Advances of Electroporation-Related Therapies and the Synergy with Immunotherapy in Cancer Treatment
Abstract
:1. History of Electroporation
2. Basic Mechanism of Electroporation
3. Advances in Electroporation Technique
4. Electroporation-Related Therapies in Cancer Treatment
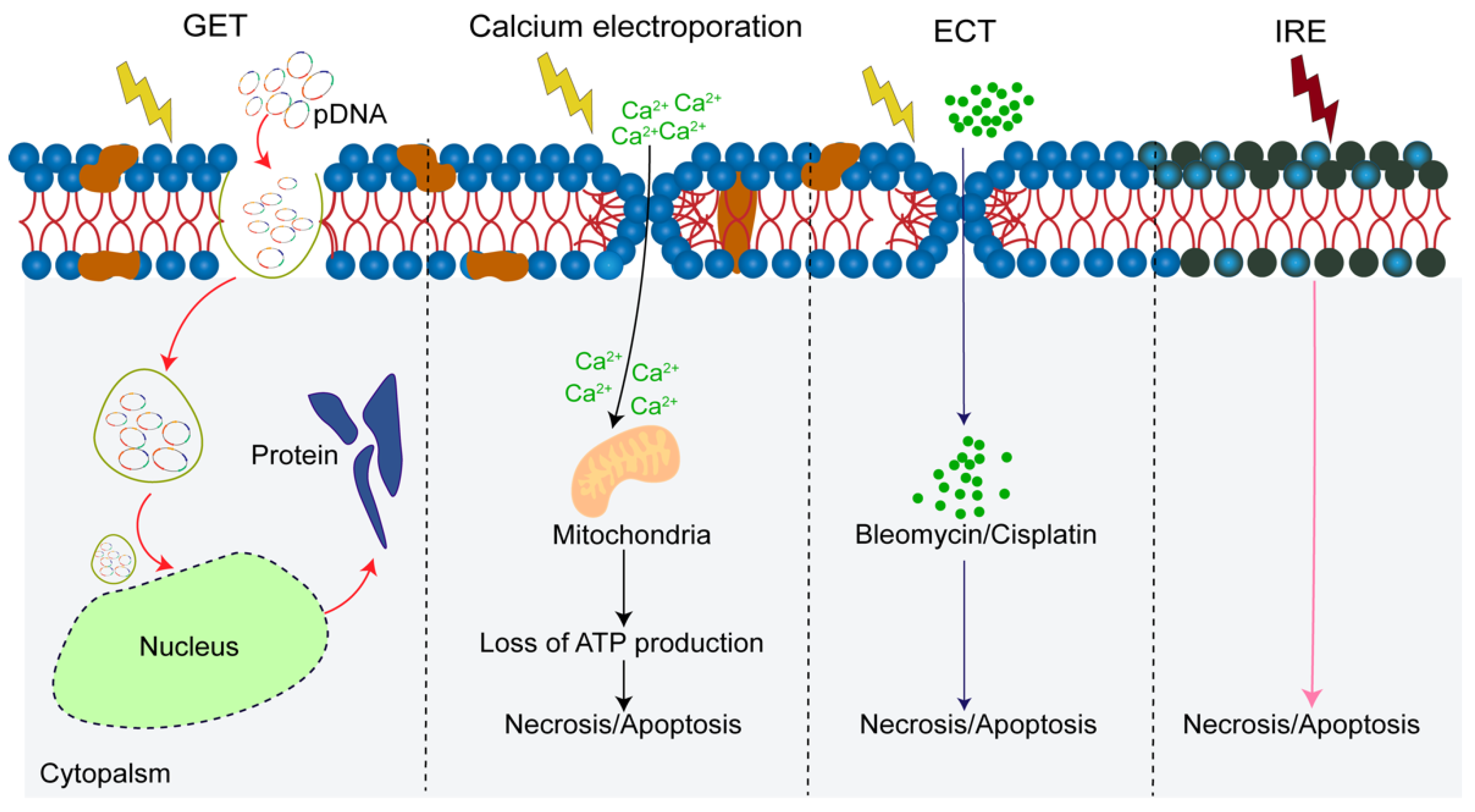
4.1. Reversible Electroporation (RE)
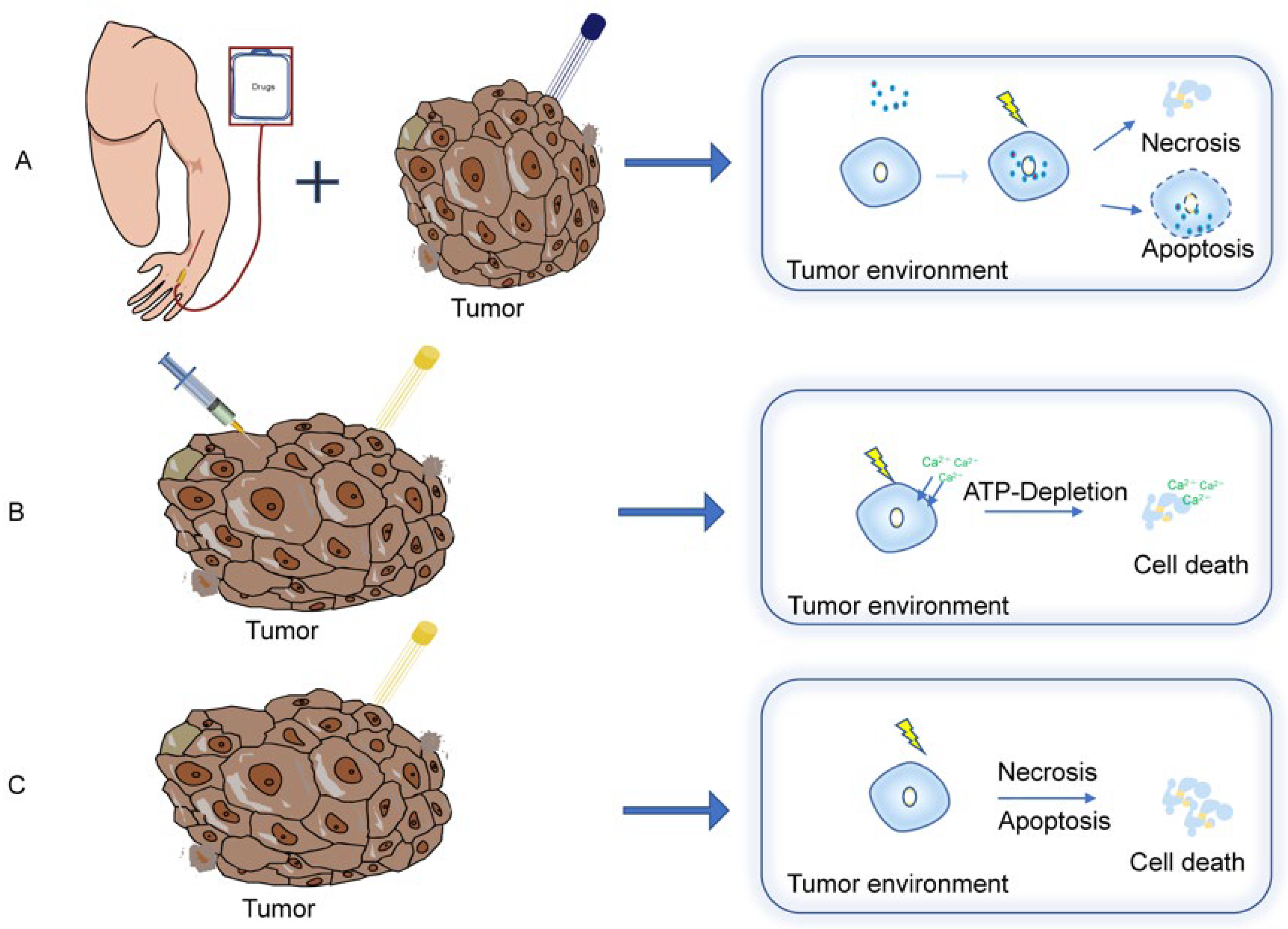
4.1.1. Electrochemotherapy (ECT)
4.1.2. Gene Electrotransfer (GET)
4.1.3. The Synergy with ECT and GET
4.1.4. Calcium Electroporation
4.2. Irreversible Electroporation (IRE)
4.3. Tumor-Treating Fields (TTFields)
5. Combination of Electroporation-Related Treatment and Immunotherapy
5.1. Cancer Immunotherapy
5.2. Immunological Aspects of RE and IRE
5.3. RE plus Immunotherapy
5.4. IRE and Immunotherapy
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.J.T.E.J. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef]
- Tsong, T.J.B.j. Electroporation of cell membranes. Electroporation Electrofusion Cell Biol. 1991, 60, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Lapinska, Z.; Szwedowicz, U.; Choromanska, A.; Saczko, J. Electroporation and Electrochemotherapy in Gynecological and Breast Cancer Treatment. Molecules 2022, 27, 2476. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Zhou, C.; Teng, L.; Ren, W.; Yang, Z.; Shih, C.H.; Wang, T.; Lee, R.J.; Tang, S.; et al. Insight into mechanisms of cellular uptake of lipid nanoparticles and intracellular release of small RNAs. Pharm. Res. 2014, 31, 2685–2695. [Google Scholar] [CrossRef]
- Yang, X.; Yang, S.; Chai, H.; Yang, Z.; Lee, R.J.; Liao, W.; Teng, L. A Novel Isoquinoline Derivative Anticancer Agent and Its Targeted Delivery to Tumor Cells Using Transferrin-Conjugated Liposomes. PLoS ONE 2015, 10, e0136649. [Google Scholar] [CrossRef]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, J.C. Electroporation theory. Concepts and mechanisms. Methods Mol. Biol. 1995, 55, 3–28. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Arancia, G.; Porrello, A.; Colone, M.; Formisano, G.; Stringaro, A.; Citro, G.; Molinari, A. Ultrastructural modifications of cell membranes induced by “electroporation” on melanoma xenografts. Microsc. Res. Tech. 2007, 70, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Wang, M.A.; Weaver, J.C. Theory of electroporation of planar bilayer membranes: Predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys. J. 1994, 67, 42–56. [Google Scholar] [CrossRef]
- Chen, C.; Smye, S.W.; Robinson, M.P.; Evans, J.A. Membrane electroporation theories: A review. Med. Biol. Eng. Comput. 2006, 44, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.F.; Sapay, N.; Tieleman, D.P. Atomistic simulations of pore formation and closure in lipid bilayers. Biophys. J. 2014, 106, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Calvet, C.Y.; Andre, F.M.; Mir, L.M. Dual therapeutic benefit of electroporation-mediated DNA vaccination in vivo: Enhanced gene transfer and adjuvant activity. Oncoimmunology 2014, 3, e28540. [Google Scholar] [CrossRef] [Green Version]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef] [Green Version]
- Onik, G.; Mikus, P.; Rubinsky, B. Irreversible electroporation: Implications for prostate ablation. Technol. Cancer Res. Treat. 2007, 6, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.; Leor, J.; Rubinsky, B. Cancer cells ablation with irreversible electroporation. Technol. Cancer Res. Treat. 2005, 4, 699–705. [Google Scholar] [CrossRef]
- Cemazar, M.K.T.; Sersa, G.; Miklavcic, D. Electroporation for electrochemotherapy and gene therapy. Electromagn. Fields Biol. Med. 2015, 24, 395–413. [Google Scholar]
