Real-World Immunogenicity and Reactogenicity of Two Doses of Pfizer-BioNTech COVID-19 Vaccination in Children Aged 5–11 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort and Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Ethical Statement
2.4. Vaccines
2.5. Immunogenicity
2.5.1. SARS-CoV-2 IgG II Quant (Abbott, IL, USA)
2.5.2. SARS-CoV-2 Pseudo Virus (psSARS-2) Neutralization Assay
2.6. PCR Testing
2.7. Statistical Methods
3. Results
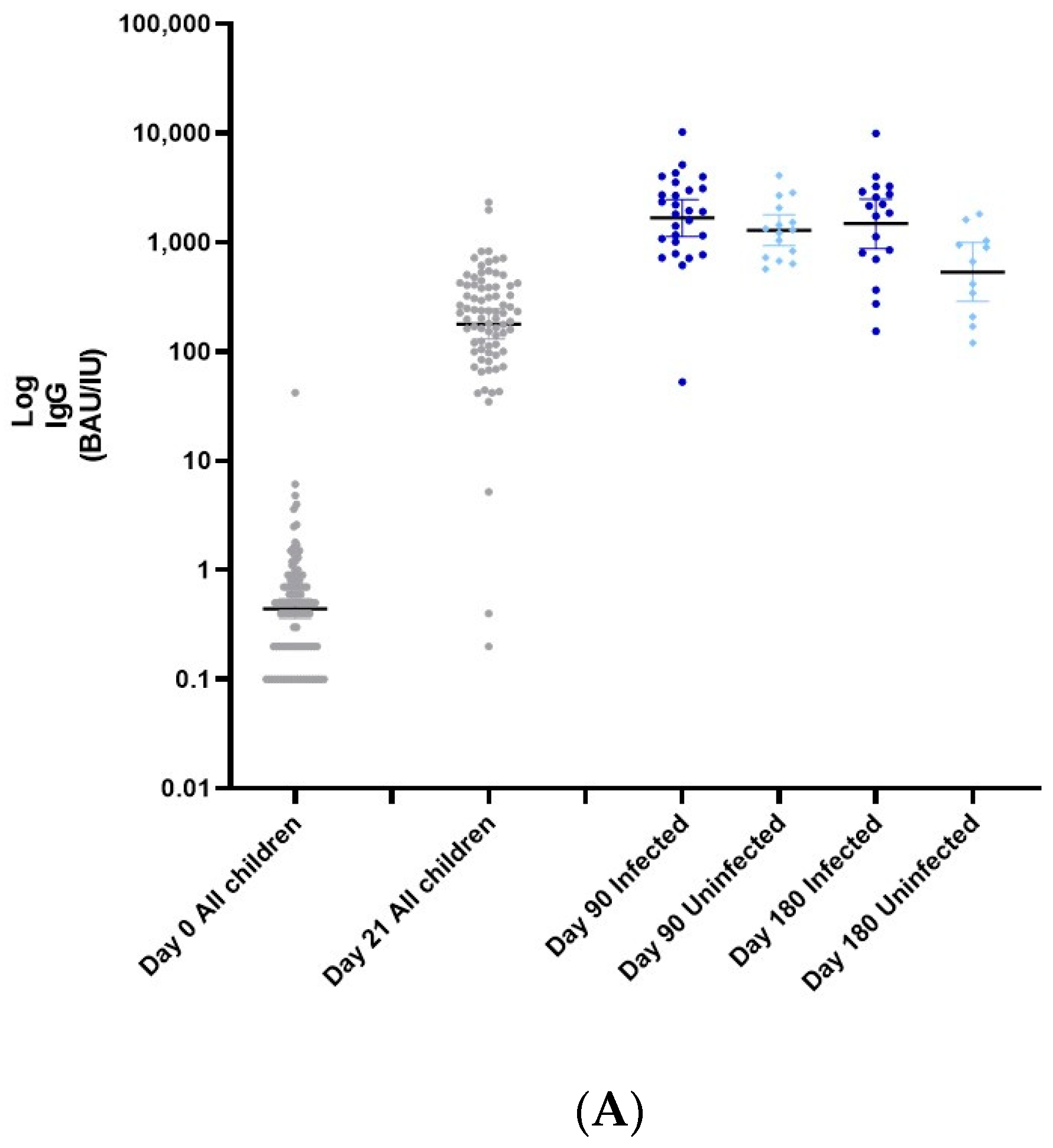
