Is the BCG Vaccine an Answer to Future Pandemic Preparedness?
Abstract
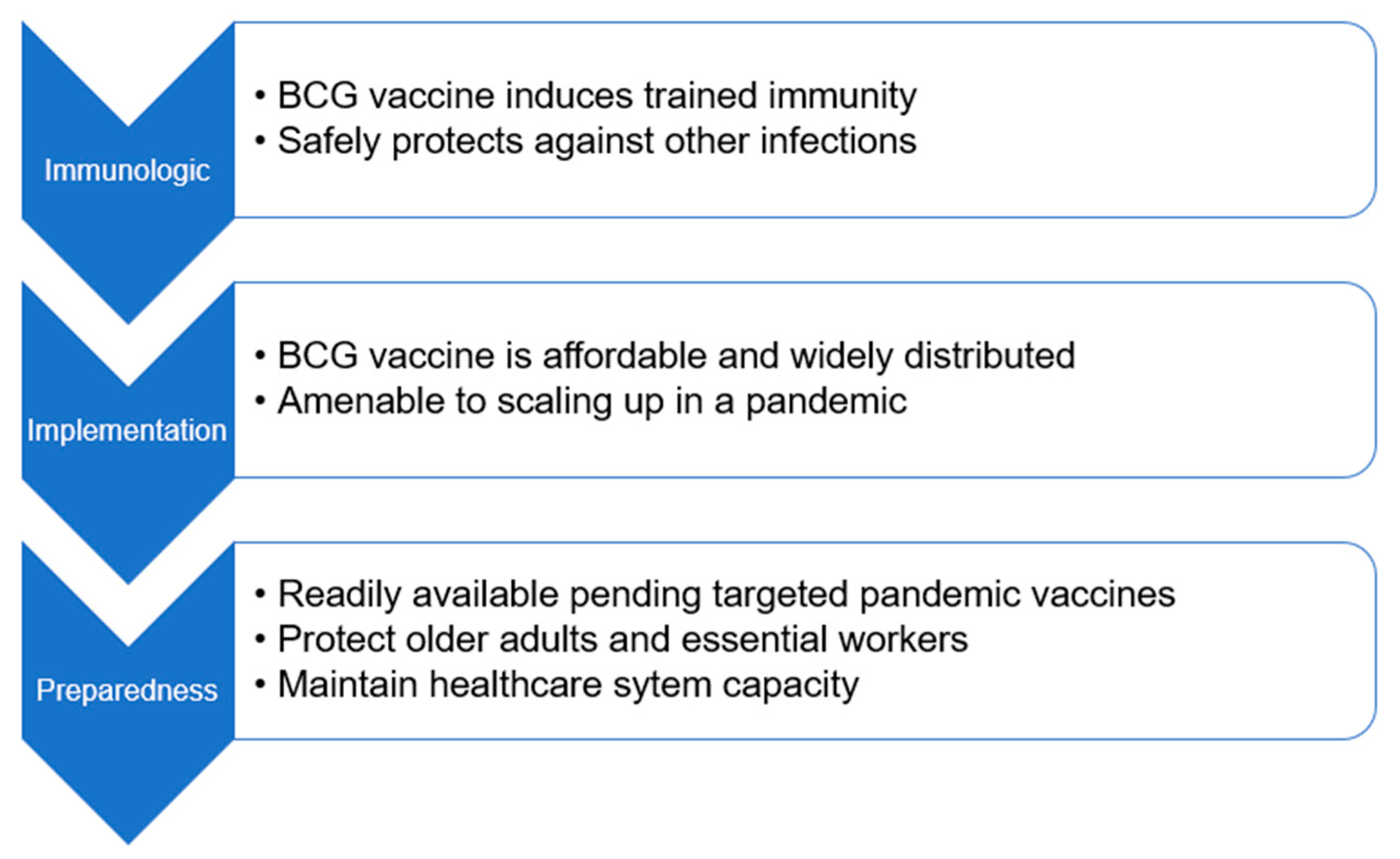
:1. Introduction
2. BCG, Trained Immunity, and Clinical Infection
3. BCG Implementation
4. BCG for Future Pandemic Preparedness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 19 January 2022).
- Bregu, M.; Draper, S.J.; Hill, A.V.S.; Greenwood, B.M. Accelerating vaccine development and deployment: Report of a Royal Society satellite meeting. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Edara, V.V.; Norwood, C.; Floyd, K.; Lai, L.; Davis-Gardner, M.E.; Hudson, W.H.; Mantus, G.; Nyhoff, L.E.; Adelman, M.W.; Fineman, R.; et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 2021, 29, 516–521.e2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, G.L.; Shen, Y.; Wang, Z.Y.; Zhan, B.D.; Duan, L.J.; Lu, B.; Shi, C.; Gao, Y.M.; Peng, H.H.; et al. Persistence of Antibody and Cellular Immune Responses in COVID-19 patients over Nine Months after Infection. J. Infect. Dis. 2021, 224, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Bettencourt, P.J.; Joosten, S.A.; Arlehamn, C.S.L.; Behr, M.A.; Locht, C.; Neyrolles, O. 100 years of the Bacillus Calmette-Guérin vaccine. Vaccine 2021, 39, 7221–7222. [Google Scholar] [CrossRef]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M.; et al. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Novakovic, B.; Habibi, E.; Wang, S.Y.; Arts, R.J.; Davar, R.; Megchelenbrink, W.; Kim, B.; Kuznetsova, T.; Kox, M.; Zwaag, J.; et al. beta-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 2016, 167, 1354–1368.e14. [Google Scholar] [CrossRef] [Green Version]
- Fanucchi, S.; Fok, E.T.; Dalla, E.; Shibayama, Y.; Börner, K.; Chang, E.Y.; Stoychev, S.; Imakaev, M.; Grimm, D.; Wang, K.C.; et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat. Genet. 2019, 51, 138–150. [Google Scholar] [CrossRef]
- DiNardo, A.R.; Netea, M.G.; Musher, D.M. Postinfectious Epigenetic Immune Modifications—A Double-Edged Sword. N. Engl. J. Med. 2021, 384, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; Van Loenhout, J.; Xavier, R.J.; Aaby, P.; Van Der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, A.; Garly, M.L.; Jensen, H.; Nielsen, J.; Aaby, P. Bacillus Calmette-Guérin vaccination and infant mortality. Expert Rev. Vaccines 2006, 5, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef]
- Prentice, S.; Nassanga, B.; Webb, E.L.; Akello, F.; Kiwudhu, F.; Akurut, H.; Elliott, A.M.; Arts, R.J.; Netea, M.G.; Dockrell, H.M.; et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: An investigator-blind randomised controlled trial. Lancet Infect. Dis. 2021, 21, 993–1003. [Google Scholar] [CrossRef]
