Urological Safety and COVID-19 Vaccinations
Abstract
:1. Introduction
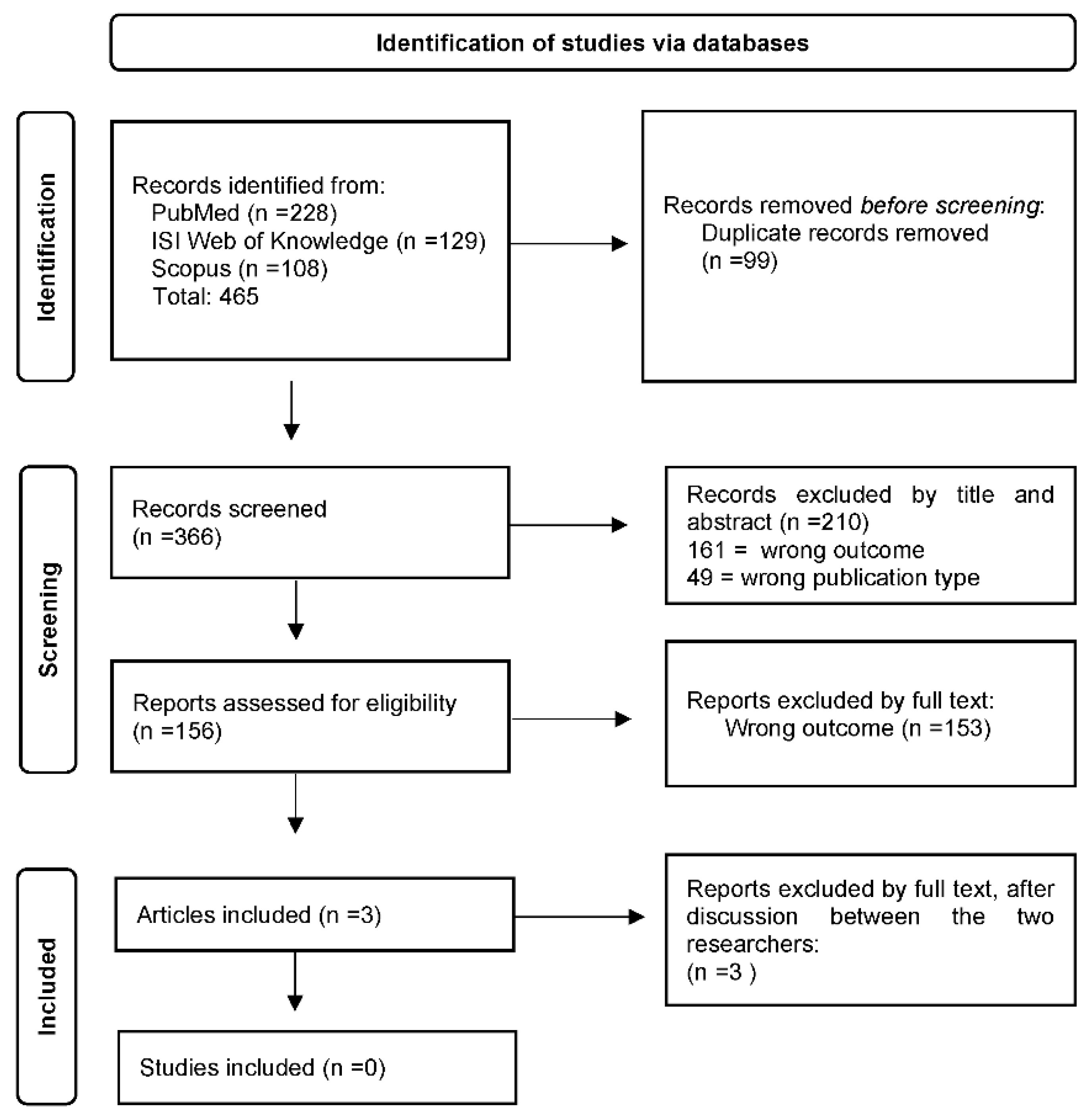
2. Materials and Methods
3. Results
3.1. Andrology
3.2. Oncology
3.3. Kidney Transplant
3.4. Miscellaneous
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puliatti, S.; Eissa, A.; Eissa, R.; Amato, M.; Mazzone, E.; Dell’Oglio, P.; Sighinolfi, M.C.; Zoeir, A.; Micali, S.; Bianchi, G.; et al. COVID-19 and urology: A comprehensive review of the literature: COVID-19 and urology. BJU Int. 2020, 125, E7–E14. [Google Scholar] [CrossRef] [PubMed]
- Hevia, V.; Lorca, J.; Hevia, M.; Domínguez, A.; López-Plaza, J.; Artiles, A.; Álvarez, S.; Sánchez, Á.; Fraile, A.; López-Fando, L.; et al. Pandemia COVID-19: Impacto y reacción rápida de la Urología. Actas Urol. Esp. 2020, 44, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Yeo, S.E.K.; Chong, Y.L.; Lee, Y.M. Stepping Forward: Urologists’ Efforts During the COVID-19 Outbreak in Singapore. Eur Urol. 2020, 78, e38–e39. [Google Scholar] [CrossRef] [Green Version]
- Campi, R.; Amparore, D.; Checcucci, E.; Claps, F.; Teoh, J.Y.C.; Serni, S.; Scarpa, R.M.; Porpiglia, F.; Carrion, D.M.; Gomez Rivas, J.; et al. Explorando la perspectiva de los residentes sobre las modalidades y contenidos de aprendizaje inteligente para la educación virtual de urología: Lección aprendida durante la pandemia de la COVID-19. Actas Urol. Esp. 2021, 45, 39–48. [Google Scholar] [CrossRef] [PubMed]
- On Behalf of European Society of Residents in Urology (ESRU); Claps, F.; Amparore, D.; Esperto, F.; Cacciamani, G.; Fiori, C.; Minervini, A.; Liguori, G.; Trombetta, C.; Porpiglia, F.; et al. Smart Learning for Urology Residents during the COVID-19 Pandemic and beyond: Insights from a Nationwide Survey in Italy. Minerva Urol. Nefrol. 2020, 72, 647–649. Available online: https://www.minervamedica.it/index2.php?show=R19Y2020N06A0647 (accessed on 27 October 2022).
- Bientinesi, R.; Coluzzi, S.; Gavi, F.; Nociti, V.; Gandi, C.; Marino, F.; Moretto, S.; Mirabella, M.; Bassi, P.; Sacco, E. The Impact of Neurogenic Lower Urinary Tract Symptoms and Erectile Dysfunctions on Marital Relationship in Men with Multiple Sclerosis: A Single Cohort Study. J. Clin. Med. 2022, 11, 5639. [Google Scholar] [CrossRef]