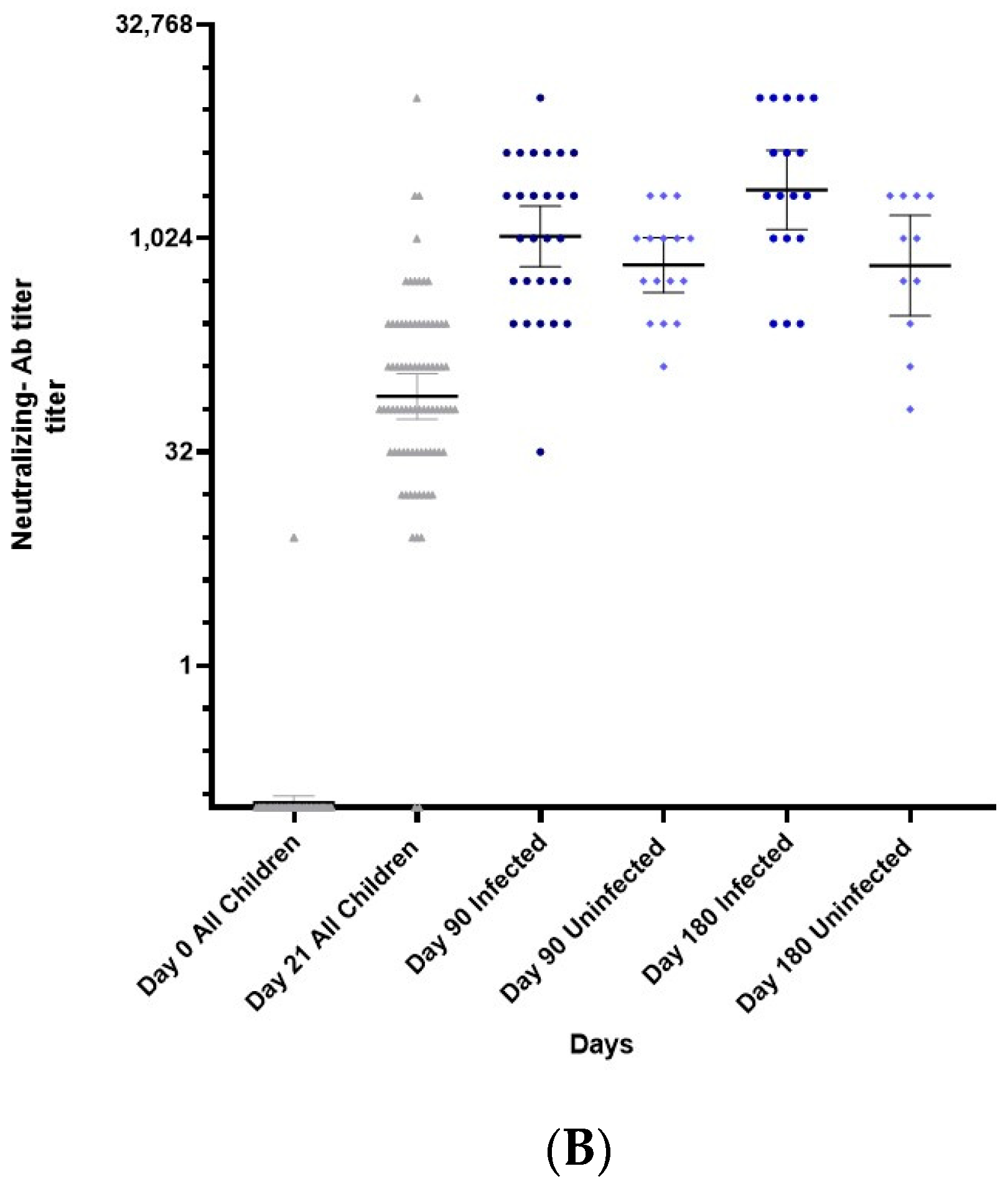
3.1. Immunogenicity
3.2. Safety
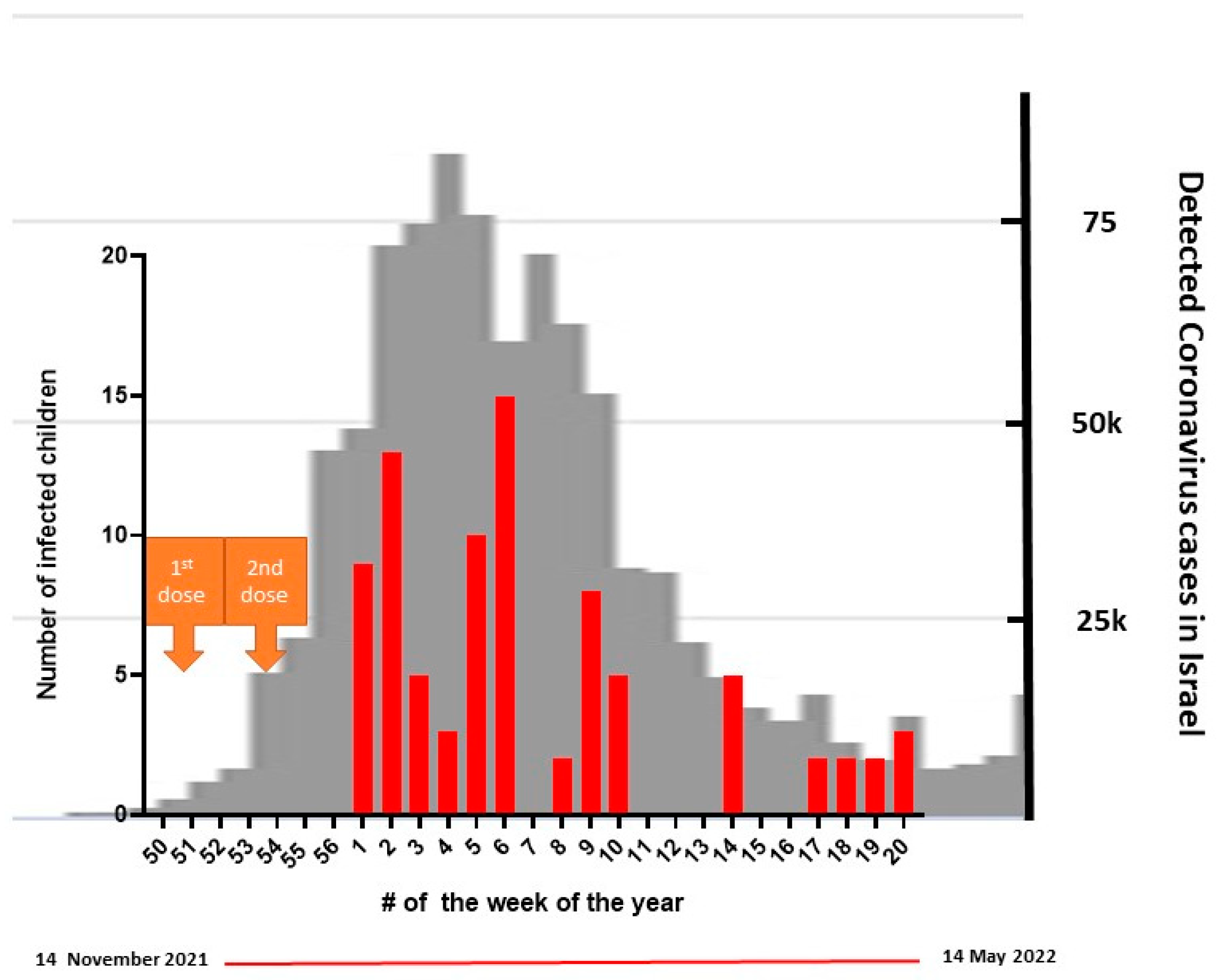
3.3. Breakthrough Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020 [Internet]. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 9 October 2022).
