1. Introduction
Salmonella enterica is an important gastroenteric pathogen, causing worldwide outbreaks in the human population. It is estimated that
Salmonella enterica gastroenteritis is responsible for about 93.8 million illnesses and 155,000 deaths each year; of these, it is estimated that 80.3 million cases are foodborne, with very high associated costs [
1]. Animal food products, especially eggs and poultry meat, have been the most common vehicles of
Salmonella infections. Outbreaks might be caused by several serotypes of non-typhoidal
Salmonella (NTS).
Salmonella Enteritidis,
Salmonella Typhimurium,
Salmonella Newport,
Salmonella Heidelberg and
Salmonella Infantis are among the most important.
S. enterica, serovar Enteritidis is implicated in 60% of salmonellosis in European people and is the world’s leading cause of salmonellosis. In the United States,
S. Typhimurium is mostly associated with salmonellosis. In Europe,
S. Infantis has emerged and is a frequently reported serovar from chicken meat (36.5%) and broilers (56.7%). In the USA,
S. Infantis is commonly isolated from sick humans and poultry meat products. The European Union summary reported on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016 [
2,
3]. In recent years, multi-drug-resistant
Salmonella have emerged and complicate the treatment of at-risk groups. Drug resistance is prevalent in
S. Typhimurium and
S. Infantis serotypes and is successfully spread from broilers to humans through certain clones [
4].
Salmonella is devastating to public health and has a negative economic impact on the poultry industry. To reduce the contamination of eggs and meat with
Salmonella and thus prevent contamination in the human population, producing farms have comprehensive
Salmonella control programs that include surveillance, biosecurity, management and vaccination [
5]. Vaccination programs for poultry are frequently complex, involving multiple vaccinations with a range of different vaccines. Commercial
Salmonella vaccines for poultry include live attenuated vaccines, inactivated (killed) vaccines and subunit vaccines and are typically based on strains of
S. Enteritidis and
S. Typhimurium. Multivalent vaccines are required to provide protection against the broad range of serovars found on poultry farms [
6]. Subunit vaccines are safer because they only contain the antigens of the pathogen. However, multiple doses with these acellular fractions might be needed to confer long-lasting immunity against
Salmonella [
7]. One strategy to improve the immunogenicity of subunit vaccines is to use cochleate systems. Cochleates are phospholipid–calcium precipitates derived from the interaction of anionic lipid vesicles with divalent cations. They have a defined multilayered structure consisting of a solid, lipid bilayer sheet rolled up in a spiral [
8]. The plasmatic membrane from
Salmonella contains phospholipids that can be transformed into cochleates and several other molecules, such as proteins and lipopolysaccharides which can be used as a source of antigenic and immunogenic molecules [
9,
10].
The objective of our research was to study the safety and efficacy of a multivalent, subunit, cochleate-based vaccine against S. Enteritidis, S. Typhimurium and S. Infantis by vaccinating chickens in a controlled environment. Post-vaccination, chickens were separately challenged with virulent strains of each of the Salmonella serotypes. Cecum contamination and immune responses were analyzed.
2. Materials and Methods
2.1. Isolation and Serotyping of Salmonella Spp.
The
Salmonella Enteritidis,
S. Typhimurium and
S. Infantis strains used were obtained through cloacal swabs from chickens belonging to commercial poultry flocks in the Metropolitan, Valparaíso and Libertador General Bernardo O’Higgins regions from Chile. The swabs were placed in sterile tubes containing 5 mL of Phosphate-Buffered Water (PBW) with Novobiocin (20 µg/mL) and incubated at 37 °C for 24 h. Tubes showing suspicious growth were sampled via streak-plate in XLD agar (Xylose lysine deoxycholate agar, Difco
®, Merck, Kenilworth, NJ, USA), then incubated at 37 °C for 24 h. Samples that showed black or translucent colonies isolated via seeding on XLD agar were suspected to be
Salmonella spp. The suspected
Salmonella colonies were subjected to traditional morphological and biochemical testing including Gram staining and the use of triple sugar iron agar slopes and API 20E strips (bioMérieux, Marcy l’Etoile City, France). If two suspected colonies were confirmed as
Salmonella spp., one of them was selected for a Polymerase Chain Reaction (PCR) to confirm the genus via the amplification of the
invA gene [
11]. After biochemical and genotypic confirmation,
Salmonella isolates were sent to the National Reference Laboratory (Institute Public Health, Santiago Chile) for serotype characterization using the Kauffman–White classification scheme [
12].