- Messina, N.L.; Pittet, L.F.; Gardiner, K.; Freyne, B.; Francis, K.L.; Zufferey, C.; Abruzzo, V.; Morrison, C.; Allen, K.J.; Flanagan, K.L.; et al. Neonatal BCG vaccination and infections in the first year of life: The MIS BAIR randomised controlled trial. J. Infect. Dis. 2021, 224, 1115–1127. [Google Scholar] [CrossRef]
- Prentice, S.; Dockrell, H.M. BCG Specific and Nonspecific Effects: Different Questions, Similar Challenges. J. Infect. Dis. 2021, 224, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; Van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef] [Green Version]
- Blok, B.A.; de Bree, L.C.; Diavatopoulos, D.A.; Langereis, J.D.; Joosten, L.A.; Aaby, P.; van Crevel, R.; Benn, C.S.; Netea, M.G. Interacting, Nonspecific, Immunological Effects of Bacille Calmette-Guerin and Tetanus-diphtheria-pertussis Inactivated Polio Vaccinations: An Explorative, Randomized Trial. Clin. Infect. Dis. 2020, 70, 455–463. [Google Scholar] [CrossRef]
- Hirve, S.; Bavdekar, A.; Juvekar, S.; Benn, C.S.; Nielsen, J.; Aaby, P. Non-specific and sex-differential effects of vaccinations on child survival in rural western India. Vaccine 2012, 30, 7300–7308. [Google Scholar] [CrossRef]
- Broset, E.; Pardo-Seco, J.; Kanno, A.I.; Aguilo, N.; Dacosta, A.I.; Rivero-Calle, I.; Gonzalo-Asensio, J.; Locht, C.; Leite, L.C.; Martin, C.; et al. BCG vaccination improves DTaP immune responses in mice and is associated with lower pertussis incidence in ecological epidemiological studies. EBioMedicine 2021, 65, 103254. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Benn, C.S. Stopping live vaccines after disease eradication may increase mortality. Vaccine 2020, 38, 10–14. [Google Scholar] [CrossRef]
- World Health Organization. Preferred Product Characteristics for New Tuberculosis Vaccines; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Arts, R.J.; Moorlag, S.J.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walk, J.; De Bree, L.C.J.; Graumans, W.; Stoter, R.; Van Gemert, G.-J.; Van De Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.C.; Arts, R.J.W.; Behet, M.C.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Domínguez-Andrés, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.E.; Damoraki, G.; et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell 2020, 183, 315–323.e9. [Google Scholar] [CrossRef] [PubMed]
- Wardhana Datau, E.A.; Sultana, A.; Mandang, V.V.; Jim, E. The efficacy of Bacillus Calmette-Guerin vaccinations for the preven-tion of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011, 43, 185–190. [Google Scholar]
- Bannister, S.; Sudbury, E.; Villanueva, P.; Perrett, K.; Curtis, N. The safety of BCG revaccination: A systematic review. Vaccine 2021, 39, 2736–2745. [Google Scholar] [CrossRef]
- Moorlag, S.J.; van Deuren, R.C.; van Werkhoven, C.H.; Jaeger, M.; Debisarun, P.; Taks, E.; Mourits, V.P.; Koeken, V.A.; de Bree, L.C.; Ten Doesschate, T.; et al. Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: A Retrospective Cohort Study. Cell. Rep. Med. 2020, 1, 100073. [Google Scholar] [CrossRef]
