Assessment of Crosslinkers between Peptide Antigen and Carrier Protein for Fusion Peptide-Directed Vaccines against HIV-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Protocols and Immunization
2.3. Cell Lines
2.4. Fusion Peptide Immunogens
2.5. HIV-1 Envelope Trimers
2.6. Antigenic Characterization
2.7. Anti-Trimer (BG505 DS-SOSIP.664) Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Sera Antigenic Analysis
2.9. Flow Cytometry and B Cell Staining
2.10. Neutralization Assays
2.11. Statistical Analyses
3. Results
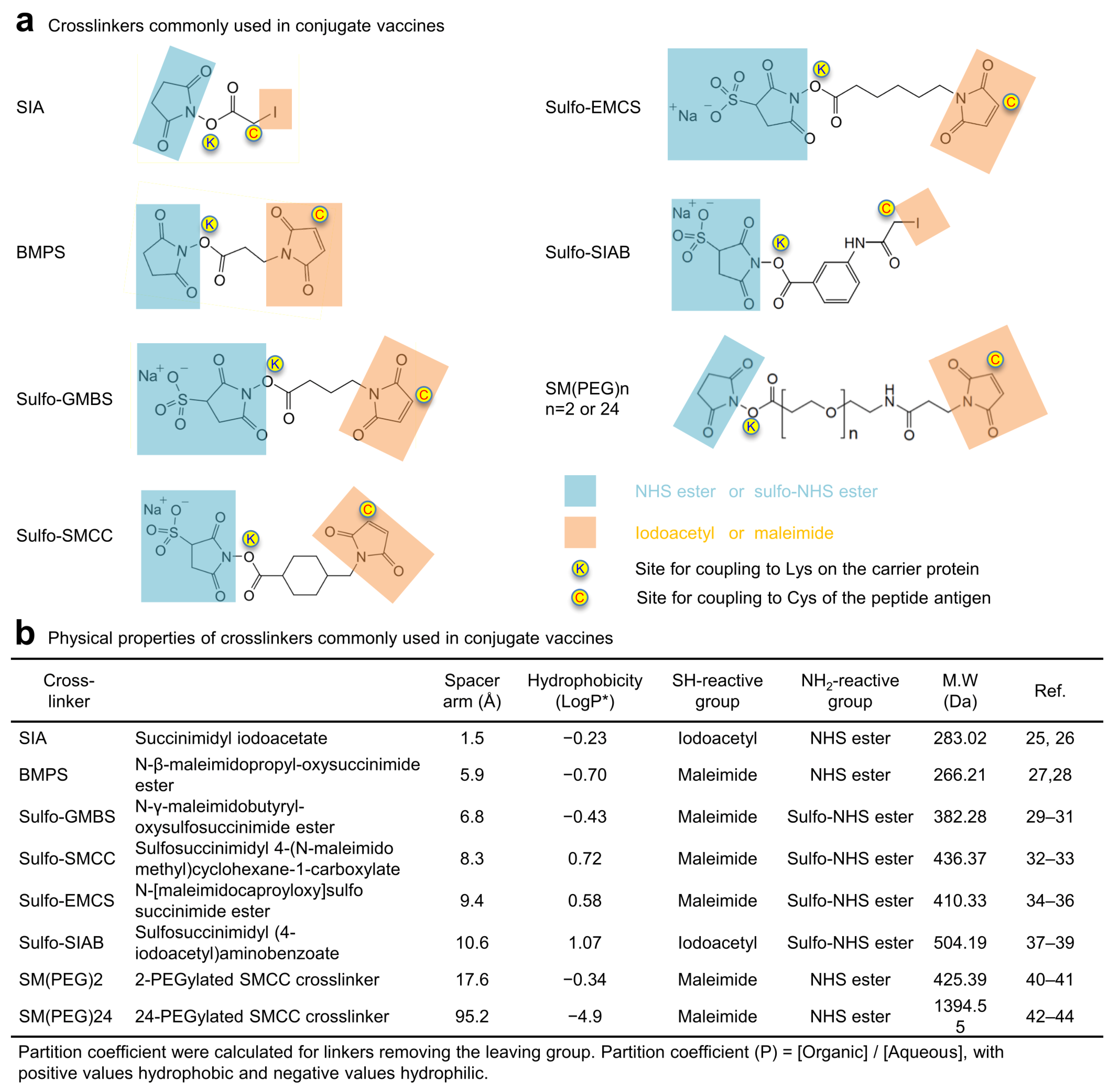
3.1. Physical and Antigenic Properties of FP8v1-rTTHC Conjugates with Eight Linkers with Diverse Chemical Properties
3.2. Immunogenicity Assessments Indicate FP8v1-rTTHC Conjugates to Be Robust to Most Linker Properties
3.3. Neutralization Assessment Reveals FP8v1-rTTHC with Hydrophobic Linker of 5–10 A to Be Preferred
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwong, P.D.; Mascola, J.R.; Nabel, G.J. Rational Design of Vaccines to Elicit Broadly Neutralizing Antibodies to HIV-1. Cold Spring Harb. Perspect Med. 2011, 1, a007278. [Google Scholar] [CrossRef] [PubMed]
- Kwong, P.D.; Mascola, J.R. HIV-1 Vaccines Based on Antibody Identification, B Cell Ontogeny, and Epitope Structure. Immunity 2018, 48, 855–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Taeye, S.W.; Ozorowski, G.; Torrents de la Pena, A.; Guttman, M.; Julien, J.P.; van den Kerkhof, T.L.; Burger, J.A.; Pritchard, L.K.; Pugach, P.; Yasmeen, A.; et al. Immunogenicity of Stabilized HIV-1 Envelope Trimers with Reduced Exposure of Non-neutralizing Epitopes. Cell 2015, 163, 1702–1715. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Xu, K.; Kong, R.; Chuang, G.Y.; Corrigan, A.R.; Geng, H.; Hill, K.R.; Jafari, A.J.; O’Dell, S.; Ou, L.; et al. Consistent elicitation of cross-clade HIV-neutralizing responses achieved in guinea pigs after fusion peptide priming by repetitive envelope trimer boosting. PLoS ONE 2019, 14, e0215163. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Acharya, P.; Kong, R.; Cheng, C.; Chuang, G.Y.; Liu, K.; Louder, M.K.; O’Dell, S.; Rawi, R.; Sastry, M.; et al. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat. Med. 2018, 24, 857–867. [Google Scholar] [CrossRef]
- Kong, R.; Duan, H.; Sheng, Z.; Xu, K.; Acharya, P.; Chen, X.; Cheng, C.; Dingens, A.S.; Gorman, J.; Sastry, M.; et al. Antibody Lineages with Vaccine-Induced Antigen-Binding Hotspots Develop Broad HIV Neutralization. Cell 2019, 178, 567–584 e19. [Google Scholar] [CrossRef]
- Chuang, G.Y.; Lai, Y.T.; Boyington, J.C.; Cheng, C.; Geng, H.; Narpala, S.; Rawi, R.; Schmidt, S.D.; Tsybovsky, Y.; Verardi, R.; et al. Development of a 3Mut-Apex-Stabilized Envelope Trimer That Expands HIV-1 Neutralization Breadth When Used To Boost Fusion Peptide-Directed Vaccine-Elicited Responses. J. Virol. 2020, 94, e00074-20. [Google Scholar] [CrossRef]