2.2. Selection of the Strains of Each Salmonella Serotype to Be Used for the Vaccine Formulation
Six strains of each serotype were selected and analyzed for the following virulence genes:
gipA, trhH, spvC, sirA, SEN1417,
pagK, sipA, mgtC and prot6e via quantitative PCR (qPCR). The strains of each serotype that amplified the most of these virulence genes were evaluated for their ability to grow at different times in a broth at pH 5, as indicated by Choi and Groisman [
13] and Retamal et al. [
14]. One strain of each serotype with the highest OD at 24 h of growth at pH 5 (data not shown) was chosen for vaccine formulation.
2.3. Vaccine Preparation
A bacterial sediment was prepared from S. Enteritidis, S. Typhimurium and S. Infantis culture in broth Terrific (Merck®) with glycerol 0.5% v/v in a 15 L bioreactor (New Brunswick bioflo 415, Enfield, CT, USA) with automatically controlled conditions (oxygenation of 40%, pH of 7.0, temperature of 37 °C and agitation of 500 rpm) to obtain the highest yield of biomass. Bacteria were harvested via centrifugation, and the bacterial sediment was washed with sterile Phosphate-Buffered Saline (PBS) and was maintained in a frozen state until the membranes were purified. To purify the bacterial membrane fraction, bacterial sediment was suspended in a buffer containing 30 mmol/L of tris (hydroxymethyl) aminomethane (TRIS) at a ratio of 1:10 volume of bacterial sediment per TRIS. This suspension was continuously sonicated at 400 watts and 24 kHz (Hielscher Ultrasonic Processor UP400S; IU-P02, Germany). The suspension was added to 1.5% (w/v) sodium deoxycholate (DOC) and stirred at 150 rpm at 20 °C for 72 h and centrifuged at 250× g for 5 min and 15 °C. The supernatant was adjusted to a concentration of 5 mg of soluble protein/mL using the bicinchoninic acid method (BCA) (Merck, USA).
To induce calcium–cochleate formation, the bacterial membrane suspension was dripped into a solution containing 0.3%
w/
v Tris, 0.5%
w/
v NaCl and 0.2%
w/
v CaCl2 at pH 10. A wash solution was added to remove the DOC (containing 0.12%
w/
v Tris and 0.08%
w/
v NaCl at pH 10). Finally, the solution was centrifuged at 2900×
g for 40 min at 4 °C, and the sediment with the cochleates was stored at 4 °C [
10].
Every dose of vaccine was composed of 20 µg of each Salmonella serotype cochleate and 350 µL of Montanide™ (Paris, France) ISA 71 VG (ISA 71) as adjuvant and saline solution to a final volume of 500 µL as excipient. The vaccine formulations were made under GMP pilot plant conditions and were tested to assure quality regarding the following parameters: sterility, via microbiological culture; Mycoplasma spp. absence, using a MycoSensor qPCR Assay Kit (Agilent, Santa Clara, CA, USA); quantification of total protein using a BCA Protein Assay Kit (Merck); verification of specific protein production via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE); and pH determination using pH-indicator strips (Invitrogen, Waltham, MA, USA).
