The Impact and Progression of the COVID-19 Pandemic in Bulgaria in Its First Two Years
Abstract
1. Introduction
2. Methods
2.1. Data Sources
2.2. Data Availability
2.3. SARS-CoV-2 Variants Analysis
2.4. Excess Mortality and P-Scores
2.5. Potential Years of Life Lost (PYLL), Aged-Standardized Years of Life Lost Rate (ASYR), and Working Years of Life Lost (WYLL) Estimates
2.6. Limitations
2.6.1. Limitation of Excess Mortality Measures
2.6.2. Limitations of PYLL/ASYR/WYLL
3. Results
3.1. Loss of Life as a Result of the COVID-19 Pandemic
3.2. Temporal Trajectory of the Pandemic in Bulgaria
3.3. Regional Mortality Patterns in Bulgaria
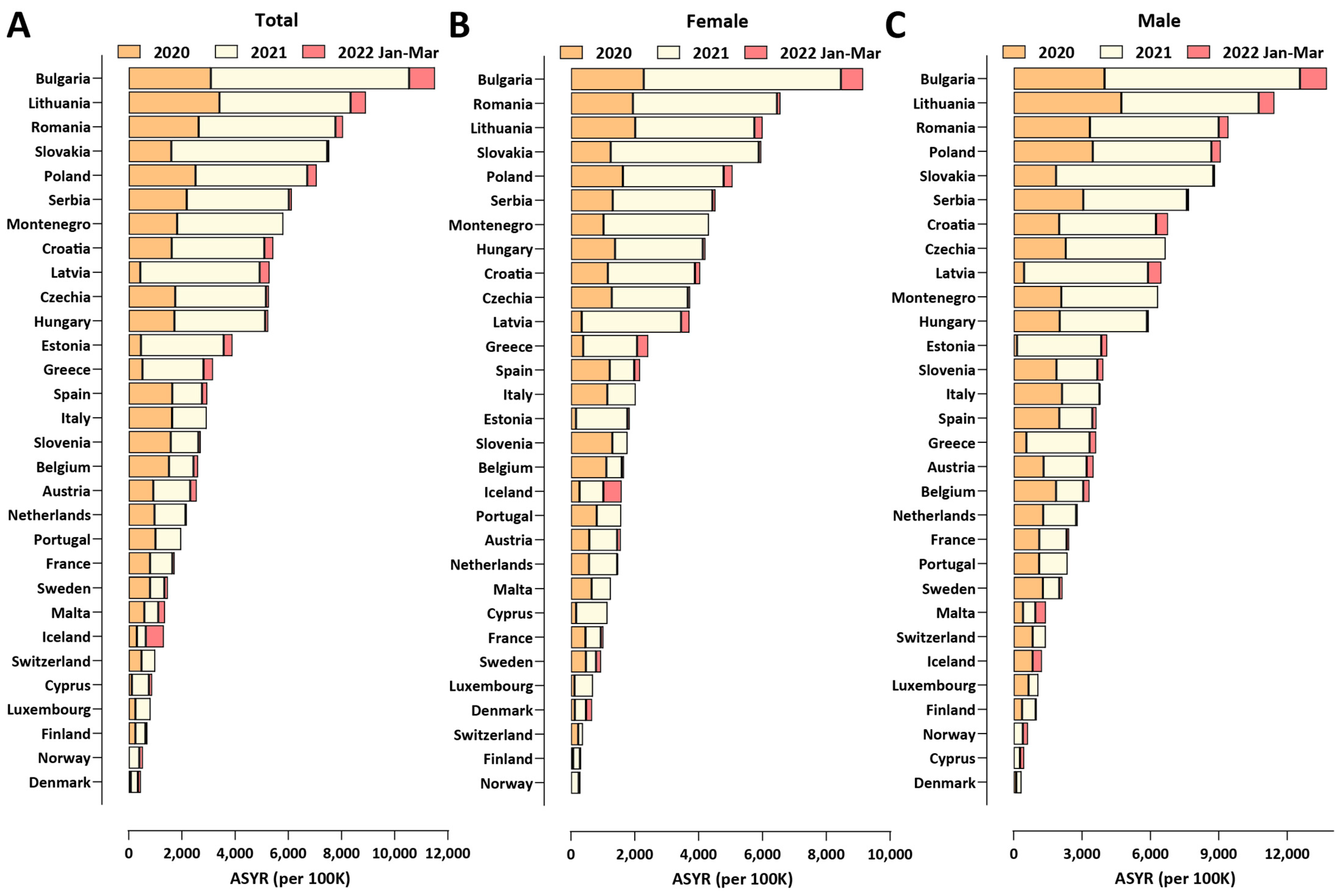
3.4. COVID-19-Related Working-Age Excess Mortality in Bulgaria and Europe
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Karlinsky, A.; Kobak, D. The World Mortality Dataset: Tracking excess mortality across countries during the COVID-19 pandemic. Elife 2021, 10, e69336. [Google Scholar] [CrossRef]
- Pifarré, I.; Arolas, H.; Acosta, E.; López-Casasnovas, G.; Lo, A.; Nicodemo, C.; Riffe, T.; Myrskylä, M. Years of life lost to COVID-19 in 81 countries. Sci. Rep. 2021, 11, 3504. [Google Scholar] [CrossRef]
- Rangachev, A.; Marinov, G.K.; Mladenov, M. The demographic and geographic impact of the COVID pandemic in Bulgaria and Eastern Europe in 2020. Sci. Rep. 2021, 12, 6333. [Google Scholar] [CrossRef]
- Eurostat. Deaths by Week, Sex, 5-Year Age Group. Available online: https://ec.europa.eu/eurostat/databrowser/view/demo_r_mwk_05/default/table?lang=en (accessed on 1 April 2022).