- Pakhomova, O.N.; Gregory, B.W.; Khorokhorina, V.A.; Bowman, A.M.; Xiao, S.; Pakhomov, A.G. Electroporation-induced electrosensitization. PLoS ONE 2011, 6, e17100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklavcic, D.; Pucihar, G.; Pavlovec, M.; Ribaric, S.; Mali, M.; Macek-Lebar, A.; Petkovsek, M.; Nastran, J.; Kranjc, S.; Cemazar, M.; et al. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency in vivo for a potential use in clinical electrochemotherapy. Bioelectrochemistry 2005, 65, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chang, L.; Chiang, C.L.; Lee, L.J. Micro-/nano-electroporation for active gene delivery. Curr. Pharm. Des. 2015, 21, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Hu, X.; Choi, H.W.; Wang, S.; Farson, D.; Lee, L.J. Micronozzle array enhanced sandwich electroporation of embryonic stem cells. Anal. Chem. 2010, 82, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, M.; Flisar, K.; Kanduser, M. The role of electrophoresis in gene electrotransfer. J. Membr. Biol. 2010, 236, 75–79. [Google Scholar] [CrossRef]
- Xie, C.; Lin, Z.; Hanson, L.; Cui, Y.; Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol. 2012, 7, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Xu, A.M.; Leal-Ortiz, S.; Cao, Y.; Garner, C.C.; Melosh, N.A. Nanostraw-electroporation system for highly efficient intracellular delivery and transfection. ACS Nano 2013, 7, 4351–4358. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Yavari, F.; Minary-Jolandan, M.; Giraldo-Vela, J.P.; Safi, A.; McNaughton, R.L.; Parpoil, V.; Espinosa, H.D. Nanofountain probe electroporation (NFP-E) of single cells. Nano Lett. 2013, 13, 2448–2457. [Google Scholar] [CrossRef] [Green Version]
- Escoffre, J.M.; Rols, M.P. Electrochemotherapy: Progress and prospects. Curr. Pharm. Des. 2012, 18, 3406–3415. [Google Scholar] [CrossRef]
- Tounekti, O.; Pron, G.; Belehradek, J., Jr.; Mir, L.M. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res. 1993, 53, 5462–5469. [Google Scholar]
- Tounekti, O.; Kenani, A.; Foray, N.; Orlowski, S.; Mir, L.M. The ratio of single- to double-strand DNA breaks and their absolute values determine cell death pathway. Br. J. Cancer 2001, 84, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Miklavcic, D.; Mali, B.; Kos, B.; Heller, R.; Sersa, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Mir, M.U. Bases and rationale of the electrochemotherapy. Eur. J. Cancer Suppl. 2006, 4, 38–44. [Google Scholar] [CrossRef]
- Campana, L.G.; Miklavcic, D.; Bertino, G.; Marconato, R.; Valpione, S.; Imarisio, I.; Dieci, M.V.; Granziera, E.; Cemazar, M.; Alaibac, M.; et al. Electrochemotherapy of superficial tumors—Current status: Basic principles, operating procedures, shared indications, and emerging applications. Semin. Oncol. 2019, 46, 173–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Enhancement of cytotoxicity by electropermeabilization: An improved method for screening drugs. Anti-Cancer Drugs 1998, 9, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Jaroszeski, M.J.; Dang, V.; Pottinger, C.; Hickey, J.; Gilbert, R.; Heller, R. Toxicity of anticancer agents mediated by electroporation in vitro. Anti-Cancer Drugs 2000, 11, 201–208. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldi, A.M.; Simeone, E.; Giannarelli, D.; Muto, P.; Falivene, S.; Borzillo, V.; Giugliano, F.M.; Sandomenico, F.; Petrillo, A.; Curvietto, M.; et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014, 3, e28780. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Teissie, J.; Cemazar, M.; Signori, E.; Kamensek, U.; Marshall, G.; Miklavcic, D. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol. Immunother. 2015, 64, 1315–1327. [Google Scholar] [CrossRef] [Green Version]
- Falk, H.; Lambaa, S.; Johannesen, H.H.; Wooler, G.; Venzo, A.; Gehl, J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma—A case report. Acta Oncol. 2017, 56, 1126–1131. [Google Scholar] [CrossRef] [Green Version]
- Bellard, E.; Markelc, B.; Pelofy, S.; Le Guerroué, F.; Sersa, G.; Teissié, J.; Cemazar, M.; Golzio, M. Intravital microscopy at the single vessel level brings new insights of vascular modification mechanisms induced by electropermeabilization. J. Control. Release Off. J. Control. Release Soc. 2012, 163, 396–403. [Google Scholar] [CrossRef]
- Edhemovic, I.; Gadzijev, E.M.; Brecelj, E.; Miklavcic, D.; Kos, B.; Zupanic, A.; Mali, B.; Jarm, T.; Pavliha, D.; Marcan, M.; et al. Electrochemotherapy: A new technological approach in treatment of metastases in the liver. Technol. Cancer Res. Treat. 2011, 10, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of bleeding metastases. Expert Rev. Anticancer Ther. 2010, 10, 729–746. [Google Scholar] [CrossRef]