- Kinoshita, M.; Tanaka, M. Impact of Routine Infant BCG Vaccination on COVID-19. J. Infect. 2020, 81, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef]
- Abdulah, D.M.; Hassan, A.B. Exploration of Association Between Respiratory Vaccinations With Infection and Mortality Rates of COVID-19. Disaster Med. Public Heal. Prep. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Blass, I.; Rappoport, N.; Linial, M. Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vac-cination Coverage: A Multivariable Analysis. Vaccines 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Lindestam Arlehamn, C.S.; Sette, A.; Peters, B. Lack of evidence for BCG vaccine protection from severe COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 25203–25204. [Google Scholar] [CrossRef] [PubMed]
- de Chaisemartin, C.; de Chaisemartin, L. BCG vaccination in infancy does not protect against COVID-19. Evidence from a natural experiment in Sweden. Clin. Infect. Dis. 2021, 72, e501–e505. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.; Labbé, A.C.; Carignan, A.; Parent, M.E.; Yu, J.; Grenier, C.; Beauchemin, S.; De Wals, P.; Valiquette, L.; Rousseau, M.C. Does BCG provide long-term protection against SARS-CoV-2 infection? A case-control study in Quebec, Canada. Vaccine 2021, 39, 7300–7307. [Google Scholar] [CrossRef] [PubMed]
- Hamiel, U.; Kozer, E.; Youngster, I. SARS-CoV-2 Rates in BCG-Vaccinated and Unvaccinated Young Adults. JAMA 2020, 323, 2340–2341. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Epidemiological transcriptomic data supports BCG protection in viral diseases including COVID-19. Gene 2021, 783, 145574. [Google Scholar] [CrossRef]
- Tsilika, M.; Taks, E.; Dolianitis, K.; Kotsaki, A.; Leventogiannis, K.; Damoulari, C.; Kostoula, M.; Paneta, M.; Adamis, G.; Papanikolaou, I.C.; et al. Activate-2: A double-blind randomized trial of bcg vaccination against covid19 in individuals at risk. medRxiv 2021. [Google Scholar] [CrossRef]
- Machlaurin, A.; Dolk, F.C.K.; Setiawan, D.; Van Der Werf, T.S.; Postma, M.J. Cost-Effectiveness Analysis of BCG Vaccination against Tuberculosis in Indonesia: A Model-Based Study. Vaccines 2020, 8, 707. [Google Scholar] [CrossRef]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef]
- Tu, H.-A.T.; Vu, H.; Rozenbaum, M.H.; Woerdenbag, H.; Postma, M.J. A review of the literature on the economics of vaccination against TB. Expert Rev. Vaccines 2012, 11, 303–317. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Immunization Policy: BCG. Available online: https://www.who.int/immunization/policy/bcg.pdf (accessed on 22 June 2021).
- The Global Fund. Results Report. 2021. Available online: https://www.theglobalfund.org/media/11304/corporate_2021resultsreport_report_en.pdf (accessed on 19 January 2022).