- Eurostat. Deaths by week, sex, 5-Year Age Group and NUTS-3 Region. Available online: https://ec.europa.eu/eurostat/databrowser/view/demo_r_mweek3/default/table?lang=en (accessed on 1 April 2022).
- Eurostat. Population on 1 January by Age Group and Sex Region. Available online: https://ec.europa.eu/eurostat/databrowser/view/demo_pjangroup/default/table?lang=en (accessed on 1 April 2022).
- UNdata Data Service. Population by Age, Sex and Urban/Rural Residence. Available online: http://data.un.org/Data.aspx?d=POP&f=tableCode%3A22 (accessed on 1 April 2022).
- Bulgarian National Statistical Institute. Preliminary Assessment of the Population of Bulgaria at 7 September 2021. Available online: https://www.nsi.bg/en/content/6643/mortality-and-life-expectancy-sex-and-place%20-residence (accessed on 1 April 2022).
- Bulgarian National Statistical Institute. Mortality and Life Expectancy by Sex and Place of Residence. Available online: https://www.nsi.bg/en/content/3018/mortality-and-life-expectancy-sex-and-place-residence (accessed on 1 April 2022).
- World Health Organization. Expectation of Life at Age X (Mortality and Global Health Estimates). Available online: https://apps.who.int/gho/data/node.imr.LIFE_0000000035?lang=en (accessed on 1 April 2022).
- Office for National Statistics. Health State Life Expectancy Estimates Template. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/datasets/healthstatelifeexpectancytemplate (accessed on 1 April 2022).
- Our World in Data. Excess Mortality during the Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/excess-mortality-covid#how-is-excess-mortality-measured (accessed on 1 April 2022).
- Our World in Data. Total COVID-19 Tests per 1000 People, Our World in Data. Available online: https://ourworldindata.org/grapher/full-list-cumulative-total-tests-per-thousand?time=2020-03-01.2020-12-31 (accessed on 1 April 2022).
- Preedy, V.; Watson, R. (Eds.) Handbook of Disease Burdens and Quality of Life Measures; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Martinez-Piedra, R.; Soliz, P.; Caixeta, R.; Ordunez, P. Reflection on modern methods: Years of life lost due to premature mortality—A versatile and comprehensive measure for monitoring non-communicable disease mortality. Int. J. Epidemiol. 2019, 48, 1367–1376. [Google Scholar] [CrossRef]
- Tang, J.W.; Bialasiewicz, S.; Dwyer, D.E.; Dilcher, M.; Tellier, R.; Taylor, J.; Hua, H.; Jennings, L.; Kok, J.; Levy, A.; et al. Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID-19 pandemic. J. Med. Virol. 2021, 93, 4099–4101. [Google Scholar] [CrossRef]
- Sunagawa, S.; Iha, Y.; Kinjo, T.; Nakamura, K.; Fujita, J. Successive disappearance of summer influenza in the Okinawa prefecture during the severe acute respiratory syndrome coronavirus 2 pandemic. Respir. Investig. 2022, 60, 184–186. [Google Scholar] [CrossRef]
- Vittucci, A.C.; Piccioni, L.; Coltella, L.; Ciarlitto, C.; Antilici, L.; Bozzola, E.; Midulla, F.; Palma, P.; Perno, C.F.; Villani, A. The Disappearance of Respiratory Viruses in Children during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 9550. [Google Scholar] [CrossRef]
- Ippolito, G.; La Vecchia, A.; Umbrello, G.; Di Pietro, G.; Bono, P.; Scalia Catenacci, S.; Pinzani, R.; Tagliabue, C.; Bosis, S.; Agostoni, C.; et al. Disappearance of Seasonal Respiratory Viruses in Children Under Two Years Old During COVID-19 Pandemic: A Monocentric Retrospective Study in Milan, Italy. Front. Pediatr. 2021, 9, 721005. [Google Scholar] [CrossRef] [PubMed]
- Finnish Center for Pensions. Retirement Ages. Available online: https://www.etk.fi/en/work-and-pensions-abroad/international-comparisons/retirement-ages/ (accessed on 1 April 2022).