- Gori, A.; Dondossola, D.; Antonelli, B.; Mangioni, D.; Alagna, L.; Reggiani, P.; Bandera, A.; Rossi, G. Coronavirus disease 2019 and transplantation: A view from the inside. Am. J. Transpl. 2020, 20, 1939–1940. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.G.; Walls, R.M. Supporting the Health Care Workforce During the COVID-19 Global Epidemic. JAMA 2020, 323, 1439. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, M.; Recupero, S.M.; Marzio, V.; De Dominicis, M.; Pinto, F.; Foschi, N.; Di Gianfrancesco, L.; Bassi, P.; Ragonese, M. El impacto de la COVID-19 en las admisiones al servicio de urgencias, hospitalizaciones y manejo clínico de la urolitiasis en el centro de Italia: Análisis multicéntrico. Actas Urol. Esp. 2020, 44, 611–616. [Google Scholar] [CrossRef]
- Novara, G.; Checcucci, E.; Crestani, A.; Abrate, A.; Esperto, F.; Pavan, N.; De Nunzio, C.; Galfano, A.; Giannarini, G.; Gregori, A.; et al. Telehealth in Urology: A Systematic Review of the Literature. How Much Can Telemedicine Be Useful During and After the COVID-19 Pandemic? Eur. Urol. 2020, 78, 786–811. [Google Scholar] [CrossRef]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar]
- EpiCentro. Sviluppo, Valutazione e Approvazione dei Vaccini Contro COVID-19. Available online: https://www.epicentro.iss.it/vaccini/covid-19-sviluppo-valutazione-approvazione (accessed on 27 October 2022).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 27 October 2022).
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, P.G.; Thomas, K.; Shah, M.D.; Vizueta, N.; Cui, Y.; Vangala, S.; Kapteyn, A. National Trends in the US Public’s Likelihood of Getting a COVID-19 Vaccine—April 1 to December 8, 2020. JAMA 2021, 325, 396. [Google Scholar] [CrossRef]
- Gonzalez, D.C.; Nassau, D.E.; Khodamoradi, K.; Ibrahim, E.; Blachman-Braun, R.; Ory, J.; Ramasamy, R. Sperm Parameters Before and After COVID-19 mRNA Vaccination. JAMA 2021, 326, 273. [Google Scholar] [CrossRef]
- Law, B.; Matthew, D. SO2-D2.1.1 Priority List of COVID-19 Adverse Events of Special Interest: Quarterly Update. Available online: https://brightoncollaboration.us/wp-content/uploads/2021/01/SO2_D2.1.1_V1.1_COVID-19_AESI-update-Sep2020.pdf (accessed on 1 September 2020).