- Markelc, B.; Sersa, G.; Cemazar, M. Differential mechanisms associated with vascular disrupting action of electrochemotherapy: Intravital microscopy on the level of single normal and tumor blood vessels. PLoS ONE 2013, 8, e59557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sersa, G.; Jarm, T.; Kotnik, T.; Coer, A.; Podkrajsek, M.; Sentjurc, M.; Miklavcic, D.; Kadivec, M.; Kranjc, S.; Secerov, A.; et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br. J. Cancer 2008, 98, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Mittal, L.; Aryal, U.K.; Camarillo, I.G.; Ferreira, R.M.; Sundararajan, R. Quantitative proteomic analysis of enhanced cellular effects of electrochemotherapy with Cisplatin in triple-negative breast cancer cells. Sci. Rep. 2019, 9, 13916. [Google Scholar] [CrossRef] [Green Version]
- Reinhold, U. Electrochemotherapy of skin tumors. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2011, 62, 549–558. [Google Scholar] [CrossRef]
- Skarlatos, I.; Kyrgias, G.; Mosa, E.; Provatopoulou, X.; Spyrou, M.; Theodorou, K.; Lepouras, A.; Gounaris, A.; Koukourakis, M. Electrochemotherapy in cancer patients: First clinical trial in Greece. In Vivo 2011, 25, 265–274. [Google Scholar] [PubMed]
- Muñoz Madero, V.; Ortega Pérez, G. Electrochemotherapy for treatment of skin and soft tissue tumours. Update and definition of its role in multimodal therapy. Clin. Transl. Oncol. 2011, 13, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Bennassar, A.; Ishioka, P.; Dalle, S.; Vilalta, A.; Fuertes, I.; Alos, L.; Thomas, L.; Puig, S.; Malvehy, J. Desmoplastic melanoma on the nose: Electrochemotherapy as an alternative treatment to local advanced disease. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 424–432. [Google Scholar] [CrossRef]
- Wiater, K.; Zdzienicki, M.; Morysiński, T.; Koseła, H.; Klimczak, A.; Obrębski, M.; Ptaszyński, K.; Rutkowski, P. Effective treatment of recurrent, advanced dermatofibrosarcoma protuberans by electrochemotherapy. Eur. J. Dermatol. 2013, 23, 260–261. [Google Scholar] [CrossRef]
- Tellado, M.; Mir, L.M.; Maglietti, F. Veterinary Guidelines for Electrochemotherapy of Superficial Tumors. Front. Vet. Sci. 2022, 9, 868989. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Campana, L.G.; Mocellin, S.; Basso, M.; Puccetti, O.; De Salvo, G.L.; Chiarion-Sileni, V.; Vecchiato, A.; Corti, L.; Rossi, C.R.; Nitti, D. Bleomycin-based electrochemotherapy: Clinical outcome from a single institution’s experience with 52 patients. Ann. Surg. Oncol. 2009, 16, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Snoj, M.; Cemazar, M.; Slekovec Kolar, B.; Sersa, G. Effective treatment of multiple unresectable skin melanoma metastases by electrochemotherapy. Croat. Med. J. 2007, 48, 391–395. [Google Scholar] [PubMed]
- Seyed Jafari, S.M.; Jabbary Lak, F.; Gazdhar, A.; Shafighi, M.; Borradori, L.; Hunger, R.E. Application of electrochemotherapy in the management of primary and metastatic cutaneous malignant tumours: A systematic review and meta-analysis. Eur. J. Dermatol. 2018, 28, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Marolt Music, M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Grcar Kuzmanov, B.; et al. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Djokic, M.; Cemazar, M.; Popovic, P.; Kos, B.; Dezman, R.; Bosnjak, M.; Zakelj, M.N.; Miklavcic, D.; Potrc, S.; Stabuc, B.; et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur. J. Surg. Oncol. 2018, 44, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Djokic, M.; Dezman, R.; Cemazar, M.; Stabuc, M.; Petric, M.; Smid, L.M.; Jansa, R.; Plesnik, B.; Bosnjak, M.; Tratar, U.L.; et al. Percutaneous image guided electrochemotherapy of hepatocellular carcinoma: Technological advancement. Radiol. Oncol. 2020, 54, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Czymek, R.; Nassrallah, J.; Gebhard, M.; Schmidt, A.; Limmer, S.; Kleemann, M.; Bruch, H.P.; Hildebrand, P. Intrahepatic radiofrequency ablation versus electrochemical treatment in vivo. Surg. Oncol. 2012, 21, 79–86. [Google Scholar] [CrossRef]