- Mogus, A.T.; Liu, L.; Jia, M.; Ajayi, D.T.; Xu, K.; Kong, R.; Huang, J.; Yu, J.; Kwong, P.D.; Mascola, J.R.; et al. Virus-Like Particle Based Vaccines Elicit Neutralizing Antibodies against the HIV-1 Fusion Peptide. Vaccines 2020, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; De Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Durando, P.; Faust, S.N.; Fletcher, M.; Krizova, P.; Torres, A.; Welte, T. Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults. Clin. Microbiol. Infect. 2013, 19 (Suppl. 1), 1–9. [Google Scholar] [CrossRef]
- Radtke, A.J.; Anderson, C.F.; Riteau, N.; Rausch, K.; Scaria, P.; Kelnhofer, E.R.; Howard, R.F.; Sher, A.; Germain, R.N.; Duffy, P. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci. Rep. 2017, 7, 40312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichichero, M.E. Protein carriers of conjugate vaccines: Characteristics, development, and clinical trials. Hum. Vaccin. Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.H. Recent advances in the molecular design of synthetic vaccines. Nat. Chem. 2015, 7, 952–960. [Google Scholar] [CrossRef]
- Buskas, T.; Li, Y.; Boons, G.J. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry 2004, 10, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Yu, W.; Hu, C.; Shen, L.; Hu, T. The phenyl linker markedly increases the immunogenicity of the pneumococcal polysaccharide conjugate vaccine. Biotechnol. Lett. 2018, 40, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Kong, W.P.; Chuang, G.Y.; Ghosh, M.; Gulla, K.; O’Dell, S.; Varriale, J.; Barefoot, N.; Changela, A.; Chao, C.W.; et al. Preclinical Development of a Fusion Peptide Conjugate as an HIV Vaccine Immunogen. Sci. Rep. 2020, 10, 3032. [Google Scholar] [CrossRef] [Green Version]
- Gulla, K.; Cibelli, N.; Cooper, J.W.; Fuller, H.C.; Schneiderman, Z.; Witter, S.; Zhang, Y.; Changela, A.; Geng, H.; Hatcher, C.; et al. A non-affinity purification process for GMP production of prefusion-closed HIV-1 envelope trimers from clades A and C for clinical evaluation. Vaccine 2021, 39, 3379–3387. [Google Scholar] [CrossRef]
- Dalal, J.; Rana, R.; Harale, K.; Hanif, S.; Kumar, N.; Singh, D.; Chhikara, M.K. Development and pre-clinical evaluation of a synthetic oligosaccharide-protein conjugate vaccine against Neisseria meningitidis serogroup C. Vaccine 2019, 37, 5297–5306. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 18—PEGylation and Synthetic Polymer Modification. In Bioconjugate Techniques, 3rd, ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 787–838. [Google Scholar]
- Cho, S.; Zammarchi, F.; Williams, D.G.; Havenith, C.E.G.; Monks, N.R.; Tyrer, P.; D’Hooge, F.; Fleming, R.; Vashisht, K.; Dimasi, N.; et al. Antitumor Activity of MEDI3726 (ADCT-401), a Pyrrolobenzodiazepine Antibody-Drug Conjugate Targeting PSMA, in Preclinical Models of Prostate Cancer. Mol. Cancer 2018, 17, 2176–2186. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Stevens, V.C.; Schwendeman, S.P. Injectable polymer microspheres enhance immunogenicity of a contraceptive peptide vaccine. Vaccine 2007, 25, 500–509. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Zheng, W.; Jiang, X. Hollow carbon nanospheres for targeted delivery of chemotherapeutics in breast cancer therapy. J. Mater. Chem. B 2017, 5, 6601–6607. [Google Scholar] [CrossRef]