- Kim, L.; Whitaker, M.; O’Halloran, A.; Kambhampati, A.; Chai, S.J.; Reingold, A.; Armistead, I.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; et al. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19-COVID-NET, 14 States, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020, 69, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Kitano, M.; Krueger, C.; Jamal, H.; Al Rawahi, H.; Lee-Krueger, R.; Sun, R.D.; Isabel, S.; García-Ascaso, M.T.; Hibino, H.; et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: A systematic review of fatality and ICU admission in children worldwide. PLoS ONE 2021, 16, e0246326. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull. World Health Organ. 2021, 99, 19–33F. [Google Scholar] [CrossRef]
- Smith, C.; Odd, D.; Harwood, R.; Ward, J.; Linney, M.; Clark, M.; Hargreaves, D.; Ladhani, S.N.; Draper, E.; Davis, P.J.; et al. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat. Med. 2022, 28, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Ashkenazi-Hoffnung, L.; Greenberg, D.; Dalal, I.; Livni, G.; Chapnick, G.; Stein-Zamir, C.; Ashkenazi, S.; Hecht-Sagie, L.; Grossman, Z. The Burden of COVID-19 in Children and Its Prevention by Vaccination: A Joint Statement of the Israeli Pediatric Association and the Israeli Society for Pediatric Infectious Diseases. Vaccines 2022, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Epidemic Treatment Team and the Corona Vaccine Committee Discussions-IMoH [Internet]. Israeli Ministry of Health. 2021. Available online: https://www.gov.il/he/departments/publications/reports/vaccine-priorities-board (accessed on 20 October 2022).
- Frenkel, L.D.; Gomez, F.; Bellanti, J.A. COVID-19 in children: Pathogenesis and current status. Allergy Asthma Proc. 2021, 42, 8–15. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.D.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; Mancini, A.; et al. COVID-19 Infection in Children and Infants: Current Status on Therapies and Vaccines. Children 2022, 9, 249. [Google Scholar] [CrossRef]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Calò Carducci, F.I.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- Bellino, S.; Punzo, O.; Rota, M.C.; Del Manso, M.; Urdiales, A.M.; Andrianou, X.; Fabiani, M.; Boros, S.; Vescio, F.; Riccardo, F.; et al. COVID-19 Disease Severity Risk Factors for Pediatric Patients in Italy. Pediatrics 2020, 146, e2020009399. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, J.; Hong, L.; Luo, Y.; Song, Q.; Chen, D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect Dis. 2020, 20, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Assaker, R.; Colas, A.E.; Julien-Marsollier, F.; Bruneau, B.; Marsac, L.; Greff, B.; Tri, N.; Fait, C.; Brasher, C.; Dahmani, S. Presenting symptoms of COVID-19 in children: A meta-analysis of published studies. Br. J. Anaesth. 2020, 125, e330–e332. [Google Scholar] [CrossRef] [PubMed]
- Cotugno, N.; Franzese, E.; Angelino, G.; Amodio, D.; Romeo, E.F.; Rea, F.; Faraci, S.; Tambucci, R.; Profeti, E.; Manno, E.C.; et al. Evaluation of Safety and Immunogenicity of BNT162B2 mRNA COVID-19 Vaccine in IBD Pediatric Population with Distinct Immune Suppressive Regimens. Vaccines 2022, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Liguoro, I.; Pilotto, C.; Bonanni, M.; Ferrari, M.E.; Pusiol, A.; Nocerino, A.; Vidal, E.; Cogo, P. SARS-COV-2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020, 179, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S. EtR Framework: Pfizer-BioNTech COVID-19 Vaccine in Children Aged 5–11 Years [Internet]. CDC ACIP Meeting; 2 November 2021. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/08-COVID-Oliver-508.pdf (accessed on 28 November 2021).
- Somekh, E.; Gleyzer, A.; Heller, E.; Lopian, M.; Kashani-Ligumski, L.; Czeiger, S.; Schindler, Y.; Lessing, J.B.; Stein, M. The role of children in the dynamics of intra family coronavirus 2019 spread in densely populated area. Pediatr. Infect. Dis. J. 2020, 39, e202–e204. [Google Scholar] [CrossRef]
- Somekh, I.; Stein, M.; Karakis, I.; Simões, E.A.F.; Somekh, E. Characteristics of SARS-CoV-2 Infections in Israeli Children during the Circulation of Different SARS-CoV-2 Variants. JAMA Netw Open 2021, 4, e2124343. [Google Scholar] [CrossRef]
- Swann, O.V.; Holden, K.A.; Turtle, L.; Pollock, L.; Fairfield, C.J.; Drake, T.M.; Seth, S.; Egan, C.; Hardwick, H.E.; Halpin, S.; et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: Prospective multicentre observational cohort study. BMJ 2020, 370, m3249. [Google Scholar] [CrossRef]
- Interim Clinical Considerations for Use of COVID-19 Vaccines|CDC [Internet]. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html (accessed on 9 October 2022).