2.4. Characterization by Transmission Electron Microscopy (TEM) and SDS–PAGE
To obtain images via TEM, an aliquot of 10 µL of cochleate was deposited on grids (300 Mesh Formvar/Carbon 50/pk, Bussines Electronics SPA, Legnano, Italy) and stained with 1% v/v aqueous uranyl acetate for 1 min. Cochleates were observed using a transmission electron microscope (Philips Tecnai 12 BioTwin, FEI Company, Eindhoven, The Netherlands), operated at 80 kV. The photographs were processed through Megaview G2 Software. Five parts of the cochleates were mixed with one part of 6 × protein electrophoresis loading buffer (6 × Laemmli SDS Sample Buffer, Visual Protein), boiled for 5 min and run on a 15% SDS–PAGE denaturing gel. AccuRuler RGB PLUS Prestained Protein Ladder (AccuRuler RGB PLUS Prestained Protein Ladder) was used as a weight standard. Finally, the gel was stained with staining solution (0.1% p/v Coomassie Blue R-250, 7% v/v acetic acid and 40% v/v methanol).
A 15% SDS–PAGE of the whole bacterial
Salmonella serotypes was transferred to a nitrocellulose membrane and blocked with 5%
w/
v nonfat milk. They were incubated with serum from vaccinated animals or controls (day 35 of the assay), diluted 1:10,000 for 2 h at 37 °C, then incubated with HRP-bound chicken rabbit anti-IgY antibody (AbCam ab97140) 1:5000 for 45 min at 37 °C. Finally, the membranes were developed with 1-Step Ultra TMB-Blotting Solution (Thermo Scientific 37574, Waltham, MA, USA) (
Figure 1 and
Figure S1). In addition, lipopolysaccharide was measured through purpald analysis [
15], while DNA content was measured using a spectrophotometer and agarose gel. These results are included as supplementary materials (
Table S1).
2.5. Evaluation of Vaccine Safety and Adverse Effects
The safety of the trivalent parenteral vaccine was evaluated in 15 chickens on a single-dose schedule and 15 chickens on a triple-dose schedule. These white leghorn females were received at one day of age from a commercial hatchery, reared until six weeks of age, when they were separated into two groups (single-dose and triple-dose) and inoculated once in the breast muscle. The animals were followed for 15 days. Behavioral, physiological and histological parameters were measured to determine vaccine safety. Chickens were observed daily, and any change was scored as presented in
Table 1, which is modified from the Guidelines on the recognition of pain, distress, and discomfort in experimental animals and a hypothesis for assessment [
16].
If scores in a trial group reached 6–9, the observation frequency would be doubled and environmental parameters verified. However, if the score reached 10–12, the experiment would be terminated, the birds would be euthanized, and experimental conditions would be re-evaluated before performing a new assay. At the end of the experiment, all animals were euthanized, and a necropsy was performed for histopathology in different tissues.
2.6. Evaluation of Vaccine Efficacy
Three independent vaccination/challenge experiments were carried out, as summarized in
Table 2. One-day-old
Salmonella-free white Leghorn chickens were received at the Avian Pathology Laboratory (Universidad de Chile) from a commercial hatchery.
Salmonella spp. absence was verified via feces culture. Chickens were reared according to genetic line parameters until six weeks. Three different vaccination and challenge experiments were performed with the three
Salmonella serotypes. Chickens were randomly separated into 2 groups of 14 chickens according to Wilde et al. [
17] and the available space in the community cages inside different isolated rooms. The groups were: vaccinated group (VG) or only adjuvant control group (CG). At 6 weeks, the vaccine or adjuvant was administered into the breast muscle via intramuscular injection. At nine weeks, chickens received a second dose. At ten weeks, the chickens were deprived of food and water overnight (12 h) and the next morning, i.e., one week after the booster vaccination [
18], they were orally infected with 10
9 colony-forming units (CFUs) of a
Salmonella serotype suspended in 0.5 mL of PBS. All animals were challenged, both control and vaccinated groups. For each challenge, an oral gavage needle was used to avoid inoculum losses. All chickens were challenged with each
Salmonella serovar, which were administered one at a time. The experiment finished at 11 weeks, the final samples of cecum and blood were taken, and the chickens were euthanized via cervical dislocation. Blood samples were taken from the brachial vein of each bird prior to vaccination and one week after the challenge. In addition, a sample of litter was taken prior to vaccination and challenge from each floor pen to screen for the presence of environmental
Salmonella. This evaluation of the presence of environmental Salmonella was performed via conventional microbiological culture, based on the standard ISO 6579:2017. Throughout the experiment, the chickens had food according to nutrient requirements and water ad libitum. Only authorized personnel were allowed to manipulate the chickens.
