Single-Shot ChAd3-MARV Vaccine in Modified Formulation Buffer Shows 100% Protection of NHPs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Production and Formulation
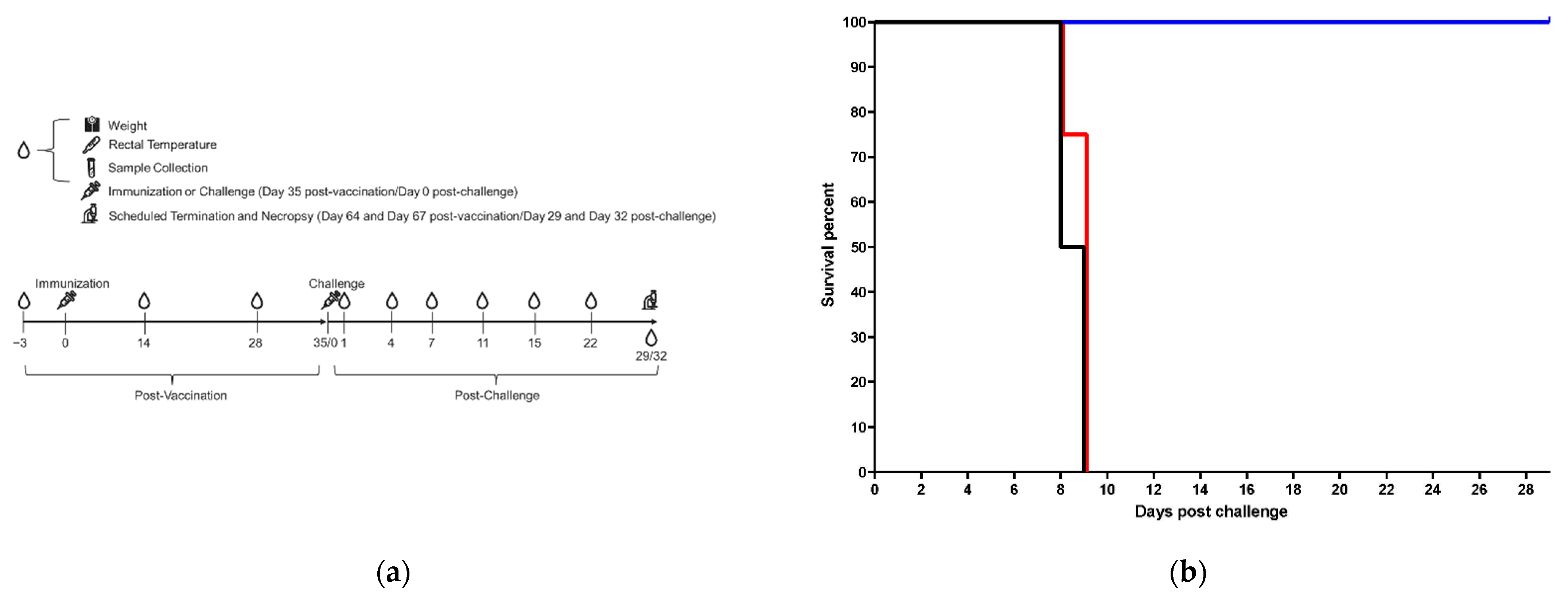
2.2. Animal Study
2.3. Vaccine Material and Control Preparation
2.4. Challenge Virus, Preparation and Back-Titration
2.5. Blood for Clinical Chemistry, Hematology and Coagulation
2.6. Serum and Tissue Viral Load Analysis by Plaque Assay and qRT-PCR
2.7. Histopathology
2.8. Anti-MARV GP IgG ELISA
2.9. NHP Immunization for PBMC Harvest and ELISpot
3. Results
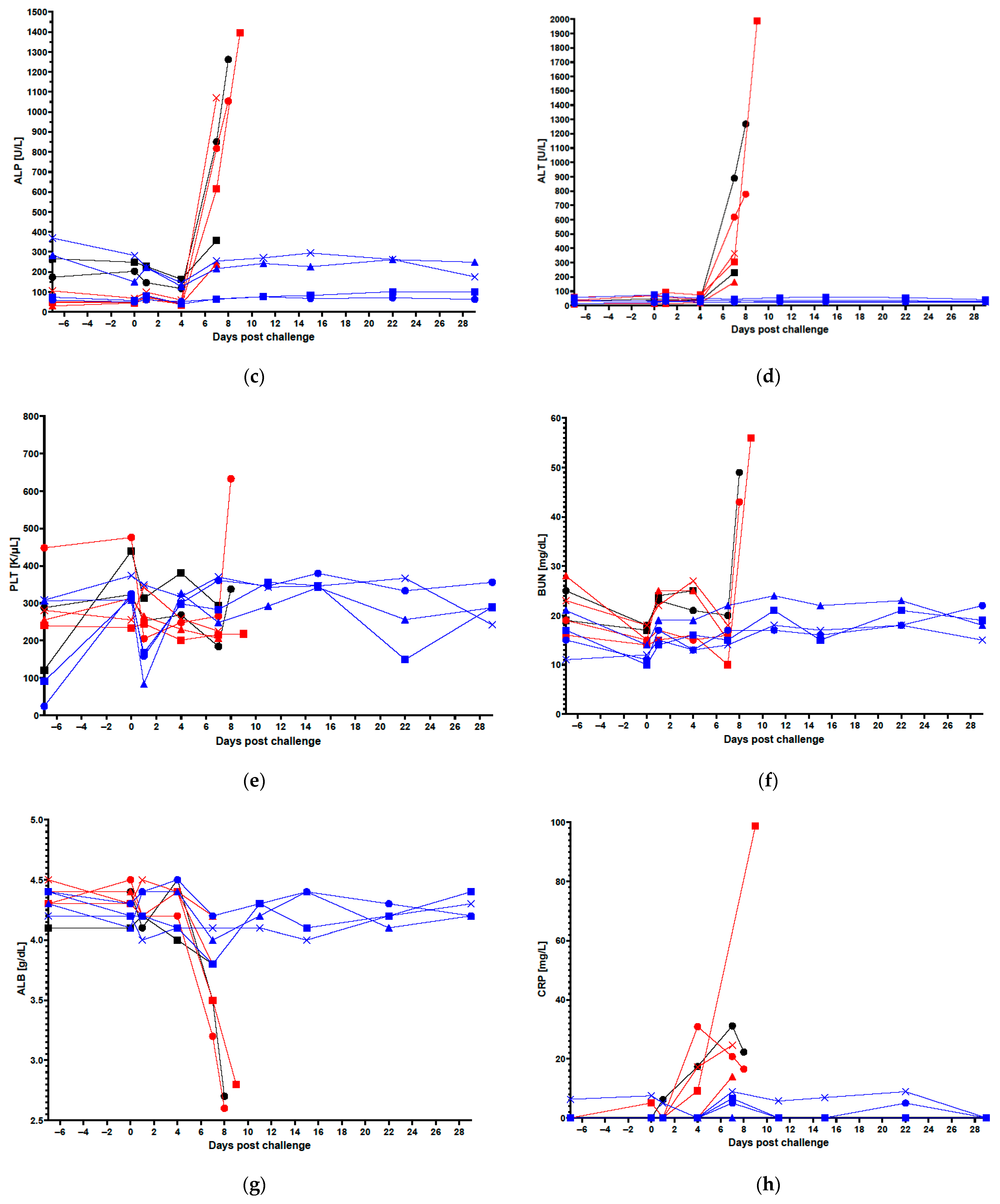
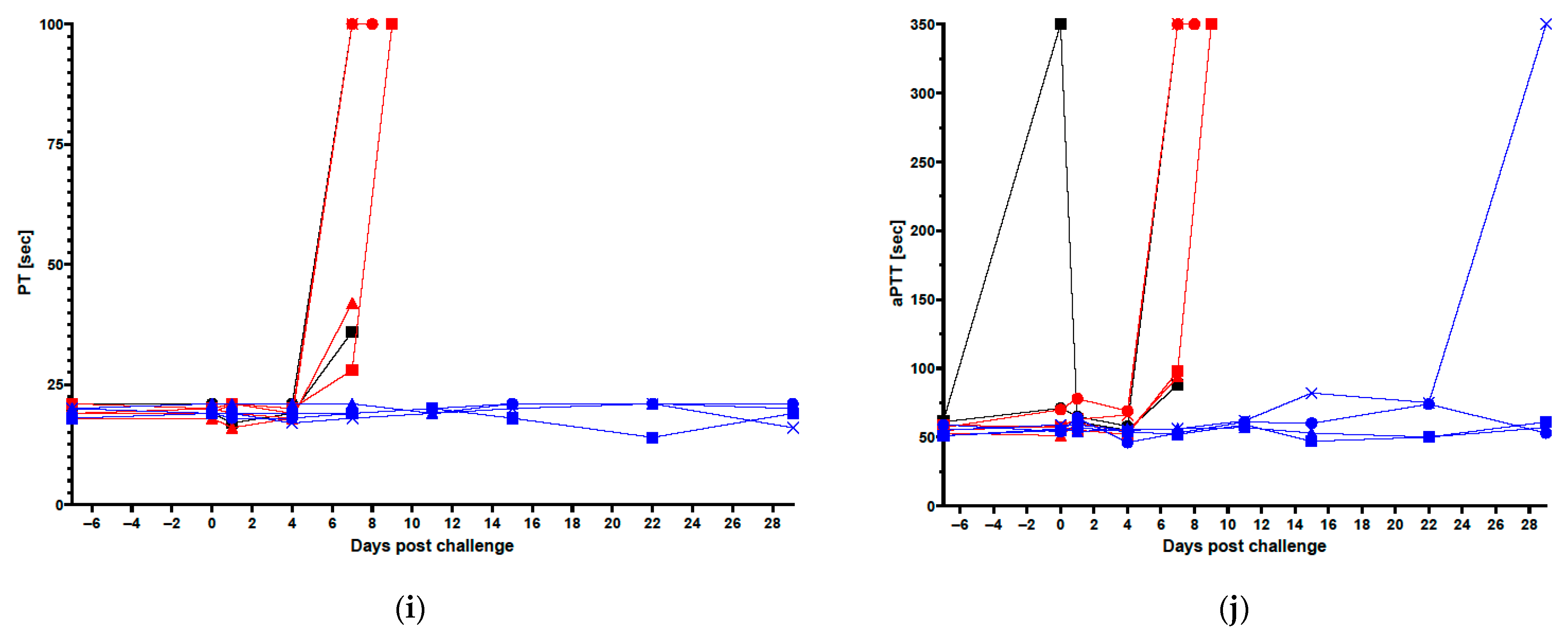
3.1. Clinical Data in Vaccinated and Control Animals
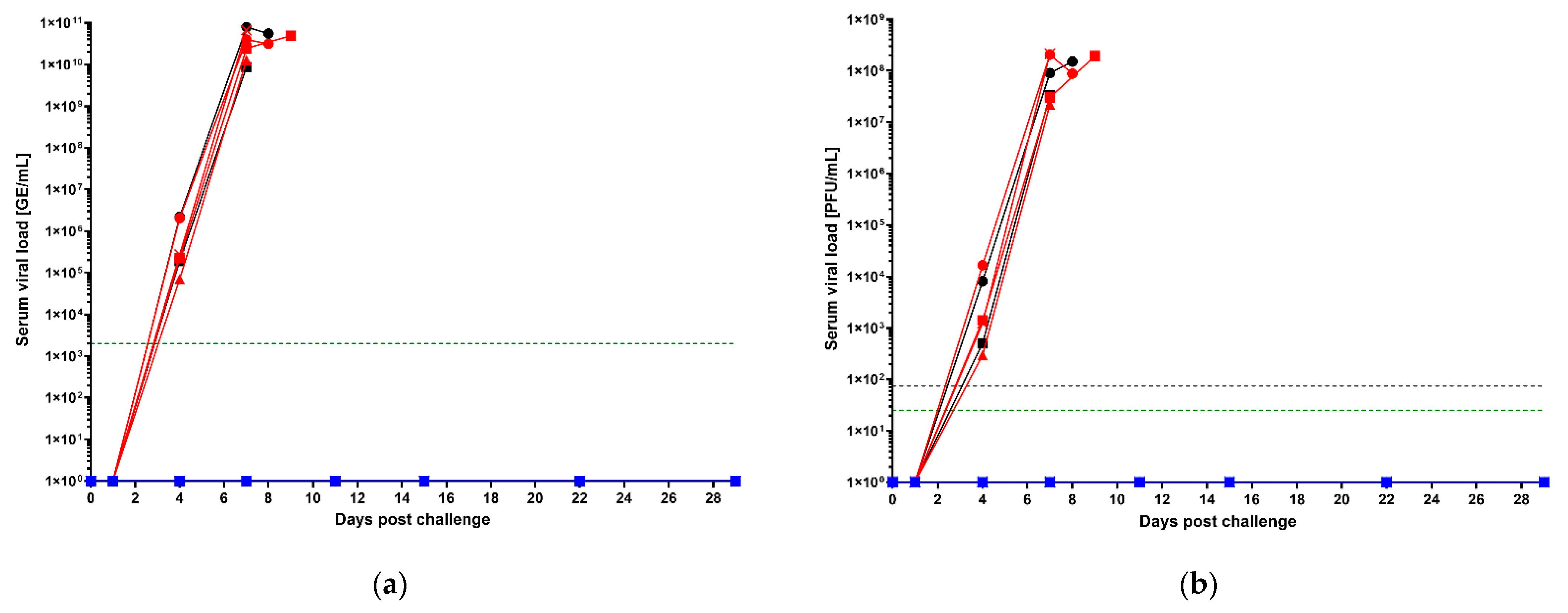
3.2. Post-Challenge Serum Viral Loads by qRT-PCR and Plaque Assay
3.3. Tissue Viral Loads by qRT-PCR and Plaque Assay
3.4. Histopathological Findings in Select Tissues
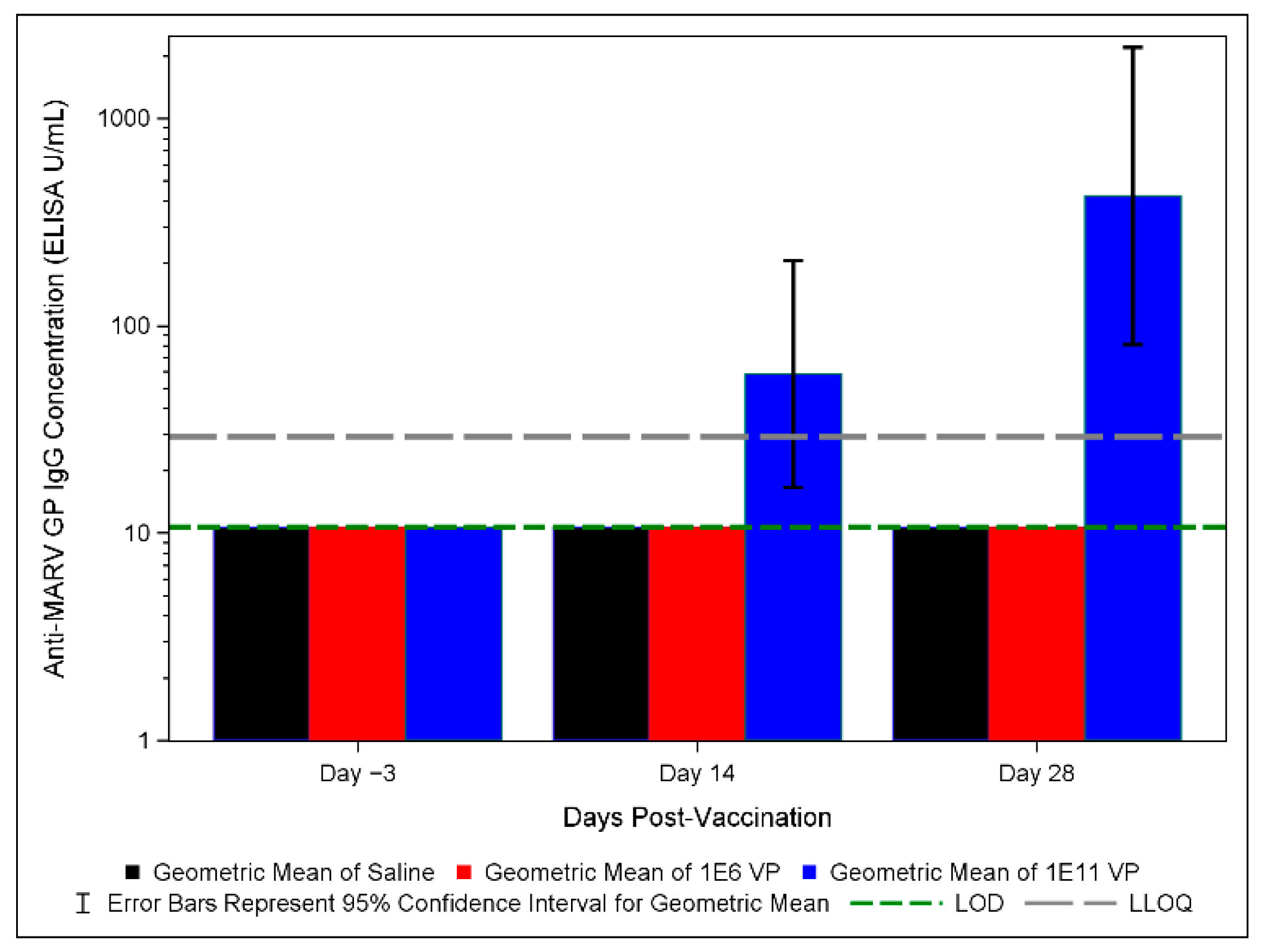