- Lu, D.S.; Raman, S.S.; Vodopich, D.J.; Wang, M.; Sayre, J.; Lassman, C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: Assessment of the “heat sink” effect. Am. J. Roentgenol. 2002, 178, 47–51. [Google Scholar] [CrossRef]
- Falk Hansen, H.; Bourke, M.; Stigaard, T.; Clover, J.; Buckley, M.; O’Riordain, M.; Winter, D.C.; Hjorth Johannesen, H.; Hansen, R.H.; Heebøll, H.; et al. Electrochemotherapy for colorectal cancer using endoscopic electroporation: A phase 1 clinical study. Endosc. Int. Open 2020, 8, E124–E132. [Google Scholar] [CrossRef] [Green Version]
- Egeland, C.; Baeksgaard, L.; Johannesen, H.H.; Löfgren, J.; Plaschke, C.C.; Svendsen, L.B.; Gehl, J.; Achiam, M.P. Endoscopic electrochemotherapy for esophageal cancer: A phase I clinical study. Endosc. Int. Open 2018, 6, E727–E734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agerholm-Larsen, B.; Iversen, H.K.; Ibsen, P.; Moller, J.M.; Mahmood, F.; Jensen, K.S.; Gehl, J. Preclinical validation of electrochemotherapy as an effective treatment for brain tumors. Cancer Res. 2011, 71, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Hjouj, M.; Last, D.; Guez, D.; Daniels, D.; Sharabi, S.; Lavee, J.; Rubinsky, B.; Mardor, Y. MRI study on reversible and irreversible electroporation induced blood brain barrier disruption. PLoS ONE 2012, 7, e42817. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, S.; Last, D.; Guez, D.; Daniels, D.; Hjouj, M.I.; Salomon, S.; Maor, E.; Mardor, Y. Dynamic effects of point source electroporation on the rat brain tissue. Bioelectrochemistry 2014, 99, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, S.; Kos, B.; Last, D.; Guez, D.; Daniels, D.; Harnof, S.; Mardor, Y.; Miklavcic, D. A statistical model describing combined irreversible electroporation and electroporation-induced blood-brain barrier disruption. Radiol. Oncol. 2016, 50, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Scheffer, H.; Graybill, P.; Ruarus, A.; Nieuwenhuizen, S.; Puijk, R.; van den Tol, P.; Davalos, R.; Rubinsky, B.; de Gruijl, T.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Hirao, L.A.; Wu, L.; Khan, A.S.; Satishchandran, A.; Draghia-Akli, R.; Weiner, D.B. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 2008, 26, 440–448. [Google Scholar] [CrossRef]
- Wells, D.J. Gene therapy progress and prospects: Electroporation and other physical methods. Gene Ther. 2004, 11, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Préat, V.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Babiuk, S.; Baca-Estrada, M.E.; Foldvari, M.; Middleton, D.M.; Rabussay, D.; Widera, G.; Babiuk, L.A. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J. Biotechnol. 2004, 110, 1–10. [Google Scholar] [CrossRef]
- Chiarella, P.; Massi, E.; De Robertis, M.; Sibilio, A.; Parrella, P.; Fazio, V.M.; Signori, E. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin. Biol. Ther. 2008, 8, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Cemazar, M.; Jarm, T.; Sersa, G. Cancer electrogene therapy with interleukin-12. Curr. Gene Ther. 2010, 10, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Greaney, S.K.; Algazi, A.P.; Tsai, K.K.; Takamura, K.T.; Chen, L.; Twitty, C.G.; Zhang, L.; Paciorek, A.; Pierce, R.H.; Le, M.H.; et al. Intratumoral Plasmid IL12 Electroporation Therapy in Patients with Advanced Melanoma Induces Systemic and Intratumoral T-cell Responses. Cancer Immunol. Res. 2020, 8, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Daud, A.I.; DeConti, R.C.; Andrews, S.; Urbas, P.; Riker, A.I.; Sondak, V.K.; Munster, P.N.; Sullivan, D.M.; Ugen, K.E.; Messina, J.L.; et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5896–5903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, S.; Longino, N.V.; Miller, N.J.; Kulikauskas, R.; Iyer, J.G.; Ibrani, D.; Blom, A.; Byrd, D.R.; Parvathaneni, U.; Twitty, C.G.; et al. Intratumoral Delivery of Plasmid IL12 Via Electroporation Leads to Regression of Injected and Noninjected Tumors in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 598–607. [Google Scholar] [CrossRef] [Green Version]