2.7. Antibody ELISA
Determination of serum IgY levels against specific antigen from
Salmonella serotypes were measured using an indirect ELISA on day 0 and days 21 and 35 after first vaccination (
Table 2). Serum was separated from blood samples via centrifugation. Then, 96-well Polysorp plates (Nunc, Thermo Scientific, Waltham, MA, USA) were coated with 2 µg of whole
Salmonella, which were frozen and thawed before use, in each well with 50 µL of coating buffer (150 mM Na
2CO
3, 350 mM NaHCO
3, pH 9.6) overnight at 4 °C. Then, the plates were washed with washing buffer (0.02% Triton × 100
v:
v in PBS) and blocked with 200 µL of blocking buffer (5%
w:
v skim milk in PBS) for 2 h at 37 °C. Subsequently, the plates were incubated with 100 µL of diluted 1:10000 serum in diluent buffer (0.5% skim milk
w:
v, 0.02% Triton × 100
v:
v in PBS) for 2 h at 37 °C. Plates were then washed and incubated with 100 µL of diluted rabbit anti-Chicken IgY peroxidase-conjugated antibodies (1:5000) for 1 h at 37 °C. The plates were washed and 100 µL of TMB substrate solution was added (1-Step Ultra TMB-ELISA, Pierce) for 2.5 min at room temperature, and the reaction was stopped with 100 µL of 2N sulfuric acid. Finally, the absorbance was measured at 450 nm (
Figure 2A).
2.8. Flow Cytometric Phenotyping of Peripheral Lymphocytes
To examine the effective induction of a T lymphocyte response upon vaccination, peripheral blood samples were collected from each animal after they were euthanized (7 days after bacterial challenge,
Table 2). Lymphocytes from peripheral blood were purified by Ficoll-Paque plus (GE Healthcare). Briefly, 2 mL of the whole blood in 2 mL of PBS with 3 mL of Ficoll-Paque were centrifuged at 400
g for 40 min at 18 °C. The lymphocyte layer (whitish phase) was washed in PBS and resuspended in 200 µL of 1 × PBS.
The frequency of helper (CD3+CD4+) and cytotoxic (CD3+CD8+) lymphocytes in vaccinated and control groups were evaluated via flow cytometry. For surface labeling of the different lymphocyte populations, 1 µL of anti-CD3+ (PE), anti-CD4+ (FITC) and anti-CD8+ (PeC5) antibodies were added and incubated for 70 min at 4 °C in the dark. Samples were centrifuged at 500
g for 5 min. The supernatant was discarded and resuspended in 2 mL of Focusing buffer (Invitrogen). Analysis was performed using a Attune NxT Flow Cytometer and for compensation, the AbC™ Total Antibody Compensation Bead Kit (Invitrogen) was used according to the manufacturer’s instructions. The results were expressed as the CD4+/CD8+ cell ratio, which represents the ratio between CD3+CD4+ and CD3+CD8+ lymphocytes. The results are shown
Figure 2B
2.9. Bacterial Counts in the Cecum of Poultry
For the determination of cecum colonization of the