3.5. Anti-MARV GP IgG ELISA Results in Vaccinated and Control Groups
3.6. MARV GP-Specific IFNγ Responses in Vaccinated Animals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Marburg Virus Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease (accessed on 8 January 2022).
- Kortepeter, M.G.; Dierberg, K.; Shenoy, E.S.; Cieslak, T.J. Marburg virus disease: A summary for clinicians. Int. J. Infect. Dis. 2020, 99, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.; Sprecher, A.; Geisbert, T.W. Ebola. N. Engl. J. Med. 2020, 382, 1832–1842. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Africa West Africa’s First-Ever Case of Marburg Virus Disease Confirmed in Guinea. Available online: https://www.afro.who.int/news/west-africas-first-ever-case-marburg-virus-disease-confirmed-guinea (accessed on 13 March 2022).
- World Health Organization. Marburg Virus—Ghana. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON402 (accessed on 14 August 2022).
- Towner, J.S.; Pourrut, X.; Albariño, C.G.; Nkogue, C.N.; Bird, B.H.; Grard, G.; Ksiazek, T.G.; Gonzalez, J.P.; Nichol, S.T.; Leroy, E.M. Marburg virus infection detected in a common African bat. PLoS ONE 2007, 2, e764. [Google Scholar] [CrossRef] [PubMed]
- Pawęska, J.T.; Storm, N.; Markotter, W.; Di Paola, N.; Wiley, M.R.; Palacios, G.; van Vuren, P.J. Shedding of marburg virus in naturally infected egyptian rousette bats, South Africa, 2017. Emerg. Infect. Dis. 2020, 26, 3051–3055. [Google Scholar] [CrossRef]
- Wolf, J.; Jannat, R.; Dubey, S.; Troth, S.; Onorato, M.T.; Coller, B.A.; Hanson, M.E.; Simon, J.K. Development of pandemic vaccines: ERVEBO case study. Vaccines 2021, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. First FDA-Approved Vaccine for the Prevention of Ebola Virus Disease, Marking a Critical Milestone in Public Health Preparedness and Response. Available online: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health (accessed on 8 January 2022).
- European Medicines Agency. First Vaccine to Protect Against Ebola. Available online: https://www.ema.europa.eu/en/news/first-vaccine-protect-against-ebola (accessed on 8 January 2022).
- Lui, A. Vaccines China Approves Domestic Ebola Vaccine Developed from Recent Outbreak. Available online: https://www.fiercepharma.com/vaccines/china-approves-self-developed-ebola-vaccine-from-2014-outbreak-virus-type (accessed on 9 December 2021).
- Kuhn, J.H.; Adachi, T.; Adhikari, N.K.J.; Arribas, J.R.; Bah, I.E.; Bausch, D.G.; Bhadelia, N.; Borchert, M.; Brantsæter, A.B.; Brett-Major, D.M.; et al. New filovirus disease classification and nomenclature. Nat. Rev. Microbiol. 2019, 17, 261–263. [Google Scholar] [CrossRef]
- European Medicines Agency. New Vaccine for Prevention of Ebola Virus Disease Recommended for Approval in the European Union. Available online: https://www.ema.europa.eu/en/news/new-vaccine-prevention-ebola-virus-disease-recommended-approval-european-union (accessed on 8 December 2021).