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Infectious Diseases. COVID-19 vaccine equity and booster doses. Lancet Infect. Dis. 2021, 21, 1193. [Google Scholar] [CrossRef]
- Marin-Hernandez, D.; Nixon, D.F.; Hupert, N. Anticipated reduction in COVID-19 mortality due to population-wide BCG vac-cination: Evidence from Germany. Hum. Vaccines Immunother. 2021, 17, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Kiravu, A.; Osawe, S.; Happel, A.U.; Nundalall, T.; Wendoh, J.; Beer, S.; Dontsa, N.; Alinde, O.B.; Mohammed, S.; Datong, P.; et al. Bacille Calmette-Guerin Vaccine Strain Modulates the Ontogeny of Both Mycobacteri-al-Specific and Heterologous T Cell Immunity to Vaccination in Infants. Front. Immunol. 2019, 10, 2307. [Google Scholar] [CrossRef]
| Title | Locations | Identifier | N |
|---|---|---|---|
| BCG to Reduce Absenteeism Among Health Care Workers During the COVID-19 Pandemic | Cape Verde Guinea-Bissau Mozambique | NCT04641858 | 1050 |
| Outcome of COVID-19 Cases Based on Tuberculin Test: Can Previous BCG Alter the Prognosis? | Egypt | NCT04347876 | 100 |
| Use of BCG Vaccine as a Preventive Measure for COVID-19 in Health Care Workers | Brazil | NCT04659941 | 1000 |
| Clinical Trial Evaluating the Effect of BCG Vaccination on the Incidence and Severity of SARS-CoV-2 Infections Among Healthcare Professionals During the COVID-19 Pandemic in Poland | Poland | NCT04659941 | 1000 |
| Prevention, Efficacy and Safety of BCG Vaccine in COVID-19 Among Healthcare Workers | Mexico | NCT04461379 | 908 |
| BCG Vaccine in Reducing Morbidity and Mortality in Elderly Individuals in COVID-19 Hotspots | India | NCT04475302 | 2175 |
| Prevention of Respiratory Tract Infection and COVID-19 through BCG Vaccination in Vulnerable Older Adults | Netherlands | NCT04537663 | 5200 |
| Application of BCG Vaccine for Immune-prophylaxis Among Egyptian Healthcare Workers During the Pandemic of COVID-19 | Egypt | NCT04350931 | 900 |
| Reducing COVID-19 Related Hospital Admission in Elderly by BCG Vaccination | Netherlands | NCT04417335 | 2014 |
| BCG Against COVID-19 for Prevention and Amelioration of Severity Trial (BAC to the PAST) | United States | NCT04534803 | 2100 |
| COVID-19: BCG As Therapeutic Vaccine, Transmission Limitation, and Immunoglobulin Enhancement | Brazil | NCT04369794 | 1000 |
| BCG Vaccination for Healthcare Workers in COVID-19 Pandemic | South Africa | NCT04379336 | 500 |
| Reducing Health Care Workers Absenteeism in COVID-19 Pandemic Through BCG Vaccine | Netherlands | NCT04328441 | 1500 |
| BCG Vaccination to Protect Healthcare Workers Against COVID-19 | Australia | NCT04327206 | 10078 |
| Using BCG Vaccine to Protect Health Care Workers in the COVID-19 Pandemic | Denmark | NCT04373291 | 1293 |
| Using BCG to Protect Senior Citizens During the COVID-19 Pandemic | Denmark | NCT04542330 | 1900 |
| Efficacy of BCG Vaccination in the Prevention of COVID-19 via the Strengthening of Innate Immunity in Health Care Workers | France | NCT04384549 | 1120 |
| BCG Vaccine for Health Care Workers as Defense Against COVID-19 | United States | NCT04348370 | 1800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.H.; Rouphael, N.G.; Baden, L.R.; Graciaa, D.S. Is the BCG Vaccine an Answer to Future Pandemic Preparedness? Vaccines 2022, 10, 201. https://doi.org/10.3390/vaccines10020201
Khan NH, Rouphael NG, Baden LR, Graciaa DS. Is the BCG Vaccine an Answer to Future Pandemic Preparedness? Vaccines. 2022; 10(2):201. https://doi.org/10.3390/vaccines10020201
Chicago/Turabian StyleKhan, Nadia H., Nadine G. Rouphael, Lindsey R. Baden, and Daniel S. Graciaa. 2022. "Is the BCG Vaccine an Answer to Future Pandemic Preparedness?" Vaccines 10, no. 2: 201. https://doi.org/10.3390/vaccines10020201