- Beccia, F.; Amantea, C.; Rossi, M.F.; Daniele, A.; Santoro, P.E.; Borrelli, I.; Marazza, M.; Boccia, S.; Ricciardi, W.; Moscato, U. Legal responsibility of vaccinating doctor. G. Ital. Med. Lav. Ergon. 2021, 43, 93–98. [Google Scholar]
- Amantea, C.; Rossi, M.F.; Santoro, P.E.; Beccia, F.; Gualano, M.R.; Borrelli, I.; Pinto da Costa, J.; Daniele, A.; Tumminello, A.; Boccia, S.; et al. Medical Liability of the Vaccinating Doctor: Comparing Policies in European Union Countries during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 7191. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Winston, J.; Munien, K. Superficial Thrombophlebitis of the Penis following AstraZeneca ChAdOx1-S Vaccination: A Rare Venous Thromboembolic Complication. Eur. J. Case Rep. Intern. Med. 2022, 9, 3. Available online: https://www.ejcrim.com/index.php/EJCRIM/article/view/3258 (accessed on 28 September 2022).
- Öztürk, H. Penile Mondor’s disease. Basic Clin. Androl. 2014, 24, 5. [Google Scholar] [CrossRef] [Green Version]
- Eren, M.T.; Özveri, H.; Kurtoğlu, H. Penile Mondor’s in a COVID-19 patient on prophylactic anti-thrombosis with rivaroxaban: A case report. Afr. J. Urol. 2021, 27, 97. [Google Scholar] [CrossRef] [PubMed]
- Lessiani, G.; Boccatonda, A.; D’Ardes, D.; Cocco, G.; di Marco, G.; Schiavone, C. Mondor’s Disease in SARS-CoV-2 Infection: A Case of Superficial Vein Thrombosis in the Era of COVID-19. Eur. J. Case Rep. Intern. Med. 2020, 7, 001803. Available online: https://www.ejcrim.com/index.php/EJCRIM/article/view/1803 (accessed on 28 September 2022). [PubMed]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ 2021, 5, n1114. [Google Scholar] [CrossRef] [PubMed]
- Ramessur, R.; Saffar, N.; Czako, B.; Agarwal, A.; Batta, K. Cutaneous thrombosis associated with skin necrosis following Oxford-AstraZeneca COVID-19 vaccination. Clin. Exp. Dermatol. 2021, 46, 1610–1612. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Yoneyama, T.; Hamaya, T.; Togashi, K.; Narita, T.; Fujita, N.; Yamamoto, H.; Yoneyama, T.; Hashimoto, Y.; Ohyama, C. Antibody responses to BNT162b2 mRNA COVID-19 vaccine in healthcare workers and patients with urological diseases in Japan. Eur. Urol. 2022, 81, S47–S48. [Google Scholar] [CrossRef]
- Nawwar, A.A.; Searle, J.; Hopkins, R.; Lyburn, I.D. False-Positive Axillary Lymph Nodes on FDG PET/CT Resulting From COVID-19 Immunization. Clin. Nucl. Med. 2021, 46, 1004–1005. [Google Scholar] [CrossRef]
- Özütemiz, C.; Krystosek, L.A.; Church, A.L.; Chauhan, A.; Ellermann, J.M.; Domingo-Musibay, E.; Steinberger, D. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology 2021, 300, E296–E300. [Google Scholar] [CrossRef]
- Fortuin, A.; de Rooij, M.; Zamecnik, P.; Haberkorn, U.; Barentsz, J. Molecular and Functional Imaging for Detection of Lymph Node Metastases in Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 13842–13857. [Google Scholar] [CrossRef] [Green Version]
- Andresciani, F.; Ricci, M.; Grasso, R.F.; Zobel, B.B.; Quattrocchi, C.C. COVID-19 vaccination simulating lymph node progression in a patient with prostate cancer. Radiol. Case Rep. 2022, 17, 2996–2999. [Google Scholar] [CrossRef]
- Kremer, D.; Pieters, T.T.; Verhaar, M.C.; Berger, S.P.; Bakker, S.J.L.; Zuilen, A.D.; Joles, J.A.; Vernooij, R.W.M.; van Balkom, B.W.M. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am. J. Transpl. 2021, 21, 3936–3945. [Google Scholar] [CrossRef]
- Haskin, O.; Ashkenazi-Hoffnung, L.; Ziv, N.; Borovitz, Y.; Dagan, A.; Levi, S.; Koren, G.; Hamdani, G.; Levi-Erez, D.; Landau, D.; et al. Serological Response to the BNT162b2 COVID-19 mRNA Vaccine in Adolescent and Young Adult Kidney Transplant Recipients. Transplantation 2021, 105, e226–e233. [Google Scholar] [CrossRef] [PubMed]