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- COVID-19 Vaccines|FDA [Internet]. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 29 September 2022).
- Jørgensen, S.B.; Nygård, K.; Kacelnik, O.; Telle, K. Secondary Attack Rates for Omicron and Delta Variants of SARS-CoV-2 in Norwegian Households. JAMA 2022, 327, 1610–1611. [Google Scholar] [CrossRef]
- Baker, J.M.; Nakayama, J.Y.; O’Hegarty, M.; McGowan, A.; Teran, R.A.; Bart, S.M.; Mosack, K.; Roberts, N.; Campos, B.; Paegle, A.; et al. SARS-CoV-2 B.1.1.529 (Omicron) Variant Transmission within Households-Four U.S. Jurisdictions, November 2021-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 341–346. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Sheward, D.J.; Kim, C.; Ehling, R.A.; Pankow, A.; Castro Dopico, X.; Dyrdak, R.; Martin, D.P.; Reddy, S.T.; Dillner, J.; Karlsson Hedestam, G.B.; et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: A cross-sectional study. Lancet Infect. Dis. 2022, 22, 813–820. [Google Scholar] [CrossRef]
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; Da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.C.; et al. Plasma neutralization properties of the SARS-CoV-2 Omicron variant. N. Engl. J. Med. 2022, 386, 599–601. [Google Scholar] [CrossRef]
- Rössler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022, 386, 698–700. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Melendez Baez, I.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef]
- Seery, V.; Raiden, S.; Russo, C.; Borda, M.; Herrera, L.; Uranga, M.; Varese, A.; Marcó Del Pont, M.; Chirino, C.; Erramuspe, C.; et al. Antibody response against SARS-CoV-2 variants of concern in children infected with pre-Omicron variants: An observational cohort study. EBioMedicine 2022, 83, 104230. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Kliker, L.; Zuckerman, N.; Atari, N.; Barda, N.; Gilboa, M.; Nemet, I.; Abd Elkader, B.; Fratty, I.S.; Jaber, H.; Mendelson, E.; et al. COVID-19 vaccination and BA1 breakthrough infection induce neutralising antibodies which are less efficient against BA4 and BA5 Omicron variants, Israel, March to June 2022. Euro Surveill. 2022, 27, 2200559. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Su, J.R.; Hugueley, B.; Thompson, D.; Gee, J.; Shimabukuro, T.T.; Shay, D.K. Safety Monitoring of Pfizer-BioNTech COVID-19 Vaccine Booster Doses Among Children Aged 5–11 Years-United States, May 17–July 31, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Gee, J.; Su, J.R.; Zhang, B.; Thompson, D.; Shimabukuro, T.T.; Shay, D.K. COVID-19 Vaccine Safety in Children Aged 5–11 Years-United States, 3 November–19 December 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Poparn, H.; Srichumpuang, C.; Sosothikul, D.; Jantarabenjakul, W.; Lauhasurayotin, S.; Techavichit, P.; Chiangthong, K.; Poovorawan, Y. Immune Response after 2 Doses of BNT162b2 mRNA COVID-19 Vaccinations in Children and Adolescents with Cancer and Hematologic Diseases. Asian Pac. J. Cancer Prev. 2022, 23, 2049–2055. [Google Scholar] [CrossRef]
- Akgün, Ö.; Çakmak, F.; Guliyeva, V.; Demirkan, F.G.; Tanatar, A.; Hançerli Torun, S.; Çin, D.; Meşe, S.; Ağaçfidan, A.; Aktay Ayaz, N. Humoral Response and Safety of BNT162b2 mRNA Vaccine in Children with Rheumatic Diseases under Immunomodulatory Treatment: A Preliminary Study. Rheumatology 2022, 30, 4482–4490. [Google Scholar] [CrossRef]
- Shoji, K.; Funaki, T.; Yamada, M.; Mikami, M.; Miyake, K.; Ueno, S.; Tao, C.; Myojin, S.; Aiba, H.; Matsui, T.; et al. Safety of and antibody response to the BNT162b2 COVID-19 vaccine in adolescents and young adults with underlying disease. J. Infect. Chemother. 2022, 21, 61–66. [Google Scholar] [CrossRef]
- Israel, A.; Merzon, E.; Schäffer, A.A.; Shenhar, Y.; Green, I.; Golan-Cohen, A.; Ruppin, E.; Magen, E.; Vinker, S. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: Test negative design study. BMJ 2021, 375, e067873. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: A retrospective, total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the, USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Wallace, M.; Godfrey, M.; Roper, L.E.; Hall, E.; Fleming-Dutra, K.E.; Link-Gelles, R.; Pilishvili, T.; Williams, J.; Moulia, D.L. Interim Recommendations from the Advisory Committee on Immunization Practices for the Use of Bivalent Booster Doses of COVID-19 Vaccines—United States, October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Chiew, C.J.; Premikha, M.; Chong, C.Y.; Wei, W.E.; Ong, B.; Lye, D.C.; Heng, D.; Lee, V.J.; Tan, K.B. Effectiveness of primary series and booster vaccination against SARS-CoV-2 infection and hospitalisation among adolescents aged 12–17 years in Singapore: A national cohort study. Lancet Infect. Dis. 2022, 28, S1473-3099(22)00573-4. [Google Scholar] [CrossRef]
- Israeli Ministry of Health. Corona dashboard [Internet]. Available online: https://datadashboard.health.gov.il/COVID-19/ (accessed on 29 March 2021).