- Ritchie, H.; Mathieu, E.; Rod´es-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Published online at OurWorldInData.org. 2020. Available online: https://covid.ourworldindata.org/data/owid-covid-data.csv (accessed on 1 April 2022).
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Forecasting Team. Variation in the COVID-19 infection-fatality ratio by age, time, and geography during the pre-vaccine era: A systematic analysis. Lancet 2022, 399, 1469–1488. [Google Scholar] [CrossRef]
- Murison, K.R.; Grima, A.A.; Simmons, A.E.; Tuite, A.R.; Fisman, D.N. Severity of SARS-CoV-2 Infection in Pregnancy in Ontario: A Matched Cohort Analysis. Clin. Infect. Dis. 2022, ciac544. [Google Scholar] [CrossRef]
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, 372, n579. [Google Scholar] [CrossRef]
- Grima, A.A.; Murison, K.R.; Simmons, A.E.; Tuite, A.R.; Fisman, D.N. Relative Virulence of SARS-CoV-2 Among Vaccinated and Unvaccinated Individuals Hospitalized with SARS-CoV-2. Clin. Infect. Dis. 2022, ciac412. [Google Scholar] [CrossRef]
- Bhopal, S.S.; Bhopal, R. Sex differential in COVID-19 mortality varies markedly by age. Lancet 2020, 396, 532–533. [Google Scholar] [CrossRef]
- Shu, Y.; McCauley, J. GISAID: Global initiative on sharing all influenza data—From vision to reality. Euro. Surveill. 2017, 22, 30494. [Google Scholar] [CrossRef]
- Plante, J.A.; Liu, Y.; Liu, J.; Xia, H.; Johnson, B.A.; Lokugamage, K.G.; Zhang, X.; Muruato, A.E.; Zou, J.; Fontes-Garfias, C.R.; et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 2021, 592, 116–121. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Wang, X.; Pascal, K.E.; Tomkins-Tinch, C.; Nyalile, T.P.; Wang, Y.; Baum, A.; Diehl, W.E.; Dauphin, A.; Carbone, C.; et al. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 2020, 183, 739–751.e8. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Kiso, M.; Iida, S.; Imai, M.; Takashita, E.; Kuroda, M.; Halfmann, P.J.; Loeber, S.; Maemura, T.; Yamayoshi, S.; et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature 2022, 607, 119–127. [Google Scholar] [CrossRef]
- Yamasoba, D.; Kimura, I.; Nasser, H.; Morioka, Y.; Nao, N.; Ito, J.; Uriu, K.; Tsuda, M.; Zahradnik, J.; Shirakawa, K.; et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. Cell 2022, 185, 2103–2115.e19. [Google Scholar] [CrossRef]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global Omicron variant COVID-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef]
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared with Previous Waves. JAMA 2022, 327, 583–584. [Google Scholar] [CrossRef]
- Esper, F.P.; Adhikari, T.M.; Tu, Z.J.; Cheng, Y.W.; El-Haddad, K.; Farkas, D.H.; Bosler, D.; Rhoads, D.; Procop, G.W.; Ko, J.S.; et al. Alpha to Omicron: Disease Severity and Clinical Outcomes of Major SARS-CoV-2 Variants. J. Infect. Dis. 2022, jiac411. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.J.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Clinical severity of SARS-CoV-2 Omicron BA.4 and BA.5 lineages compared to BA.1 and Delta in South Africa. Nat. Commun. 2022, 13, 5860. [Google Scholar] [CrossRef]
- Butt, A.A.; Dargham, S.R.; Coyle, P.; Yassine, H.M.; Al-Khal, A.; Abou-Samra, A.B.; Abu-Raddad, L.J. COVID-19 Disease Severity in Persons Infected with Omicron BA.1 and BA.2 Sublineages and Association with Vaccination Status. JAMA Intern. Med. 2022, 182, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.; Jassat, W.; DATCOV-Gen Author Group; von Gottberg, A.; Cohen, C. Clinical severity of omicron lineage BA.2 infection compared with BA.1 infection in South Africa. Lancet 2022, 400, 93–96. [Google Scholar] [CrossRef]
- Sievers, C.; Zacher, B.; Ullrich, A.; Huska, M.; Fuchs, S.; Buda, S.; Haas, W.; Diercke, M.; An der Heiden, M.; Kröger, S. SARS-CoV-2 Omicron variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany, 2021 to 2022. Euro. Surveill. 2022, 27, 2200396. [Google Scholar] [CrossRef] [PubMed]