- Roozendaal, R.; Hendriks, J.; van Effelterre, T.; Spiessens, B.; Dekking, L.; Solforosi, L.; Czapska-Casey, D.; Bockstal, V.; Stoop, J.; Splinter, D.; et al. Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate. NPJ Vaccines 2020, 5, 112. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Fusco, M.L.; Bornholdt, Z.A.; Lee, J.E.; Flyak, A.I.; Matsuoka, R.; Kohda, D.; Yanagi, Y.; Hammel, M.; Crowe, J.E.; et al. Structural Basis for Marburg Virus Neutralization by a Cross-Reactive Human Antibody. Cell 2015, 160, 904–912. [Google Scholar] [CrossRef] [Green Version]
- Mittler, E.; Kolesnikova, L.; Hartlieb, B.; Davey, R.; Becker, S. The Cytoplasmic Domain of Marburg Virus GP Modulates Early Steps of Viral Infection. J. Virol. 2011, 85, 8188–8196. [Google Scholar] [CrossRef] [Green Version]
- Kibuuka, H.; Berkowitz, N.M.; Millard, M.; Enama, M.E.; Tindikahwa, A.; Sekiziyivu, A.B.; Costner, P.; Sitar, S.; Glover, D.; Hu, Z.; et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: A phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet 2015, 385, 1545–1554. [Google Scholar] [CrossRef]
- Albert, B. Sabin Vaccine Institute Evaluation of Safety, Tolerability and Immune Responses of Ebola-S and Marburg Vaccines in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04723602?term=NCT04723602&draw=2&rank=1 (accessed on 8 January 2022).
- National Institute of Allergy and Infectious Diseases. cAd3-Marburg Vaccine in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03475056 (accessed on 8 January 2022).
- Siragam, V.; Wong, G.; Qiu, X.G. Animal models for filovirus infections. Zool. Res. 2018, 39, 15–24. [Google Scholar] [CrossRef] [PubMed]
- St Claire, M.C.; Ragland, D.R.; Bollinger, L.; Jahrling, P.B. Animal models of Ebolavirus infection. Comp. Med. 2017, 67, 253–262. [Google Scholar] [PubMed]
- Marzi, A.; Menicucci, A.R.; Engelmann, F.; Callison, J.; Horne, E.J.; Feldmann, F.; Jankeel, A.; Feldmann, H.; Messaoudi, I. Protection against marburg virus using a recombinant VSV-vaccine depends on T and B cell activation. Front. Immunol. 2019, 10, 3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrer, A.T.; Chuang, E.; Namekar, M.; Williams, C.A.; Wong, T.A.S.; Lieberman, M.M.; Granados, A.; Misamore, J.; Yalley-Ogunro, J.; Andersen, H.; et al. Recombinant Protein Filovirus Vaccines Protect Cynomolgus Macaques From Ebola, Sudan, and Marburg Viruses. Front. Immunol. 2021, 12, 703986. [Google Scholar] [CrossRef]
- Marzi, A.; Jankeel, A.; Menicucci, A.R.; Callison, J.; O’Donnell, K.L.; Feldmann, F.; Pinski, A.N.; Hanley, P.W.; Messaoudi, I. Single Dose of a VSV-Based Vaccine Rapidly Protects Macaques From Marburg Virus Disease. Front. Immunol. 2021, 12, 774026. [Google Scholar] [CrossRef]
- Hunegnaw, R.; Honko, A.; Wang, L.; Carr, D.; Murray, T.; Shi, W.; Nguyen, L.; Storm, N.; Dulan, C.N.M.; Foulds, K.E.; et al. A Single-Shot ChAd3-MARV GP Vaccine Confers Rapid and Durable Protection Against Marburg Virus in non-human primates. Sci. Transl. Med. 2022; in press. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Bailey, M.; Geisbert, J.B.; Asiedu, C.; Roederer, M.; Grazia-Pau, M.; Custers, J.; Jahrling, P.; Goudsmit, J.; Koup, R.; et al. Vector Choice Determines Immunogenicity and Potency of Genetic Vaccines against Angola Marburg Virus in Nonhuman Primates. J. Virol. 2010, 84, 10386–10394. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, P.; Marzi, A. Ebola and Marburg virus vaccines. Virus Genes 2017, 53, 501–515. [Google Scholar] [CrossRef]
- Callendret, B.; Vellinga, J.; Wunderlich, K.; Rodriguez, A.; Steigerwald, R.; Dirmeier, U.; Cheminay, C.; Volkmann, A.; Brasel, T.; Carrion, R.; et al. A prophylactic multivalent vaccine against different filovirus species is immunogenic and provides protection from lethal infections with Ebolavirus and Marburgvirus species in non-human primates. PLoS ONE 2018, 13, e0192312. [Google Scholar] [CrossRef] [Green Version]
- Cross, R.W.; Xu, R.; Matassov, D.; Hamm, S.; Latham, T.E.; Gerardi, C.S.; Nowak, R.M.; Geisbert, J.B.; Ota-Setlik, A.; Agans, K.N.; et al. Quadrivalent VesiculoVax vaccine protects nonhuman primates from viral-induced hemorrhagic fever and death. J. Clin. Investig. 2020, 130, 539–551. [Google Scholar] [CrossRef]