S. Enteritidis,
S. Typhimurium and
S. Infantis strains, 7 days after challenge (35 days after first vaccination,
Table 2), all chickens were euthanized. Cecum was collected under aseptic conditions, weighed and homogenized on stomacher bags prior to dilution in 5 mL of Buffered Peptone Water (BPW). Suspensions were 10-fold diluted up to 10 × 10
−5 and aliquots were plated onto XLD agar as appropriate for bacterial counts. The plates were incubated at 41°C for 18 to 24 h, and the number of
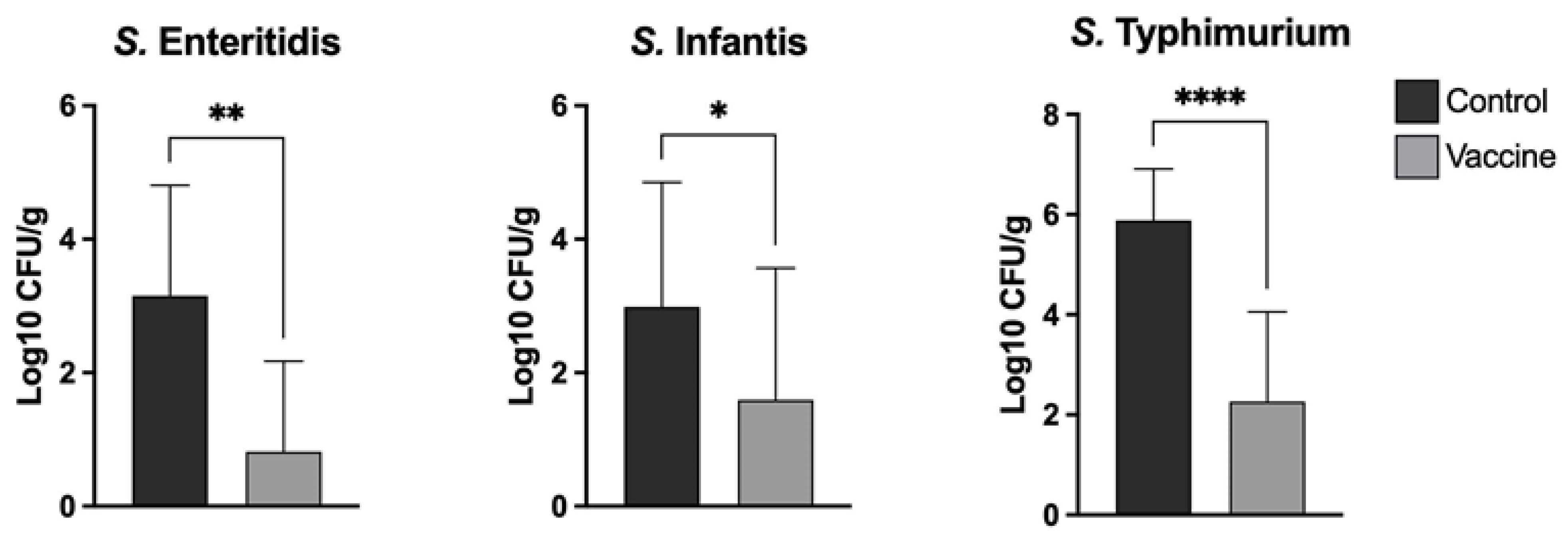
Salmonella obtained via direct culture was counted and recorded. Log10 units of protection were obtained by subtracting the mean Log10 CFU for each experimental group from the mean Log10 CFU of the control group. For samples where no colonies were detected, a value of 1 CFU was used, thus yielding a sample-specific detection limit. The results are shown
Figure 3.
2.10. Statistical Analysis
Statistical analysis was performed using the software GraphPad Prism 8 (Graphpad Software, Inc). Differences in antibodies were analyzed using the Kruskal–Wallis test followed by Dunn’s post-test for multiple comparisons. Differences in bacterial burden and immunophenotyping of peripheral blood lymphocytes were analyzed using an unpaired t-test. p ≤ 0.05 was considered significant, and all experiments were performed at least in triplicate.
4. Discussions
Salmonella contamination in poultry food products is a very important problem for both public health and the industry. The most important
Salmonella serotypes associated with poultry and that cause disease in humans are
S. Enteritidis and
S. Typhimurium and, consequently, most vaccines available for administration in poultry contain one or both serotypes. However, other zoonotic
Salmonella serotypes have emerged in recent years and there are no commercial vaccines against them. The emergence of the
S. Infantis serotype has been described in European countries and the United States [
4,
19,
20]. In South America, S. Infantis has also been described in Ecuador [
21], Perú [
22] and Chile [
23]. In Brazil,
S. Infantis has been catalogued as the second most prevalent serotype in broilers [
24]. The vaccination of breeder-pullets, along with other intervention measures, is an important strategy that is currently being used to reduce the levels of
Salmonella in poultry flocks, which will ultimately lead to lower rates of human
Salmonella infections [
6]. For this reason, it is essential that the available vaccines cover all the
Salmonella serotypes present or most frequently found in poultry farms. This study investigated the efficacy and safety of a new trivalent, subunit, cochleate-based vaccine against