- De Robertis, M.; Pasquet, L.; Loiacono, L.; Bellard, E.; Messina, L.; Vaccaro, S.; Di Pasquale, R.; Fazio, V.M.; Rols, M.P.; Teissie, J.; et al. In Vivo Evaluation of a New Recombinant Hyaluronidase to Improve Gene Electro-Transfer Protocols for DNA-Based Drug Delivery against Cancer. Cancers 2018, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Ku, G.Y.; Adamow, M.; Mu, Z.; Tandon, S.; Hannaman, D.; Chapman, P.; Schwartz, G.; Carvajal, R.; Panageas, K.S.; et al. Immunologic responses to xenogeneic tyrosinase DNA vaccine administered by electroporation in patients with malignant melanoma. J. Immunother. Cancer 2013, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [Green Version]
- Prehn, R.T.; Prehn, L.M. The flip side of immune surveillance: Immune dependency. Immunol. Rev. 2008, 222, 341–356. [Google Scholar] [CrossRef]
- He, Y.; Zha, J.; Wang, Y.; Liu, W.; Yang, X.; Yu, P. Tissue damage-associated “danger signals” influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Res. 2013, 73, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.D.; Fulmer, A.; Buckholz, J.; Zhang, B.; Cutrera, J.; Shiomitsu, K.; Li, S. Bleomycin/interleukin-12 electrochemogene therapy for treating naturally occurring spontaneous neoplasms in dogs. Cancer Gene 2010, 17, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Maglietti, F.; Tellado, M.; De Robertis, M.; Michinski, S.; Fernandez, J.; Signori, E.; Marshall, G. Electroporation as the Immunotherapy Strategy for Cancer in Veterinary Medicine: State of the Art in Latin America. Vaccines 2020, 8, 537. [Google Scholar] [CrossRef]
- Maglietti, F.; Michinski, S.; Emanuela, S.; Tellado, M.; Marshall, G. Electrochemotherapy immune response enhancement by gene electrotransfer using IL-2 and IL-12 genes in canine patients. Eur. J. Cancer 2016, 61, S548–S549. [Google Scholar] [CrossRef]
- Salvadori, C.; Svara, T.; Rocchigiani, G.; Millanta, F.; Pavlin, D.; Cemazar, M.; Lampreht Tratar, U.; Sersa, G.; Tozon, N.; Poli, A. Effects of Electrochemotherapy with Cisplatin and Peritumoral IL-12 Gene Electrotransfer on Canine Mast Cell Tumors: A Histopathologic and Immunohistochemical Study. Radiol. Oncol. 2017, 51, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, L.; Pottinger, C.; Jaroszeski, M.J.; Gilbert, R.; Heller, R. In vivo electroporation of plasmids encoding GM-CSF or interleukin-2 into existing B16 melanomas combined with electrochemotherapy induces long-term antitumour immunity. Melanoma Res. 2000, 10, 577–583. [Google Scholar] [CrossRef]
- Sedlar, A.; Dolinsek, T.; Markelc, B.; Prosen, L.; Kranjc, S.; Bosnjak, M.; Blagus, T.; Cemazar, M.; Sersa, G. Potentiation of electrochemotherapy by intramuscular IL-12 gene electrotransfer in murine sarcoma and carcinoma with different immunogenicity. Radiol. Oncol. 2012, 46, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Ursic, K.; Kos, S.; Kamensek, U.; Cemazar, M.; Miceska, S.; Markelc, B.; Bucek, S.; Staresinic, B.; Kloboves Prevodnik, V.; Heller, R.; et al. Potentiation of electrochemotherapy effectiveness by immunostimulation with IL-12 gene electrotransfer in mice is dependent on tumor immune status. J. Control. Release 2021, 332, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Lipp, P. Calcium--a life and death signal. Nature 1998, 395, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E. Calcium signalling: A historical account, recent developments and future perspectives. Cell Mol. Life Sci. 2000, 57, 354–370. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers 2022, 12, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Plaschke, C.C.; Gehl, J.; Johannesen, H.H.; Fischer, B.M.; Kjaer, A.; Lomholt, A.F.; Wessel, I. Calcium electroporation for recurrent head and neck cancer: A clinical phase I study. Laryngoscope Investig. Otolaryngol. 2019, 4, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, H.; Forde, P.F.; Bay, M.L.; Mangalanathan, U.M.; Hojman, P.; Soden, D.M.; Gehl, J. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model. Oncoimmunology 2017, 6, e1301332. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Moser, M.A.J.; Luo, Y.; Zhang, W.; Zhang, B. Chemical Enhancement of Irreversible Electroporation: A Review and Future Suggestions. Technol. Cancer Res. Treat. 2019, 18, 1533033819874128. [Google Scholar] [CrossRef]
- Davalos, R.V.; Mir, I.L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef]