- Matassov, D.; Mire, C.E.; Latham, T.; Geisbert, J.B.; Xu, R.; Ota-Setlik, A.; Agans, K.N.; Kobs, D.J.; Wendling, M.Q.S.; Burnaugh, A.; et al. Single-Dose Trivalent VesiculoVax Vaccine Protects Macaques from Lethal Ebolavirus and Marburgvirus Challenge. J. Virol. 2018, 92, e01190-17. [Google Scholar] [CrossRef] [Green Version]
- Tapia, M.D.; Sow, S.O.; Lyke, K.E.; Haidara, F.C.; Diallo, F.; Doumbia, M.; Traore, A.; Coulibaly, F.; Kodio, M.; Onwuchekwa, U.; et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: A phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-bli. Lancet Infect. Dis. 2016, 16, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Ledgerwood, J.E.; DeZure, A.D.; Stanley, D.A.; Coates, E.E.; Novik, L.; Enama, M.E.; Berkowitz, N.M.; Hu, Z.; Joshi, G.; Ploquin, A.; et al. Chimpanzee Adenovirus Vector Ebola Vaccine. N. Engl. J. Med. 2017, 376, 928–938. [Google Scholar] [CrossRef]
- Venkatraman, N.; Ndiaye, B.P.; Bowyer, G.; Wade, D.; Sridhar, S.; Wright, D.; Powlson, J.; Ndiaye, I.; Dièye, S.; Thompson, C.; et al. Safety and immunogenicity of a heterologous prime-boost ebola virus vaccine regimen in healthy adults in the United Kingdom and Senegal. J. Infect. Dis. 2019, 219, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- De Santis, O.; Audran, R.; Pothin, E.; Warpelin-Decrausaz, L.; Vallotton, L.; Wuerzner, G.; Cochet, C.; Estoppey, D.; Steiner-Monard, V.; Lonchampt, S.; et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: A randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis. 2016, 16, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Quinn, K.M.; Da Costa, A.; Yamamoto, A.; Berry, D.; Lindsay, R.W.B.; Darrah, P.A.; Wang, L.; Cheng, C.; Kong, W.-P.; Gall, J.G.D.; et al. Comparative Analysis of the Magnitude, Quality, Phenotype, and Protective Capacity of Simian Immunodeficiency Virus Gag-Specific CD8+ T Cells following Human-, Simian-, and Chimpanzee-Derived Recombinant Adenoviral Vector Immunization. J. Immunol. 2013, 190, 2720–2735. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.A.; Honko, A.N.; Asiedu, C.; Trefry, J.C.; Lau-Kilby, A.W.; Johnson, J.C.; Hensley, L.; Ammendola, V.; Abbate, A.; Grazioli, F.; et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014, 20, 1126–1129. [Google Scholar] [CrossRef]
- Sabin Vaccine Institute. GSK Grants Exclusive Technology License for Clinical-Stage Ebola Vaccines to Sabin Vaccine Institute. Available online: https://www.sabin.org/updates/pressreleases/gsk-grants-exclusive-technology-license-clinical-stage-ebola-vaccines-sabin (accessed on 12 January 2022).
- Alfson, K.J.; Avena, L.E.; Worwa, G.; Carrion, R.; Griffiths, A. Development of a lethal intranasal exposure model of ebola virus in the cynomolgus macaque. Viruses 2017, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Alfson, K.J.; Avena, L.E.; Beadles, M.W.; Staples, H.; Nunneley, J.W.; Ticer, A.; Dick, E.J.; Owston, M.A.; Reed, C.; Patterson, J.L.; et al. Particle-to-PFU Ratio of Ebola Virus Influences Disease Course and Survival in Cynomolgus Macaques. J. Virol. 2015, 89, 6773–6781. [Google Scholar] [CrossRef] [Green Version]
- Alfson, K.J.; Goez-Gazi, Y.; Gazi, M.; Staples, H.; Mattix, M.; Ticer, A.; Klaffke, B.; Stanfield, K.; Escareno, P.; Keiser, P.; et al. Development of a well-characterized rhesus macaque model of ebola virus disease for support of product development. Microorganisms 2021, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Alfson, K.J.; Avena, L.E.; Delgado, J.; Beadles, M.W.; Patterson, J.L.; Carrion, R.; Griffiths, A. A Single Amino Acid Change in the Marburg Virus Glycoprotein Arises during Serial Cell Culture Passages and Attenuates the Virus in a Macaque Model of Disease. mSphere 2018, 3, e00401-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudge, T.L.; Sankovich, K.A.; Niemuth, N.A.; Anderson, M.S.; Badorrek, C.S.; Skomrock, N.D.; Cirimotich, C.M.; Sabourin, C.L. Development, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS ONE 2019, 14, e0215457. [Google Scholar] [CrossRef] [PubMed]