- Stevens, M.W.; Gunnell, M.G.; Tawney, R.; Owens, S.M. Optimization of a methamphetamine conjugate vaccine for antibody production in mice. Int. Immunopharmacol. 2016, 35, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Veiseh, O.; Gunn, J.; Fang, C.; Hansen, S.; Lee, D.; Sze, R.; Ellenbogen, R.G.; Olson, J.; Zhang, M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small 2008, 4, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Wunderbaldinger, P.; Josephson, L.; Weissleder, R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug. Chem. 2002, 13, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.; Zhang, P.; Yuan, Z.; Dong, D.; Wu, K.; Niu, L.; McMullen, P.; Luozhong, S.; Hung, H.C.; Cheng, Y.H.; et al. Zwitterionic Polymer Conjugated Glucagon-like Peptide-1 for Prolonged Glycemic Control. Bioconjug. Chem. 2020, 31, 1812–1819. [Google Scholar] [CrossRef]
- Xiao, K.; Zhao, Y.; Choi, M.; Liu, H.; Blanc, A.; Qian, J.; Cahill, T.J., 3rd; Li, X.; Xiao, Y.; Clark, L.J.; et al. Revealing the architecture of protein complexes by an orthogonal approach combining HDXMS, CXMS, and disulfide trapping. Nat. Protoc. 2018, 13, 1403–1428. [Google Scholar] [CrossRef]
- Song, H.Y.; Zhou, X.; Hobley, J.; Su, X. Comparative study of random and oriented antibody immobilization as measured by dual polarization interferometry and surface plasmon resonance spectroscopy. Langmuir 2012, 28, 997–1004. [Google Scholar] [CrossRef]
- Westcott, M.M.; Clemens, E.A.; Holbrook, B.C.; King, S.B.; Alexander-Miller, M.A. The choice of linker for conjugating R848 to inactivated influenza virus determines the stimulatory capacity for innate immune cells. Vaccine 2018, 36, 1174–1182. [Google Scholar] [CrossRef]
- Wheeler, B.C.; Corey, J.M.; Brewer, G.J.; Branch, D.W. Microcontact printing for precise control of nerve cell growth in culture. J. Biomech. Eng. 1999, 121, 73–78. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Wang, X.; Chen, B.; Xiao, Z.; Shi, C.; Wei, Z.; Hou, X.; Wang, Q.; Dai, J. The osteogenic effect of bone morphogenetic protein-2 on the collagen scaffold conjugated with antibodies. J. Control. Release 2010, 141, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, H.; Yao, L.; Yu, R.; Feng, M.; Zou, B. Applications of mesenchymal stem cells labeled with Tat peptide conjugated quantum dots to cell tracking in mouse body. Bioconjug. Chem. 2008, 19, 421–427. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Ilyas, R.; Dodds, A.W.; Sim, R.B. Enzyme-independent, orientation-selective conjugation of whole human complement C3 to protein surfaces. J. Immunol. Methods 2008, 337, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Guo, A.; Li, M.; Liu, W.; Pan, Z.; Jiang, L.; Wu, X.; Xu, H. Salmonella flagellin is a potent carrier-adjuvant for peptide conjugate to induce peptide-specific antibody response in mice. Vaccine 2015, 33, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, J.; Morimatsu, M.; Yanagida, T.; Sako, Y. Covalent immobilization of epidermal growth factor molecules for single-molecule imaging analysis of intracellular signaling. Biomaterials 2006, 27, 3343–3350. [Google Scholar] [CrossRef]
- Patrulea, V.; Hirt-Burri, N.; Jeannerat, A.; Applegate, L.A.; Ostafe, V.; Jordan, O.; Borchard, G. Peptide-decorated chitosan derivatives enhance fibroblast adhesion and proliferation in wound healing. Carbohydr. Polym. 2016, 142, 114–123. [Google Scholar] [CrossRef]
- Schroeder, B.; Le Xuan, H.; Völzke, J.L.; Weller, M.G. Preactivation Crosslinking—An Efficient Method for the Oriented Immobilization of Antibodies. Methods Protoc. 2019, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Qian, F. Conjugation of Plasmodium falciparum Pfs25 to Pseudomonas aeruginosa ExoProtein A with different chemical linkers. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2011, 29, 254–257. [Google Scholar]
- Kitai, Y.; Fukuda, H.; Enomoto, T.; Asakawa, Y.; Suzuki, T.; Inouye, S.; Handa, H. Cell selective targeting of a simian virus 40 virus-like particle conjugated to epidermal growth factor. J. Biotechnol. 2011, 155, 251–256. [Google Scholar] [CrossRef]
- Noteborn, W.E.; Zwagerman, D.N.; Talens, V.S.; Maity, C.; van der Mee, L.; Poolman, J.M.; Mytnyk, S.; van Esch, J.H.; Kros, A.; Eelkema, R.; et al. Crosslinker-Induced Effects on the Gelation Pathway of a Low Molecular Weight Hydrogel. Adv. Mater. 2017, 29, 1603769. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, M. A smart viral vector for targeted delivery of hydrophobic drugs. Sci. Rep. 2021, 11, 7030. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Loh, K.C. Immobilization of hydrophobic peptidic ligands to hydrophilic chromatographic matrix: A preconcentration approach. Anal. Biochem. 2012, 423, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, L.; Zhou, Y.; Yin, H.; Ai, S. Enhanced Photoelectrochemical Method for Sensitive Detection of Protein Kinase A Activity Using TiO2/g-C3N4, PAMAM Dendrimer, and Alkaline Phosphatase. Anal. Chem. 2017, 89, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.M.; Schwaar, T.; Bienwald, F.; Weller, M.G. Predictable Peptide Conjugation Ratios by Activation of Proteins with Succinimidyl Iodoacetate (SIA). Methods Protoc. 2017, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Kong, R.; Xu, K.; Zhou, T.; Acharya, P.; Lemmin, T.; Liu, K.; Ozorowski, G.; Soto, C.; Taft, J.D.; Bailer, R.T.; et al. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science 2016, 352, 828–833. [Google Scholar] [CrossRef] [Green Version]
