. Chemother. 2008, 61, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Oviaño, M.; Bou, G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for the rapid detection of antimicrobial resistance mechanisms and beyond. Clin. Microbiol. Rev. 2018, 32, e00037-18. [Google Scholar] [CrossRef] [Green Version]
- Oviaño, M.; Sparbier, K.; Barba, M.J.; Kostrzewa, M.; Bou, G. Universal protocol for the rapid automated detection of carbapenem-resistant Gram-negative bacilli directly from blood cultures by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF/MS). Int. J. Antimicrob. Agents 2016, 48, 655–660. [Google Scholar] [CrossRef]
- Hrabák, J. Detection of carbapenemases using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) meropenem hydrolysis assay. In Sepsis; Springer: Berlin/Heidelberg, Germany, 2015; pp. 91–96. [Google Scholar]
- Camara, J.E.; Hays, F.A. Discrimination between wild-type and ampicillin-resistant Escherichia coli by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 1633–1638. [Google Scholar] [CrossRef]
- Bizzini, A.; Jaton, K.; Romo, D.; Bille, J.; Prod’hom, G.; Greub, G. Matrix-assisted laser desorption ionization–time of flight mass spectrometry as an alternative to 16S rRNA gene sequencing for identification of difficult-to-identify bacterial strains. J. Clin. Microbiol. 2011, 49, 693–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justesen, U.S.; Holm, A.; Knudsen, E.; Andersen, L.B.; Jensen, T.G.; Kemp, M.; Skov, M.N.; Gahrn-Hansen, B.; Møller, J.K. Species identification of clinical isolates of anaerobic bacteria: A comparison of two matrix-assisted laser desorption ionization–time of flight mass spectrometry systems. J. Clin. Microbiol. 2011, 49, 4314–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Rotz, M.; Dierig, A.; Heininger, U.; Chrobak, C.; Baettig, V.; Egli, A. Case report: When two and ½ men go camping…. BMC Infect. Dis. 2017, 17, 102. [Google Scholar]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Pincus, D.; Orenga, S.; Chatellier, S. Yeast identification—Past, present, and future methods. Med. Mycol. 2007, 45, 97–121. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Ellis, B.; Lee, R.; Stamper, P.; Zhang, S.; Carroll, K. Prospective evaluation of a matrix-assisted laser desorption ionization–time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: A bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J. Clin. Microbiol. 2012, 50, 3301–3308. [Google Scholar]
- Pupo, G.M.; Lan, R.; Reeves, P.R. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc. Natl. Acad. Sci. USA 2000, 97, 10567–10572. [Google Scholar] [CrossRef] [Green Version]
- Paauw, A.; Jonker, D.; Roeselers, G.; Heng, J.M.; Mars-Groenendijk, R.H.; Trip, H.; Molhoek, E.M.; Jansen, H.-J.; van der Plas, J.; de Jong, A.L. Rapid and reliable discrimination between Shigella species and Escherichia coli using MALDI-TOF mass spectrometry. Int. J. Med. Microbiol. 2015, 305, 446–452. [Google Scholar] [CrossRef]
- Khot, P.D.; Fisher, M.A. Novel approach for differentiating Shigella species and Escherichia coli by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 3711–3716. [Google Scholar] [CrossRef] [Green Version]
- Branda, J.; Fritsche, T.; Burnham, C.; Butler-Wu, S.; Doern, C.; Fedorko, D.; Frana, T.; Gawoski, J.; Ginocchio, C.; Hamula, C. Methods for the identification of cultured microorganisms using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. In CLSI Guideline; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Karlsson, R.; Gonzales-Siles, L.; Boulund, F.; Svensson-Stadler, L.; Skovbjerg, S.; Karlsson, A.; Davidson, M.; Hulth, S.; Kristiansson, E.; Moore, E.R. Proteotyping: Proteomic characterization, classification and identification of microorganisms—A prospectus. Syst. Appl. Microbiol. 2015, 38, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Body, B.A.; Beard, M.A.; Slechta, E.S.; Hanson, K.E.; Barker, A.P.; Babady, N.E.; McMillen, T.; Tang, Y.-W.; Brown-Elliott, B.A.; Iakhiaeva, E. Evaluation of the Vitek MS v3.0 matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of Mycobacterium and Nocardia species. J. Clin. Microbiol. 2018, 56, e00237-18. [Google Scholar] [CrossRef] [PubMed]
- Rychert, J.; Slechta, E.S.; Barker, A.P.; Miranda, E.; Babady, N.E.; Tang, Y.-W.; Gibas, C.; Wiederhold, N.; Sutton, D.; Hanson, K.E. Multicenter evaluation of the Vitek MS v3.0 system for the identification of filamentous fungi. J. Clin. Microbiol. 2018, 56, e01353-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, M.; Yousefi, L.; Pakdel, F.; Ghotaslou, R.; Rezaee, M.A.; Khodadadi, E.; Oskouei, M.A.; Barhaghi, M.H.S.; Kafil, H.S. MALDI-TOF Mass Spectroscopy Applications in Clinical Microbiology. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 9928238. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbehiry, A.; Aldubaib, M.; Abalkhail, A.; Marzouk, E.; ALbeloushi, A.; Moussa, I.; Ibrahem, M.; Albazie, H.; Alqarni, A.; Anagreyyah, S.; et al. How MALDI-TOF Mass Spectrometry Technology Contributes to Microbial Infection Control in Healthcare Settings. Vaccines 2022, 10, 1881. https://doi.org/10.3390/vaccines10111881
Elbehiry A, Aldubaib M, Abalkhail A, Marzouk E, ALbeloushi A, Moussa I, Ibrahem M, Albazie H, Alqarni A, Anagreyyah S, et al. How MALDI-TOF Mass Spectrometry Technology Contributes to Microbial Infection Control in Healthcare Settings. Vaccines. 2022; 10(11):1881. https://doi.org/10.3390/vaccines10111881
Chicago/Turabian StyleElbehiry, Ayman, Musaad Aldubaib, Adil Abalkhail, Eman Marzouk, Ahmad ALbeloushi, Ihab Moussa, Mai Ibrahem, Hamad Albazie, Abdullah Alqarni, Sulaiman Anagreyyah, and et al. 2022. "How MALDI-TOF Mass Spectrometry Technology Contributes to Microbial Infection Control in Healthcare Settings" Vaccines 10, no. 11: 1881. https://doi.org/10.3390/vaccines10111881