- Rudge, T.L.; Machesky, N.J.; Sankovich, K.A.; Lemmon, E.E.; Badorrek, C.S.; Overman, R.; Niemuth, N.A.; Anderson, M.S. Assays for the Evaluation of the Immune Response to Marburg and Ebola Sudan Vaccination—Filovirus Animal Nonclinical Group Anti-Marburg Virus Glycoprotein Immunoglobulin G Enzyme-Linked Immunosorbent Assay and a Pseudovirion Neutralization Assay. Vaccines 2022, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Jakubik, J.; Holsberg, F.; Shulenin, S.; Smock, P.; Albanese, K.A.; Rudge, T.; Xu, R.; Rustomjeee, R. Challenges in Production of High Purity Marburg Recombinant Glycoprotein for ELISA. In Proceedings of the Immunogenicity & Bioassays Summit: Optimizing Bioassays for Biologics Conference, Washington, DC, USA, 18–22 October 2021. [Google Scholar]
- Guide for Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- Bente, D.; Gren, J.; Strong, J.E.; Feldmann, H. Disease modeling for Ebola and Marburg viruses. DMM Dis. Model. Mech. 2009, 2, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.L.; Twenhafel, N.A.; Connor, J.H.; Cashman, K.A.; Shamblin, J.D.; Donnelly, G.C.; Esham, H.L.; Wlazlowski, C.B.; Johnson, J.C.; Honko, A.N.; et al. Temporal Characterization of Marburg Virus Angola Infection following Aerosol Challenge in Rhesus Macaques. J. Virol. 2015, 89, 9875–9885. [Google Scholar] [CrossRef] [Green Version]
- Blair, P.W.; Keshtkar-Jahromi, M.; Psoter, K.J.; Reisler, R.B.; Warren, T.K.; Johnston, S.C.; Goff, A.J.; Downey, L.G.; Bavari, S.; Cardile, A.P. Virulence of marburg virus Angola compared to Mt. Elgon (Musoke) in macaques: A pooled survival analysis. Viruses 2018, 10, 658. [Google Scholar] [CrossRef] [Green Version]
- Alfson, K.J.; Goez-Gazi, Y.; Gazi, M.; Chou, Y.-L.; Niemuth, N.A.; Mattix, M.E.; Staples, H.M.; Klaffke, B.; Rodriguez, G.F.; Bartley, C.; et al. Development of a Well-Characterized Cynomolgus Macaque Model of Marburg Virus Disease for Support of Vaccine and Therapy Development. Vaccines 2022, 10, 1314. [Google Scholar] [CrossRef]
- Reynard, S.; Gloaguen, E.; Baillet, N.; Madelain, V.; Guedj, J.; Raoul, H.; de Lamballerie, X.; Mullaert, J.; Baize, S. Early control of viral load by favipiravir promotes survival to ebola virus challenge and prevents cytokine storm in non-human primates. PLoS Negl. Trop. Dis. 2021, 15, e0009300. [Google Scholar] [CrossRef]
- Thi, E.P.; Lee, A.C.H.; Geisbert, J.B.; Ursic-Bedoya, R.; Agans, K.N.; Robbins, M.; Deer, D.J.; Fenton, K.A.; Kondratowicz, A.S.; MacLachlan, I.; et al. Rescue of non-human primates from advanced Sudan ebolavirus infection with lipid encapsulated siRNA. Nat. Microbiol. 2016, 1, 16142. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, N.J.; Hensley, L.; Asiedu, C.; Geisbert, T.W.; Stanley, D.; Johnson, J.; Honko, A.; Olinger, G.; Bailey, M.; Geisbert, J.B.; et al. CD8 + cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 2011, 17, 1128–1131. [Google Scholar] [CrossRef]
- Cross, R.W.; Longini, I.M.; Becker, S.; Bok, K.; Boucher, D.; Carroll, M.W.; Díaz, J.V.; Dowling, W.E.; Draghia-Akli, R.; Duworko, J.T.; et al. An introduction to the Marburg virus vaccine consortium, MARVAC. PLoS Pathog. 2022, 18, e1010805. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.; Rampling, T.; Venkatraman, N.; Bowyer, G.; Wright, D.; Lambe, T.; Imoukhuede, E.B.; Payne, R.; Fehling, S.K.; Strecker, T.; et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N. Engl. J. Med. 2016, 374, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Mire, C.E.; Geisbert, J.B.; Agans, K.N.; Satterfield, B.A.; Versteeg, K.M.; Fritz, E.A.; Feldmann, H.; Hensley, L.E.; Geisbert, T.W. Durability of a vesicular stomatitis virus-based marburg virus vaccine in nonhuman primates. PLoS ONE 2014, 9, e0094355. [Google Scholar] [CrossRef] [PubMed]

| Hematology | ||
|---|---|---|
| Parameter | Abbreviation | Units |
| Platelet Count | PLT/PCT | K/µL, % |
| Serum Chemistry Parameters | ||
| Parameter | Abbreviation | Units |
| Alanine Aminotransferase | ALT | U/L |
| Albumin | ALB | g/dL |
| Alkaline Phosphatase | ALP | U/L |
| Blood Urea Nitrogen | BUN | mg/dL |
| C-Reactive Protein | CRP | mg/L |
| Reagent | Virus/Variant | Supplier | Lot Number | Coating Amount per Well |
|---|---|---|---|---|
| rGP | MARV/Angola | Integrated Biotherapeutics Rockville, MD, USA | 9 January 2021 | 20 ng |
| Reagent | Lot Number | Supplier | Concentration (ELISA Units/mL) | Starting Dilution on Plate |
| Reference Standard | BMIMARV107 | Battelle | 1058 | 1:105.8 |
| Quality Control High | BMIMARV108 | Battelle | 427.58 | 1:50 |
| Quality Control Low | BMIMARV109 | Battelle | 156.09 | 1:50 |