- Blattner, C.; Lee, J.H.; Sliepen, K.; Derking, R.; Falkowska, E.; de la Pena, A.T.; Cupo, A.; Julien, J.P.; van Gils, M.; Lee, P.S.; et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 2014, 40, 669–680. [Google Scholar] [CrossRef] [Green Version]
- van Gils, M.J.; van den Kerkhof, T.L.; Ozorowski, G.; Cottrell, C.A.; Sok, D.; Pauthner, M.; Pallesen, J.; de Val, N.; Yasmeen, A.; de Taeye, S.W.; et al. An HIV-1 antibody from an elite neutralizer implicates the fusion peptide as a site of vulnerability. Nat. Microbiol. 2016, 2, 16199. [Google Scholar] [CrossRef] [Green Version]
- Qian, F.; Aebig, J.A.; Reiter, K.; Barnafo, E.; Zhang, Y.; Shimp, R.L., Jr.; Rausch, K.M.; Jones, D.S.; Zhu, D.; Lambert, L.; et al. Enhanced antibody responses to Plasmodium falciparum Pfs28 induced in mice by conjugation to ExoProtein A of Pseudomonas aeruginosa with an improved procedure. Microbes Infect. 2009, 11, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Joyce, M.G.; Georgiev, I.S.; Yang, Y.; Druz, A.; Geng, H.; Chuang, G.Y.; Kwon, Y.D.; Pancera, M.; Rawi, R.; Sastry, M.; et al. Soluble Prefusion Closed DS-SOSIP.664-Env Trimers of Diverse HIV-1 Strains. Cell Rep. 2017, 21, 2992–3002. [Google Scholar] [CrossRef] [Green Version]
- Kothe, D.L.; Li, Y.; Decker, J.M.; Bibollet-Ruche, F.; Zammit, K.P.; Salazar, M.G.; Chen, Y.; Weng, Z.; Weaver, E.A.; Gao, F.; et al. Ancestral and consensus envelope immunogens for HIV-1 subtype C. Virology 2006, 352, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Duan, H.; Xu, K.; Chuang, G.Y.; Corrigan, A.R.; Geng, H.; O’Dell, S.; Ou, L.; Chambers, M.; Changela, A.; et al. Immune Monitoring Reveals Fusion Peptide Priming to Imprint Cross-Clade HIV-Neutralizing Responses with a Characteristic Early B Cell Signature. Cell Rep. 2020, 32, 107981. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, A.R.; Duan, H.; Cheng, C.; Gonelli, C.A.; Ou, L.; Xu, K.; DeMouth, M.E.; Geng, H.; Narpala, S.; O’Connell, S.; et al. Fusion peptide priming reduces immune responses to HIV-1 envelope trimer base. Cell Rep. 2021, 35, 108937. [Google Scholar] [CrossRef] [PubMed]
- Galabova, G.; Brunner, S.; Winsauer, G.; Juno, C.; Wanko, B.; Mairhofer, A.; Luhrs, P.; Schneeberger, A.; von Bonin, A.; Mattner, F.; et al. Peptide-based anti-PCSK9 vaccines—An approach for long-term LDLc management. PLoS ONE 2014, 9, e114469. [Google Scholar] [CrossRef] [Green Version]
- Ponte, J.F.; Sun, X.; Yoder, N.C.; Fishkin, N.; Laleau, R.; Coccia, J.; Lanieri, L.; Bogalhas, M.; Wang, L.; Wilhelm, S.; et al. Understanding How the Stability of the Thiol-Maleimide Linkage Impacts the Pharmacokinetics of Lysine-Linked Antibody-Maytansinoid Conjugates. Bioconjug. Chem. 2016, 27, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Tsurutani, J.; Takahashi, S.; Iwata, H.; Krop, I.E.; Redfern, C.; Sagara, Y.; Doi, T.; Park, H.; Murthy, R.K.; et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 2019, 20, 816–826. [Google Scholar] [CrossRef]