S. Enteritidis,
S. Typhimurium and
S. Infantis to be administered parenterally in laying hens.
Our subunit vaccine design utilizes an enriched extract of solubilized bacterial membranes, which upon exposure to a CaCl2 solution coiled into filamentous cochlear structures of nanometer diameters, stabilized by calcium atoms. The coiled membranes capture and retain a wide range of bacterial antigens, both membrane and cytoplasmic proteins, together with traces of immunostimulatory molecules such as lipopolysaccharide or LPS, bacterial DNA and RNA, etc. These pathogen-associated molecular patterns (PAMP) are conserved structures that bind to specific receptors on immune system cells. Lipopolysaccharide, for example, present in Gram-negative bacteria, stimulates the immune response through the binding and activation of Toll-like receptor 4, together with lipopolysaccharide binding protein (LBP), CD14 and MD-2, on antigen-presenting cells. LPS induces the expression of proinflammatory cytokines such as Il1b and TnFa, co-stimulatory molecules (CD40, CD80 and CD86) and cytokine secretion (IL12, IL 2), which increases antigenic presentation to CD4+ LTs, and consequently, the adaptive immune response. LPS has a detrimental effect as an endotoxin is due to the hyperactivation of TLR4 that occurs when present in high amounts; however, in our formulation, it is trapped in cochleates, which favor a long-term protective response [
25].
Outer-membrane protein vaccines with adjuvants have been used to decrease the shedding of
S. Enteritidis in poultry. It has been reported that immunized 9-week-old chickens with two outer-membrane proteins subcutaneously, followed by two boost immunizations with time intervals of 2 weeks, decreasing cecal colonization about 1000-fold when the animals were infected orally with 8 × 10
8 CFU of a virulent
S. Enteritidis strain [
26,
27]. Additionally, a subunit vaccine has been evaluated against the de-flagellated or SEp9 antigen of
S. Enteritidis, which decreases the bacterial colonization of the chicken intestine [
27,
28]. In another study, Desin et al. [
29] evaluated a subunit vaccine based on the type 1 pathogenicity island (SPI-1 T3SS), which resulted in high antibody titers and lead to a reduction in the levels of
S. Enteritidis in the liver, but not in the spleen and ceca. Li et al. [
18] experimentally evaluated a subunit vaccine against
S. Enteritidis in poultry to provide protection against
Salmonella Enteritidis challenge in chickens, which induced strong immune responses.
Interestingly, our study shows that the electrophoretic profile of the proteins retained in the cochleae for the three
Salmonella serotypes is different, and therefore, we expanded the range of surface antigens present in the formulation (
Figure 1C). The outer-membrane proteins (OMPs) of
Salmonella represent important virulence factors as well as being highly immunogenic [
30]. In contrast to the use of a single antigen, the use of a varied set of antigens for each serotype broadens the spectrum of specific antibodies generated by immunized animals.
The antigenic component of non-live vaccines can be killed whole organisms, purified proteins from the organism, recombinant proteins or polysaccharides [
31]. Our polyvalent vaccine based on cochleates is well characterized and contains outer-membrane proteins (OMP), LPS and bacterial DNA. As it was observed in
Figure 1C, the pattern of proteins present in each cochleate is constant and can be compared with the electrophoretic pattern of the master seeds for each Salmonella serotype from
Figures S1 and S2. The presence of LPS and bacterial DNA was also quantified, as shown in
Table S1. No specific antibodies against any Salmonella serotype were observed in the animals before the vaccination, demonstrating that maternal antibodies are no longer present at 6 weeks of age. Three weeks after the first vaccination, there was already a significant increase in specific antibodies against
Salmonella in all vaccinated animals; this increase in antibodies persisted until day 35 post first vaccination, which was the last day of sampling in our experiment (
Figure 2A). The presence of immunostimulatory molecules in the cochleae, such as LPS, favors the immune response not only with an increase in antibody levels but also in the progression of the T-lymphocyte-dependent immune response; in fact, we could see a functional B- and T-cell response in the vaccinated animals before and after the challenge.