- Cordero, E.; Bulnes-Ramos, A.; Aguilar-Guisado, M.; González Escribano, F.; Olivas, I.; Torre-Cisneros, J.; Gavaldá, J.; Aydillo, T.; Moreno, A.; Montejo, M.; et al. Effect of Influenza Vaccination Inducing Antibody Mediated Rejection in Solid Organ Transplant Recipients. Front. Immunol. 2020, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Baluch, A.; Humar, A.; Eurich, D.; Egli, A.; Liacini, A.; Hoschler, K.; Campbell, P.; Berka, N.; Urschel, S.; Wilson, L.; et al. Randomized Controlled Trial of High-Dose Intradermal Versus Standard-Dose Intramuscular Influenza Vaccine in Organ Transplant Recipients: Intradermal Vaccine in Transplant. Am. J. Transpl. 2013, 13, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.T.; Boyarsky, B.J.; Motter, J.D.; Greenberg, R.S.; Teles, A.T.; Ruddy, J.A.; Krach, M.R.; Jain, V.S.; Werbel, W.A.; Avery, R.K.; et al. Safety and Reactogenicity of 2 Doses of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation 2021, 105, 2170–2174. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Plasse, R.; Nee, R.; Gao, S.; Olson, S. Acute kidney injury with gross hematuria and IgA nephropathy after COVID-19 vaccination. Kidney Int. 2021, 100, 944–945. [Google Scholar] [CrossRef]
- Rahim, S.E.G.; Lin, J.T.; Wang, J.C. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021, 100, 238. [Google Scholar] [CrossRef]
- Perrin, P.; Bassand, X.; Benotmane, I.; Bouvier, N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021, 100, 466–468. [Google Scholar] [CrossRef]
- Rubin, E.J.; Baden, L.R.; Morrissey, S. Audio Interview: A Look at Covid-19 Prevention and Care in 2020. N. Engl. J. Med. 2020, 383, e147. [Google Scholar] [CrossRef]
- Windpessl, M.; Bruchfeld, A.; Anders, H.J.; Kramer, H.; Waldman, M.; Renia, L.; Ng, L.F.P.; Xing, Z.; Kronbichler, A. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021, 17, 291–293. [Google Scholar] [CrossRef]
- Zhao, H.; Souders, C.; Carmel, M.; Anger, J.T. Low Rates of Urologic Side Effects Following Coronavirus Disease Vaccination: An Analysis of the Food and Drug Administration Vaccine Adverse Event Reporting System. Urology 2021, 153, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Kuzumi, A.; Yoshizaki, A.; Chiba, K.; Mitsuo, S.; Matsuda, K.M.; Norimatsu, Y.; Nagai, K.; Omatsu, J.; Miyake, T.; Sato, S. Genital necrosis with cutaneous thrombosis after COVID-19 mRNA vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e185–e186. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jdv.17837 (accessed on 28 September 2022). [CrossRef] [PubMed]
- Popatia, S.; Chiu, Y.E. Vulvar aphthous ulcer after COVID-19 vaccination. Pediatr. Dermatol. 2022, 39, 153–154. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foschi, N.; Santoro, P.E.; Borrelli, I.; Gavi, F.; Amantea, C.; Russo, P.; Moscato, U. Urological Safety and COVID-19 Vaccinations. Vaccines 2022, 10, 1887. https://doi.org/10.3390/vaccines10111887
Foschi N, Santoro PE, Borrelli I, Gavi F, Amantea C, Russo P, Moscato U. Urological Safety and COVID-19 Vaccinations. Vaccines. 2022; 10(11):1887. https://doi.org/10.3390/vaccines10111887
Chicago/Turabian StyleFoschi, Nazario, Paolo Emilio Santoro, Ivan Borrelli, Filippo Gavi, Carlotta Amantea, Pierluigi Russo, and Umberto Moscato. 2022. "Urological Safety and COVID-19 Vaccinations" Vaccines 10, no. 11: 1887. https://doi.org/10.3390/vaccines10111887
APA StyleFoschi, N., Santoro, P. E., Borrelli, I., Gavi, F., Amantea, C., Russo, P., & Moscato, U. (2022). Urological Safety and COVID-19 Vaccinations. Vaccines, 10(11), 1887. https://doi.org/10.3390/vaccines10111887