- Stefanetti, G.; Rondini, S.; Lanzilao, L.; Saul, A.; MacLennan, C.A.; Micoli, F. Impact of conjugation chemistry on the immunogenicity of S. Typhimurium conjugate vaccines. Vaccine 2014, 32, 6122–6129. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Li, D.; Kang, A.; An, W.; Fan, B.; Ma, X.; Ma, G.; Su, Z.; Hu, T. PEG as a spacer arm markedly increases the immunogenicity of meningococcal group Y polysaccharide conjugate vaccine. J. Control. Release 2013, 172, 382–389. [Google Scholar] [CrossRef]
- Micoli, F.; Romano, M.R.; Tontini, M.; Cappelletti, E.; Gavini, M.; Proietti, D.; Rondini, S.; Swennen, E.; Santini, L.; Filippini, S.; et al. Development of a glycoconjugate vaccine to prevent meningitis in Africa caused by meningococcal serogroup X. Proc. Natl. Acad. Sci. USA 2013, 110, 19077–19082. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, Z.; Su, Z.; Yang, Y.; Ma, G.; Yu, R.; Zhang, S. Development of meningococcal polysaccharide conjugate vaccine that can elicit long-lasting and strong cellular immune response with hepatitis B core antigen virus-like particles as a novel carrier protein. Vaccine 2019, 37, 956–964. [Google Scholar] [CrossRef]
- Ni, J.; Song, H.; Wang, Y.; Stamatos, N.M.; Wang, L.X. Toward a carbohydrate-based HIV-1 vaccine: Synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjug. Chem. 2006, 17, 493–500. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, L.; Gulla, K.; Biju, A.; Biner, D.W.; Bylund, T.; Changela, A.; Chen, S.J.; Zheng, C.-Y.; Cibelli, N.; Corrigan, A.R.; et al. Assessment of Crosslinkers between Peptide Antigen and Carrier Protein for Fusion Peptide-Directed Vaccines against HIV-1. Vaccines 2022, 10, 1916. https://doi.org/10.3390/vaccines10111916
Ou L, Gulla K, Biju A, Biner DW, Bylund T, Changela A, Chen SJ, Zheng C-Y, Cibelli N, Corrigan AR, et al. Assessment of Crosslinkers between Peptide Antigen and Carrier Protein for Fusion Peptide-Directed Vaccines against HIV-1. Vaccines. 2022; 10(11):1916. https://doi.org/10.3390/vaccines10111916
Chicago/Turabian StyleOu, Li, Krishana Gulla, Andrea Biju, Daniel W. Biner, Tatsiana Bylund, Anita Changela, Steven J. Chen, Cheng-Yan Zheng, Nicole Cibelli, Angela R. Corrigan, and et al. 2022. "Assessment of Crosslinkers between Peptide Antigen and Carrier Protein for Fusion Peptide-Directed Vaccines against HIV-1" Vaccines 10, no. 11: 1916. https://doi.org/10.3390/vaccines10111916
APA StyleOu, L., Gulla, K., Biju, A., Biner, D. W., Bylund, T., Changela, A., Chen, S. J., Zheng, C.-Y., Cibelli, N., Corrigan, A. R., Duan, H., Gonelli, C. A., Kong, W.-P., Cheng, C., O’Dell, S., Sarfo, E. K., Shaddeau, A., Wang, S., Vinitsky, A., ... Kwong, P. D. (2022). Assessment of Crosslinkers between Peptide Antigen and Carrier Protein for Fusion Peptide-Directed Vaccines against HIV-1. Vaccines, 10(11), 1916. https://doi.org/10.3390/vaccines10111916