- Golberg, A.; Yarmush, M.L. Nonthermal irreversible electroporation: Fundamentals, applications, and challenges. IEEE Trans. Biomed. Eng. 2013, 60, 707–714. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Leor, J.; Rubinsky, B. The effect of irreversible electroporation on blood vessels. Technol. Cancer Res. Treat. 2007, 6, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Narayan, R.; Padath, T.; Rubinsky, B. Irreversible electroporation on the small intestine. Br. J. Cancer 2012, 106, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.W.; Chen, C.; Prieto, V.E.; Dry, S.M.; Loh, C.T.; Kee, S.T. Advanced hepatic ablation technique for creating complete cell death: Irreversible electroporation. Radiology 2010, 255, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.P.W.; Finch, A.; Gerigk, M.; Triantis, I.F.; Watts, C.; Malliaras, G.G. Electrotherapies for Glioblastoma. Adv. Sci. 2021, 8, e2100978. [Google Scholar] [CrossRef] [PubMed]
- Rai, Z.L.; Feakins, R.; Pallett, L.J.; Manas, D.; Davidson, B.R. Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. J. Clin. Med. 2021, 8, 1609. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C., 2nd; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: Safety and efficacy. Ann. Surg. 2015, 262, 486–494; discussion 492–484. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Aycock, K.N.; Davalos, R.V. Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology. Bioelectricity 2019, 1, 214–234. [Google Scholar] [CrossRef] [Green Version]
- Pandya, G.J.; Shelat, V.G. Radiofrequency ablation of pancreatic ductal adenocarcinoma: The past, the present and the future. World J. Gastrointest. Oncol. 2015, 7, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C., 2nd; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J. Am. Coll. Surg. 2012, 215, 361–369. [Google Scholar] [CrossRef]
- Leen, E.; Picard, J.; Stebbing, J.; Abel, M.; Dhillon, T.; Wasan, H. Percutaneous irreversible electroporation with systemic treatment for locally advanced pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2018, 9, 275–281. [Google Scholar] [CrossRef] [PubMed]
- van den Bos, W.; Scheltema, M.J.; Siriwardana, A.R.; Kalsbeek, A.M.F.; Thompson, J.E.; Ting, F.; Bohm, M.; Haynes, A.M.; Shnier, R.; Delprado, W.; et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018, 121, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, V.W.; Spiro, C.; Laurence, J.M.; Johnston, E.; Hollands, M.J.; Pleass, H.C.; Richardson, A.J. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann. Surg. Oncol. 2012, 19, 1292–1301. [Google Scholar] [CrossRef]
- Niessen, C.; Thumann, S.; Beyer, L.; Pregler, B.; Kramer, J.; Lang, S.; Teufel, A.; Jung, E.M.; Stroszczynski, C.; Wiggermann, P. Percutaneous Irreversible Electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci. Rep. 2017, 7, 43687. [Google Scholar] [CrossRef] [Green Version]
- Ball, C.; Thomson, K.R.; Kavnoudias, H. Irreversible electroporation: A new challenge in “out of operating theater” anesthesia. Anesth. Analg. 2010, 110, 1305–1309. [Google Scholar] [CrossRef]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, A.M.; Weinberg, U.; Palti, Y. Tumor treating fields: A new frontier in cancer therapy. Ann. N. Y. Acad. Sci. 2013, 1291, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Philips, A.; Henshaw, D.L.; Lamburn, G.; O’Carroll, M.J. Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995–2015 Suggests an Adverse Environmental or Lifestyle Factor. J. Enviorn. Public Health 2018, 2018, 7910754. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbaly, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Printz, C. Electrical device for patients with glioblastoma met with support, skepticism: Some question the device’s efficacy, others tout it as a new standard of care. Cancer 2015, 121, 969–970. [Google Scholar] [CrossRef]
