Toward a Multivalent Synthetic Oligosaccharide-Based Conjugate Vaccine against Shigella: State-of-the-Art for a Monovalent Prototype and Challenges
Abstract
:1. Introduction
2. From Polysaccharide Antigens to SF2a-TT15, a Shigella Synthetic Glycan Conjugate Vaccine Prototype
2.1. Concept: Synthetic Glycans as Surrogates for Shigella O-Ags
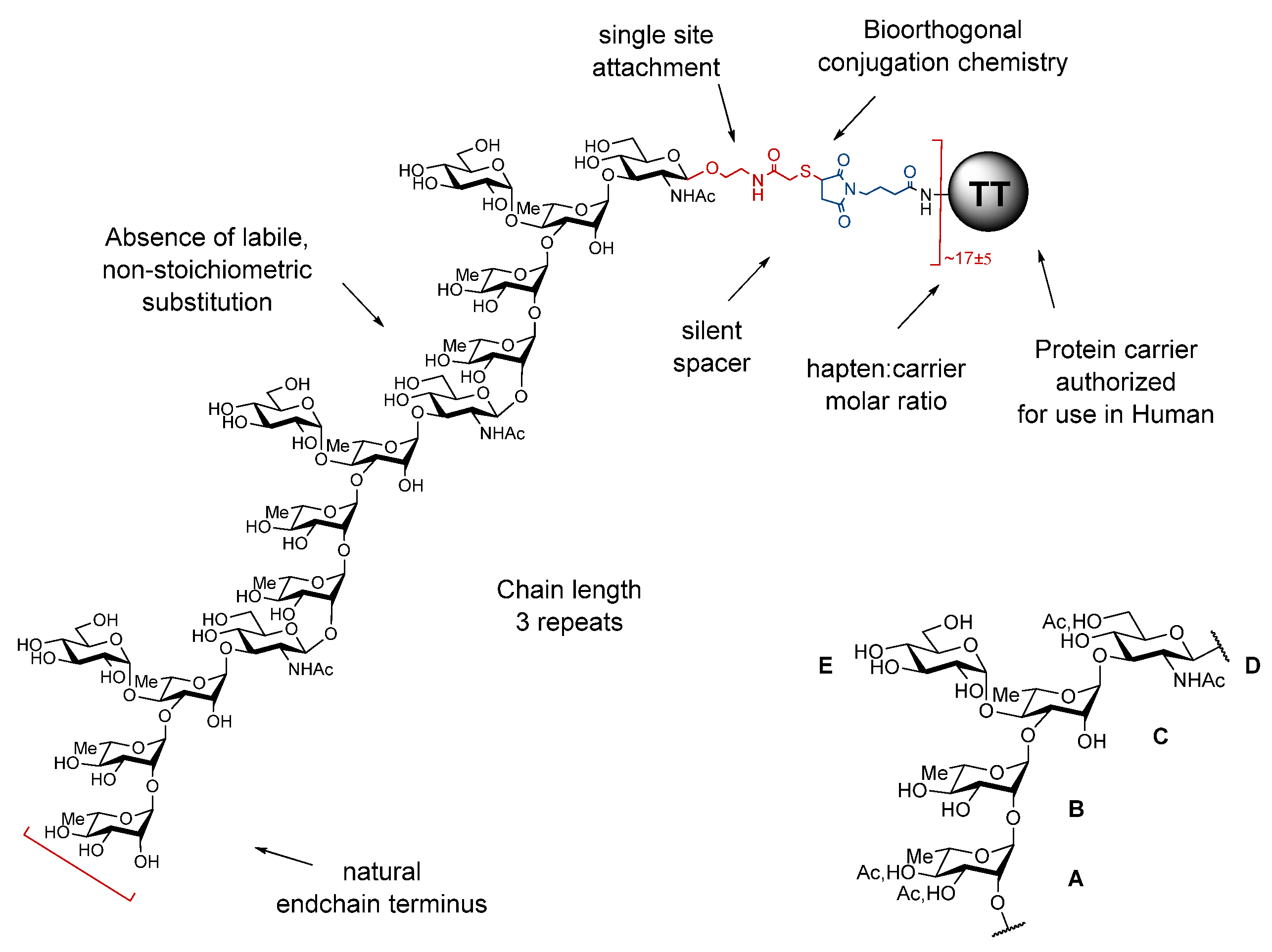
2.2. Design and Properties of SF2a-TT15, a “Sun”-Type Synthetic Oligosaccharide-Based Conjugate
- -
- While the branched B(E)CD segment was identified as a minimal antigenic determinant, the B(E)CD-TT conjugate did not induce any anti-SF2a LPS antibodies in mice despite eliciting high anti-B(E)CD IgG antibody titers. This discrepancy between antigenic mimicry and functional mimicry provided a strong support to a deeper molecular investigation on larger O-Ag segments, also taking into account conformational and structural mimicry.