- de Prost, N.; Audureau, E.; Heming, N.; Gault, E.; Pham, T.; Chaghouri, A.; de Montmollin, N.; Voiriot, G.; Morand-Joubert, L.; Joseph, A.; et al. Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. Nat. Commun 2022, 13, 6025. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat. Med. 2022, 28, 1933–1943. [Google Scholar] [CrossRef]
- Molenberghs, G.; Faes, C.; Verbeeck, J.; Deboosere, P.; Abrams, S.; Willem, L.; Aerts, J.; Theeten, H.; Devleesschauwer, B.; Bustos Sierra, N.; et al. COVID-19 mortality, excess mortality, deaths per million and infection fatality ratio, Belgium, 9 March 2020 to 28 June 2020. Euro. Surveill. 2022, 27, 2002060. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; de Larrea, N.F.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Kenyon, C. COVID-19 Infection Fatality Rate Associated with Incidence-A Population-Level Analysis of 19 Spanish Autonomous Communities. Biology 2020, 9, 128. [Google Scholar] [CrossRef]
- Ghisolfi, S.; Almås, I.; Sandefur, J.C.; von Carnap, T.; Heitner, J.; Bold, T. Predicted COVID-19 fatality rates based on age, sex, comorbidities and health system capacity. BMJ Glob. Health 2020, 5, e003094. [Google Scholar] [CrossRef]
- Russell, T.W.; Hellewell, J.; I Jarvis, C.; van Zandvoort, K.; Abbott, S.; Ratnayake, R.; CMMID COVID-19 Working Group; Flasche, S.; Eggo, R.M.; Edmunds, W.J.; et al. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Eurosurveillance 2020, 25, 2000256. [Google Scholar] [CrossRef]
- Hauser, A.; Counotte, M.J.; Margossian, C.C.; Konstantinoudis, G.; Low, N.; Althaus, C.L.; Riou, J. Estimation of SARS-CoV-2 mortality during the early stages of an epidemic: A modeling study in Hubei, China, and six regions in Europe. PLoS Med. 2020, 17, e1003189. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Merone, L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020, 101, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.S.; Marks, N.A. Estimating the case fatality ratio for COVID-19 using a time-shifted distribution analysis. Epidemiol. Infect. 2021, 149, e197. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, K.; Kagaya, K.; Chowell, G. Early epidemiological assessment of the transmission potential and virulence of coronavirus disease 2019 (COVID-19) in Wuhan City, China, January–February, 2020. BMC Med. 2020, 18, 217. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Reconciling estimates of global spread and infection fatality rates of COVID-19: An overview of systematic evaluations. Eur. J. Clin. Investig. 2021, 51, e13554. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, E.; Mulaney, B.; Sood, N.; Shah, S.; Bromley-Dulfano, R.; Lai, C.; Weissberg, Z.; Saavedra-Walker, R.; Tedrow, J.; Bogan, A.; et al. COVID-19 antibody seroprevalence in Santa Clara County, California. Int. J. Epidemiol. 2021, 50, 410–419. [Google Scholar] [CrossRef]
- Marinov, G.K.; Mladenov, M.; Rangachev, A.; Alexiev, I. SARS-CoV-2 reinfections during the first three major COVID-19 waves in Bulgaria. medRxiv 2022. medRxiv:2022.03.11.22271527. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangachev, A.; Marinov, G.K.; Mladenov, M. The Impact and Progression of the COVID-19 Pandemic in Bulgaria in Its First Two Years. Vaccines 2022, 10, 1901. https://doi.org/10.3390/vaccines10111901
Rangachev A, Marinov GK, Mladenov M. The Impact and Progression of the COVID-19 Pandemic in Bulgaria in Its First Two Years. Vaccines. 2022; 10(11):1901. https://doi.org/10.3390/vaccines10111901
Chicago/Turabian StyleRangachev, Antoni, Georgi K. Marinov, and Mladen Mladenov. 2022. "The Impact and Progression of the COVID-19 Pandemic in Bulgaria in Its First Two Years" Vaccines 10, no. 11: 1901. https://doi.org/10.3390/vaccines10111901
APA StyleRangachev, A., Marinov, G. K., & Mladenov, M. (2022). The Impact and Progression of the COVID-19 Pandemic in Bulgaria in Its First Two Years. Vaccines, 10(11), 1901. https://doi.org/10.3390/vaccines10111901