| Negative Control | 062121-KE001 | Battelle | 0.00 | 1:50 |
| Reagent | Lot Number | Catalog Number | Supplier | Dilution on Plate |
| Conjugate | 150,445 | 109-035-098 | Jackson ImmunoResearch West Grove, PA, USA | 1:30,000 |
| Selected Tissue Histopathological Findings | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site/Tissue | Finding | NHP1, 1 × 1011 vp * | NHP2, 1 × 1011 vp * | NHP3, 1 × 1011 vp * | NHP4, 1 × 1011 vp * | NHP1, 1 × 106 vp | NHP2, 1 × 106 vp | NHP3, 1 × 106 vp | NHP4, 1 × 106 vp | NHP1, Saline Control | NHP2, Saline Control |
| CHALLENGE SITE | Inflammation | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 0 |
| Necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | |
| Inflammatory cell infiltration | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Hemorrhage | 0 | 0 | 0 | 0 | P | 0 | 0 | 0 | 0 | 0 | |
| Edema | 0 | 0 | 0 | 0 | P | 0 | P | 0 | 0 | 0 | |
| Thrombosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P | |
| LYMPH NODE | Lymphoid depletion | 2 | 2 | 0 | 1 | 4 | 4 | 3 | 4 | 4 | 4 |
| Lymphocytolysis | 0 | 0 | 0 | 0 | 3 | 2 | 1 | 2 | 2 | 2 | |
| Inflammation | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 3 | 3 | 0 | |
| Necrosis | 0 | 0 | 0 | 0 | 4 | 4 | 2 | 3 | 4 | 4 | |
| Fibrin deposition | 0 | 0 | 0 | 0 | P | P | 0 | P | P | P | |
| Hemorrhage | 0 | 0 | 0 | 0 | P | P | 0 | P | P | P | |
| Sinus histiocytosis | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| Paracortical hyperplasia | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | |
| Follicular hyperplasia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SPLEEN | Lymphoid depletion | 0 | 2 | 0 | 0 | 3 | 4 | 3 | 3 | 3 | 4 |
| Lymphocytolysis | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 2 | 1 | |
| Fibrin deposition | 0 | 0 | 0 | 0 | P | P | P | P | P | P | |
| Congestion/hemorrhage | 0 | 0 | 0 | 0 | 0 | P | P | P | P | P | |
| Follicular hyperplasia | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| LIVER | Necrosis, hepatocellular | 0 | 1 | 0 | 0 | 2 | 3 | 2 | 2 | 2 | 1 |
| Inflammation | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | |
| ADRENAL | Necrosis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Inflammation | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| KIDNEY | Thrombosis | 0 | 0 | 0 | 0 | 0 | P | 0 | 0 | 0 | 0 |
| BRAIN, CEREBRUM | Hemorrhage, choroid plexus | 0 | 0 | 0 | 0 | 0 | P | 0 | 0 | 0 | 0 |
| Thrombosis, choroid plexus | 0 | 0 | 0 | 0 | 0 | P | 0 | 0 | 0 | 0 | |
| Animal ID | Days Post-ChAd3-MARV Vaccination | Mean Spot-Forming Cell Counts/1 × 106 PBMCs | |
|---|---|---|---|
| MARV Peptide Pool 1 | MARV Peptide Pool 2 | ||
| NHP-A, MARV | 56 | 305 | 363 |
| NHP-B, MARV | 53 | 868 | |
| NHP-C, MARV | 188 | 818 | |
| NHP-A, SUDV | N/A | 27 | 60 |
| NHP-B, SUDV | 28 | 32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finch, C.L.; King, T.H.; Alfson, K.J.; Albanese, K.A.; Smith, J.N.P.; Smock, P.; Jakubik, J.; Goez-Gazi, Y.; Gazi, M.; Dutton, J.W., III; et al. Single-Shot ChAd3-MARV Vaccine in Modified Formulation Buffer Shows 100% Protection of NHPs. Vaccines 2022, 10, 1935. https://doi.org/10.3390/vaccines10111935
Finch CL, King TH, Alfson KJ, Albanese KA, Smith JNP, Smock P, Jakubik J, Goez-Gazi Y, Gazi M, Dutton JW III, et al. Single-Shot ChAd3-MARV Vaccine in Modified Formulation Buffer Shows 100% Protection of NHPs. Vaccines. 2022; 10(11):1935. https://doi.org/10.3390/vaccines10111935
Chicago/Turabian StyleFinch, Courtney L., Thomas H. King, Kendra J. Alfson, Katie A. Albanese, Julianne N. P. Smith, Paul Smock, Jocelyn Jakubik, Yenny Goez-Gazi, Michal Gazi, John W. Dutton, III, and et al. 2022. "Single-Shot ChAd3-MARV Vaccine in Modified Formulation Buffer Shows 100% Protection of NHPs" Vaccines 10, no. 11: 1935. https://doi.org/10.3390/vaccines10111935
APA StyleFinch, C. L., King, T. H., Alfson, K. J., Albanese, K. A., Smith, J. N. P., Smock, P., Jakubik, J., Goez-Gazi, Y., Gazi, M., Dutton, J. W., III, Clemmons, E. A., Mattix, M. E., Carrion, R., Jr., Rudge, T., Jr., Ridenour, A., Woodin, S. F., Hunegnaw, R., Sullivan, N. J., & Xu, R. (2022). Single-Shot ChAd3-MARV Vaccine in Modified Formulation Buffer Shows 100% Protection of NHPs. Vaccines, 10(11), 1935. https://doi.org/10.3390/vaccines10111935