- -
- Antibody binding increased with chain length to reach a plateau for oligosaccharides larger than B(E)CDAB(E)C, suggesting that antigenic mimicry required oligosaccharides longer than one repeat. This observation was comforted by the determination of the crystal structure of a mIgG in complex with a synthetic 15-mer ([AB(E)CD]3) segment. The antibody binding site accommodates a 9-mer glycotope. Six residues located on two adjacent repeats make direct contact with the antibody, suggesting that a suitable O-Ag surrogate should comprise at least two contiguous repeats to achieve structural O-Ag mimicry.
- -
- NMR data in solution revealed strong signal overlap for internal residues only within the 10-mer ([AB(E)CD]2) and 15-mer ([AB(E)CD]3) segment, suggesting that O-Ag conformational mimicry was best reached for the latter.
- -
- Recognition of [AB(E)CD]3 by sera from naturally infected or vaccinated individuals, was superior to that of [AB(E)CD]2 and paralleled that of the LPS isolated from the SF2a strain used to generate mIgGs.
- -
- Moreover and comforting the above findings, binding data for all five mIgGs revealed that O-acetylation was not a critical feature of SF2a protective epitopes.
- -
- The blockwise synthesis established at the lab scale of the ready-for-conjugation 15-mer oligosaccharide equipped with an aminolinker at its reducing end [8] was not considered more demanding than that of the 10-mer equivalent, suggesting that chemical synthesis was not a limiting factor.
- -
- The immunogenicity of a non-adjuvanted [AB(E)CD]3-TT conjugate far exceeded that of a [AB(E)CD]2-TT conjugate featuring a similar average oligosaccharide:TT molar ratio, while no detrimental anti-linker antibody titers were detected. In addition, conjugates encompassing larger O-Ag synthetic segments or oligosaccharides differing by their endchain residue did not surpass the 15-mer conjugate (Mulard et al., unpublished data).
3. SF2a-TT15: Safety, Tolerability, and Immunogenicity in a First-in-Human Phase I Study
4. SF2a-TT15: Ongoing Phase IIa Clinical Study to Assess Safety, Tolerability and Immunogenicity in the Target Infant Population Living in Endemic Areas
5. Toward a Multivalent Synthetic Glycan-Based Shigella Vaccine Providing Broad Strain Coverage
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robbins, J.B.; Chu, C.; Schneerson, R. Hypothesis for vaccine development: Protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin. Infect. Dis. 1992, 15, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, M.; Berti, F.; Bzducha-Wróbel, A.; Chiodo, F.; Colombo, C.; Compostella, F.; Durlik, K.; Ferhati, X.; Holmdahl, R.; Jovanovic, D.; et al. Recent advances on smart glycoconjugate vaccines in infections and cancer. FEBS J. 2021. [CrossRef] [PubMed]
- Cohen, D.; Meron-Sudai, S.; Bialik, A.; Asato, V.; Goren, S.; Ariel-Cohen, O.; Reizis, A.; Hochberg, A.; Ashkenazi, S. Serum IgG antibodies to Shigella lipopolysaccharide antigens—A correlate of protection against shigellosis. Hum. Vaccin. Immunother. 2019, 15, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, J.B.; Schneerson, R.; Szu, S.C. Perspective: Hypothesis: Serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 1995, 171, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Barel, L.A.; Mulard, L.A. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: From concept to efficacy in human. Hum. Vaccin. Immunother. 2019, 15, 1338–1356. [Google Scholar] [CrossRef] [PubMed]
- Pozsgay, V.; Chu, C.; Pannell, L.; Wolfe, J.; Robbins, J.B.; Schneerson, R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc. Natl. Acad. Sci. USA 1999, 96, 5194–5197. [Google Scholar] [CrossRef] [Green Version]