CD4+ T lymphocytes play a major role in protective immunity during primary and secondary
Salmonella infection. CD4+ helper T cells release cytokines such as IL-2 and IFNγ to further stimulate NK cells, macrophages and CD8+ cytotoxic T cells and promote the differentiation of B cells into antibody-producing plasma cells [
32,
33,
34]. IFNγ has been reported to activate macrophages, resulting in the improved clearance of engulfed bacteria. It has been shown that decreased activity of peritoneal macrophage is associated with the increased systemic dissemination of
S. Enteritidis in chickens. In addition,
Salmonella is known to be resistant to clearance by macrophages and to even use macrophages as support for systemic dissemination [
32]. We were able to determine that after a challenge with two of the three
Salmonella serotypes (
S. Enteritidis and
S. Infantis), there was a significant increase in the CD4+/CD8+ ratio in vaccinated chickens (
Figure 2B). In the case of the group challenged with
S. Typhimurium, although the median CD4+/CD8+ ratio was higher in the vaccinated group compared to the control, no significant differences were observed, possibly due to the large dispersion of the data obtained in this group (
Figure 2B). The post hoc statistical power of the analyses was calculated for each serovar against its control; they were 0.972 (
S. Typhimurium), 0.999 (
S. Enteritidis), and 1 (
S. Infantis). Thus, the non-statistical difference observed with
S. Typhimurium is unrelated to the size of the group.
Recently, [
35] described the changes in the innate and adaptive immune system that are generated in birds after infection with
S. Enteritidis in detail. As in the control group of our study, one week after the challenge with
Salmonella, there was an increase in the proportion of CD8+ T lymphocytes and other cells, but this, according to these authors, is not sufficient to prevent the progressive colonization of the cecum and spleen with
Salmonella. However, in our study, the vaccine was able to significantly decrease
Salmonella colonization of the cecum in all challenged vaccinated chickens.
The chickens were challenged separately with a high infecting dose of each
Salmonella serotype present in the vaccine. The bacterial burden was quantified in the cecum seven days post-challenge. The results of our study show that the trivalent vaccine was able to significantly reduce bacterial colonization with each of the serovars tested (
Figure 3). The reduction in the number of UFC in the assays was highest for serovar
S. Typhimurium followed by serovar
S. Enteritidis and serovar
S. Infantis. Interestingly, the levels of spleen and liver invasion of the three
Salmonella serotypes were very low in all the animals sampled, both in the control and vaccinated groups, with the latter showing no detectable bacterial growth in any sample. This may be due to the fact that these
Salmonella serovars are zoonotic and do not produce disease in chickens. This is in agreement with the observations of Berndt et al. [
36], who found
S. Infantis to only have limited capability to invade the lamina propria in very young chickens. Considering the UFC reduction in all vaccinated groups against their respective controls, new power analyses were performed; in all analyses, the power calculated was equal to 1.
In contrast to the currently used live attenuated, commercial vaccines, the developed vaccine possesses antigens of all three Salmonella serotypes, including the currently relevant Infantis serotype. Moreover, unlike inactivated vaccines in which the antigens are denatured by formalin treatment, the antigens captured in the cochleates are preserved together with immunostimulatory molecules that induce a CD4+ LT-mediated response. This, together with a reduced production cost, enhances the novelty of this new type of formulation. In summary, our parenterally administered subunit trivalent vaccine based on cochleates was effective and did not show any adverse effect in the birds in our controlled environment trials. The doses of bacteria delivered in the challenge trials (1 × 109 CFU/mL) were generally higher than the Salmonella infection pressure to which birds are naturally exposed to in commercial farms, and our vaccine was able to significantly decrease the number of Salmonella colonizing the caecum in vaccinated birds compared to control birds.