- Wick, W. TTFields: Where does all the skepticism come from? Neuro Oncol. 2016, 18, 303–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T.; et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Italian Cooperative Study Group on Chronic Myeloid Leukemia; Tura, S.; Baccarani, M.; Zuffa, E.; Russo, D.; Fanin, R.; Zaccaria, A.; Fiacchini, M. Interferon alfa-2a as compared with conventional chemotherapy for the treatment of chronic myeloid leukemia. N. Engl. J. Med. 1994, 330, 820–825. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Strawderman, M.H.; Ernstoff, M.S.; Smith, T.J.; Borden, E.C.; Blum, R.H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 1996, 14, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef] [Green Version]
- Porter, D.L.; Hwang, W.T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Shin, S.; Dy, G. Advances in Cancer Immunotherapy in Solid Tumors. Cancers 2016, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, R.A.; Chinnasamy, N.; Abate-Daga, D.; Gros, A.; Robbins, P.F.; Zheng, Z.; Dudley, M.E.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013, 36, 133–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, R.M.; Beitel-White, N.; Davalos, R.V.; Allen, I.C. Starting a Fire Without Flame: The Induction of Cell Death and Inflammation in Electroporation-Based Tumor Ablation Strategies. Front. Oncol. 2020, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Haen, S.P.; Loffler, M.W.; Rammensee, H.G.; Brossart, P. Towards new horizons: Characterization, classification and implications of the tumour antigenic repertoire. Nat. Rev. Clin. Oncol. 2020, 17, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.Y.; Famin, D.; Andre, F.M.; Mir, L.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigi, L.; Galdo, G.; Cesinaro, A.M.; Vaschieri, C.; Marconi, A.; Pincelli, C.; Fantini, F. Electrochemotherapy induces apoptotic death in melanoma metastases: A histologic and immunohistochemical investigation. Clin. Cosmet. Investig. Derm. 2016, 9, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, A.; Wright, J.; Shirley, S.; Canton, D.A.; Burkart, C.; Connolly, R.J.; Campbell, J.S.; Pierce, R.H. Characterization of abscopal effects of intratumoral electroporation-mediated IL-12 gene therapy. Gene 2019, 26, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Mizoguchi, I.; Morishima, N.; Chiba, Y.; Mizuguchi, J.; Yoshimoto, T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin. Dev. Immunol. 2010, 2010, 832454. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.P.; Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002, 13, 155–168. [Google Scholar] [CrossRef]
- Kerkar, S.P.; Goldszmid, R.S.; Muranski, P.; Chinnasamy, D.; Yu, Z.; Reger, R.N.; Leonardi, A.J.; Morgan, R.A.; Wang, E.; Marincola, F.M.; et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Investig. 2011, 121, 4746–4757. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.L.; Heller, R. IL-12 gene therapy using an electrically mediated nonviral approach reduces metastatic growth of melanoma. DNA Cell Biol. 2003, 22, 755–763. [Google Scholar] [CrossRef]
- Shao, Q.; O’Flanagan, S.; Lam, T.; Roy, P.; Pelaez, F.; Burbach, B.J.; Azarin, S.M.; Shimizu, Y.; Bischof, J.C. Engineering T cell response to cancer antigens by choice of focal therapeutic conditions. Int. J. Hyperth. 2019, 36, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Bulvik, B.E.; Rozenblum, N.; Gourevich, S.; Ahmed, M.; Andriyanov, A.V.; Galun, E.; Goldberg, S.N. Irreversible Electroporation versus Radiofrequency Ablation: A Comparison of Local and Systemic Effects in a Small-Animal Model. Radiology 2016, 280, 413–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Huang, X.; Zhang, Y.; Lin, X.; Li, S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin. Transl. Med. 2020, 10, e39. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sun, S.; Zhang, Y.; Xie, F.; Li, S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. Oncoimmunology 2021, 10, 1897295. [Google Scholar] [CrossRef] [PubMed]
- Kos, S.; Lopes, A.; Preat, V.; Cemazar, M.; Lampreht Tratar, U.; Ucakar, B.; Vanvarenberg, K.; Sersa, G.; Vandermeulen, G. Intradermal DNA vaccination combined with dual CTLA-4 and PD-1 blockade provides robust tumor immunity in murine melanoma. PLoS ONE 2019, 14, e0217762. [Google Scholar] [CrossRef] [Green Version]