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.-G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection among Children Aged 5–11 Years and Adolescents Aged 12–15 Years-PROTECT Cohort, July 2021–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination with Symptomatic SARS-CoV-2 Infection in Children and Adolescents during Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- Cohen-Stavi, C.J.; Magen, O.; Barda, N.; Yaron, S.; Peretz, A.; Netzer, D.; Giaquinto, C.; Judd, A.; Leibovici, L.; Hernán, M.A.; et al. BNT162b2 Vaccine Effectiveness against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 227–236. [Google Scholar] [CrossRef]
- Klein, N.P.; Stockwell, M.S.; Demarco, M.; Gaglani, M.; Kharbanda, A.B.; Irving, S.A.; Rao, S.; Grannis, S.J.; Dascomb, K.; Murthy, K.; et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA Vaccination in Preventing COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Nonimmunocompromised Children and Adolescents Aged 5–17 Years-VISION Network, 10 States, April 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 352–358. [Google Scholar] [CrossRef]
- Tan, S.H.X.; Cook, A.R.; Heng, D.; Ong, B.; Lye, D.C.; Tan, K.B. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Rosa Duque, J.S.; Leung, D.; Yip, K.M.; Lee, D.H.L.; So, H.; Wong, W.H.S.; Lung, Y. Effectiveness of BNT162b2 and CoronaVac against pediatric COVID-19-associated hospitalization and moderate-to-severe disease. medRxiv 2022, 11, 2304–2314. [Google Scholar]
| Visit | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Day | Day 0 | Day 21 ± 4 | Day 90 ± 7 | Day 180 ± 7 |
| Vaccine Dose | 1st | 2nd | ||
| Number of children tested * | 110 | 86 | 45 | 29 |
| Number questionnaires responses | 53 | 30 | 110 | 110 |
| Number of infected children ** | 0 | 0 | 70 | 83 |
| Total | Infected | Uninfected | |
|---|---|---|---|
| N (%) | 110 (100%) | 83 (75.5%) | 27 (24.5%) |
| Age (years) | |||
| Mean (SD) | 9.18 (1.97) | 9.32 (1.94) | 8.65 (2.04) |
| Median (Range) | 9.39 (5–11) | 9.86 (5–11) | 8.53 (5–11) |
| Gender = female (%) | 41 (37.3%) | 32 (38.6%) | 9 (33.3%) |
| Symptoms | First Dose of BNT126b2 Vaccine N = 52 | Second Dose of BNT126b2 Vaccine N = 31 | ||
|---|---|---|---|---|
| N (%) | Symptom Duration (Days) Mean (SD) | N (%) | Symptom Duration (Days) Mean (SD), Median | |
| Any local symptom | 37 (71.1%) | 20 (64.5%) | ||
| Pain at the injection site | 37 (71.1%) | 2.07 (0.94) | 18 (58.1%) | 2.40 (1.10), 2 |
| Erythema | 3 (5.7%) | 2.07 (0.94) | 2 (6.5%) | 2.40 (1.10), 2 |
| Edema | 3 (5.7%) | 2.07 (0.94) | 0 (0%) | |
| Itching at the injection site | 1 (1.9%) | 2.07 (0.94) | 1 (3.2%) | 2.40 (1.10), 2 |
| Other local symptoms | 1 (1.9%) (abdominal pain) | 0 (0%) | ||
| Any systemic symptom | 12 (23.1%) | 7 (22.6%) | ||
| Fever over 37.5 °C | 2 (3.8%) | 4 (12.8%) | 1.6 (1.1), 1.5 | |
| Fatigue and weakness | 9 (17.0%) | 5.3 (4.4), 5 | 5 (16.0%) | 3.4 (2.1), 3 |
| Myalgia | 6 (11.4%) | 3.3 (2.3), 3 | 2 (6.4%) | 5.5 (0.7), 5.5 |
| Lymphadenopathy | 5 (9.2%) | 3 (0%), 3 | 2 (6.4%) | 1 |
| Headache | 7 (13.2%) | 3.3 (1.7), 3 | 4 (12.8%) | 2.5 (1.9), 2.0 |
| Facial nerve palsy | 0 (0%) | 0 (0%) | ||
| Paresthesia | 0 (0%) | 0 (0%) | ||
| Systemic Allergic reaction | 0 (0%) | 0 (0%) | ||
| At least one day of school absence | 8 (15.1%) | 1.63 (1.41), 1 | 7 (21%) | 1.2 (0.44), 1 |
| Medical treatment | 1 (1.9%) Visit a pediatrician | 2 (6.4%) Analgesics, antibiotics | ||
| Hospitalization | 0 (0%) | 0 (0%) | ||
| Symptom | N (%) | |
|---|---|---|
| 1. | any symptoms | 52 (62.6%) |
| 2. | fatigue or weakness | 37 (44.6%) |
| 3. | rhinorrhea | 33 (39.8%) |
| 4. | headache | 29 (34.9%) |
| 5. | sore throat | 28 (33.7%) |
| 6. | fever above 37.5 for up to 2 days | 24 (28.9%) |
| 7. | cough | 20 (24.1%) |
| 8. | Gastrointestinal symptoms | 8 (9.6%) |
| 9. | fever above 37.5 for more than 2 days | 7 (8.4%) |
| 10. | anosmia or ageusia | 2 (2.4%) |
| 11. | shortness of breath | 1 (1.2%) |
| 12. | hospitalization | 0 (0%) |
| 13. | any other symptoms | 2 (2.4%) (myalgia, hallucinations) |
| 14. | symptomatic illness length (days) COVID-19 Mean (SD), Median | 3.5 (0.71), 3.5 |
| 15. | school absence (days) Mean (SD), Median | 5.72 (1.48), 5 |
| 16. | persistent COVID symptoms (more than 2 weeks after the initial illness) | 3 (3.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, G.; Klein, E.; Lustig, Y.; Weiss-Ottolenghi, Y.; Asraf, K.; Indenbaum, V.; Amit, S.; Kriger, O.; Gilboa, M.; Levy, Y.; et al. Real-World Immunogenicity and Reactogenicity of Two Doses of Pfizer-BioNTech COVID-19 Vaccination in Children Aged 5–11 Years. Vaccines 2022, 10, 1954. https://doi.org/10.3390/vaccines10111954
Joseph G, Klein E, Lustig Y, Weiss-Ottolenghi Y, Asraf K, Indenbaum V, Amit S, Kriger O, Gilboa M, Levy Y, et al. Real-World Immunogenicity and Reactogenicity of Two Doses of Pfizer-BioNTech COVID-19 Vaccination in Children Aged 5–11 Years. Vaccines. 2022; 10(11):1954. https://doi.org/10.3390/vaccines10111954
Chicago/Turabian StyleJoseph, Gili, Elisheva Klein, Yaniv Lustig, Yael Weiss-Ottolenghi, Keren Asraf, Victoria Indenbaum, Sharon Amit, Or Kriger, Mayan Gilboa, Yuval Levy, and et al. 2022. "Real-World Immunogenicity and Reactogenicity of Two Doses of Pfizer-BioNTech COVID-19 Vaccination in Children Aged 5–11 Years" Vaccines 10, no. 11: 1954. https://doi.org/10.3390/vaccines10111954