- Algazi, A.P.; Twitty, C.G.; Tsai, K.K.; Le, M.; Pierce, R.; Browning, E.; Hermiz, R.; Canton, D.A.; Bannavong, D.; Oglesby, A.; et al. Phase II Trial of IL-12 Plasmid Transfection and PD-1 Blockade in Immunologically Quiescent Melanoma. Clin. Cancer Res. 2020, 26, 2827–2837. [Google Scholar] [CrossRef]
- Heppt, M.V.; Eigentler, T.K.; Kahler, K.C.; Herbst, R.A.; Goppner, D.; Gambichler, T.; Ulrich, J.; Dippel, E.; Loquai, C.; Schell, B.; et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: A retrospective multicenter analysis. Cancer Immunol. Immunother. 2016, 65, 951–959. [Google Scholar] [CrossRef]
- Campana, L.G.; Peric, B.; Mascherini, M.; Spina, R.; Kunte, C.; Kis, E.; Rozsa, P.; Quaglino, P.; Jones, R.P.; Clover, A.J.P.; et al. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers 2021, 13, 4289. [Google Scholar] [CrossRef]
- Babikr, F.; Wan, J.; Xu, A.; Wu, Z.; Ahmed, S.; Freywald, A.; Chibbar, R.; Wu, Y.; Moser, M.; Groot, G.; et al. Distinct roles but cooperative effect of TLR3/9 agonists and PD-1 blockade in converting the immunotolerant microenvironment of irreversible electroporation-ablated tumors. Cell Mol. Immunol. 2021, 18, 2632–2647. [Google Scholar] [CrossRef]
- He, C.; Sun, S.; Zhang, Y.; Li, S. Irreversible Electroporation Plus Anti-PD-1 Antibody versus Irreversible Electroporation Alone for Patients with Locally Advanced Pancreatic Cancer. J. Inflamm. Res. 2021, 14, 4795–4807. [Google Scholar] [CrossRef]
- Burbach, B.J.; O’Flanagan, S.D.; Shao, Q.; Young, K.M.; Slaughter, J.R.; Rollins, M.R.; Street, T.J.L.; Granger, V.E.; Beura, L.K.; Azarin, S.M.; et al. Irreversible electroporation augments checkpoint immunotherapy in prostate cancer and promotes tumor antigen-specific tissue-resident memory CD8+ T cells. Nat. Commun. 2021, 12, 3862. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; O’Neill, C.; Wang, X.; Chen, Y.; Yu, Y.; Tan, M.; Lv, G.; Li, Y.; Martin, R.C. Irreversible electroporation enhances immunotherapeutic effect in the off-target tumor in a murine model of orthotopic HCC. Am. J. Cancer Res. 2021, 11, 3304–3319. [Google Scholar] [PubMed]
- Lin, M.; Zhang, X.; Liang, S.; Luo, H.; Alnaggar, M.; Liu, A.; Yin, Z.; Chen, J.; Niu, L.; Jiang, Y. Irreversible electroporation plus allogenic Vgamma9Vdelta2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal. Transduct. Target 2020, 5, 215. [Google Scholar] [CrossRef] [PubMed]
- Partridge, B.R.; Kani, Y.; Lorenzo, M.F.; Campelo, S.N.; Allen, I.C.; Hinckley, J.; Hsu, F.C.; Verbridge, S.S.; Robertson, J.L.; Davalos, R.V.; et al. High-Frequency Irreversible Electroporation (H-FIRE) Induced Blood-Brain Barrier Disruption Is Mediated by Cytoskeletal Remodeling and Changes in Tight Junction Protein Regulation. Biomedicines 2022, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Partridge, B.; Rossmeisl, J.H.; Kaloss, A.M.; Basso, E.K.G.; Theus, M.H. Novel ablation methods for treatment of gliomas. J Neurosci. Methods 2020, 336, 108630. [Google Scholar] [CrossRef]
- Hafez, D.M.; Liekweg, C.; Leuthardt, E.C. Staged Laser Interstitial Thermal Therapy (LITT) Treatments to Left Insular Low-Grade Glioma. Neurosurgery 2020, 86, E337–E342. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lee, I.; Tatsui, C.; Elder, T.; Sloan, A.E. Laser interstitial thermotherapy (LITT) for the treatment of tumors of the brain and spine: A brief review. J. Neurooncol. 2021, 151, 429–442. [Google Scholar] [CrossRef]
- Justesen, T.F.; Orhan, A.; Raskov, H.; Nolsoe, C.; Gogenur, I. Electroporation and Immunotherapy-Unleashing the Abscopal Effect. Cancers 2022, 14, 2876. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Chen, Z.; Hu, J.J.; Liu, C. Advances of Electroporation-Related Therapies and the Synergy with Immunotherapy in Cancer Treatment. Vaccines 2022, 10, 1942. https://doi.org/10.3390/vaccines10111942
Gong X, Chen Z, Hu JJ, Liu C. Advances of Electroporation-Related Therapies and the Synergy with Immunotherapy in Cancer Treatment. Vaccines. 2022; 10(11):1942. https://doi.org/10.3390/vaccines10111942
Chicago/Turabian StyleGong, Xuan, Zhou Chen, Jason J. Hu, and Chao Liu. 2022. "Advances of Electroporation-Related Therapies and the Synergy with Immunotherapy in Cancer Treatment" Vaccines 10, no. 11: 1942. https://doi.org/10.3390/vaccines10111942