- Perepelov, A.V.; Shekht, M.E.; Liu, B.; Shevelev, S.D.; Ledov, V.A.; Senchenkova, S.N.; L’vov, V.L.; Shashkov, A.S.; Feng, L.; Aparin, P.G.; et al. Shigella flexneri O-antigens revisited: Final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol. Med. Microbiol. 2012, 66, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Belot, F.; Guerreiro, C.; Baleux, F.; Mulard, L.A. Synthesis of two linear PADRE conjugates bearing a deca- or pentadecasaccharide B epitope as potential synthetic vaccines against Shigella flexneri serotype 2a infection. Chem. Eur. J. 2005, 11, 1625–1635. [Google Scholar] [CrossRef]
- Mulard, L.A. Bacterial polysaccharides as major surface antigens: Interest in O-acetyl substitutions. Carbohydr. Chem. 2018, 43, 71–103. [Google Scholar]
- van der Put, R.M.; Kim, T.H.; Guerreiro, C.; Thouron, F.; Hoogerhout, P.; Sansonetti, P.J.; Westdijk, J.; Stork, M.; Phalipon, A.; Mulard, L.A. A Synthetic Carbohydrate Conjugate Vaccine Candidate against Shigellosis: Improved Bioconjugation and Impact of Alum on Immunogenicity. Bioconjugate Chem. 2016, 27, 883–892. [Google Scholar] [CrossRef]
- van der Put, R.M.F.; Smitsman, C.; de Haan, A.; Hamzink, M.; Timmermans, H.; Uit-tenbogaard, J.; Westdijk, J.; Stork, M.; Ophorst, O.; Thouron, F.; et al. The first-in-human synthetic glycan-based conjugate vaccine candidate against Shigella. ACS Cent. Sci. 2022. [Google Scholar] [CrossRef]
- Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M.L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2021, 21, 546–558. [Google Scholar] [CrossRef]
- Riddle, M.S.; Kaminski, R.W.; Di Paolo, C.; Porter, C.K.; Gutierrez, R.L.; Clarkson, K.A.; Weerts, H.E.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: A Single-Blind, Randomized Phase I Study. Clin. Vaccine Immunol. 2016, 23, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, K.A.; Porter, C.K.; Talaat, K.R.; Frenck, R.W., Jr.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Kaminski, R.W. Shigella-Specific Immune Profiles Induced after Parenteral Immunization or Oral Challenge with Either Shigella flexneri 2a or Shigella sonnei. mSphere 2021, 6, e0012221. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Lindsay, B.; Saha, D.; Sanogo, D.; Das, S.K.; Omore, R.; Farag, T.H.; Nasrin, D.; Li, S.; Panchalingam, S.; Levine, M.M.; et al. Association Between Shigella Infection and Diarrhea Varies Based on Location and Age of Children. Am. J. Trop. Med. Hyg. 2015, 93, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Phalipon, A.; Tanguy, M.; Grandjean, C.; Guerreiro, C.; Belot, F.; Cohen, D.; Sansonetti, P.J.; Mulard, L.A. A Synthetic Carbohydrate-Protein Conjugate Vaccine Candidate against Shigella flexneri 2a Infection. J. Immunol. 2009, 182, 2241–2247. [Google Scholar] [CrossRef] [Green Version]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella Isolates From the Global Enteric Multicenter Study Inform Vaccine Development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef]
- Theillet, F.X.; Simenel, C.; Guerreiro, C.; Phalipon, A.; Mulard, L.A.; Delepierre, M. Effects of backbone substitutions on the conformational behavior of Shigella flexneri O-antigens: Implications for vaccine strategy. Glycobiology 2011, 21, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Kenne, L.; Lindberg, B.; Petersson, K.; Katzenellenbogen, E.; Romanowska, E. Structural studies of the O-specific side-chains of the Shigella sonnei phase I lipopolysaccharide. Carbohydr. Res. 1980, 78, 119–126. [Google Scholar] [CrossRef]
- Pfister, H.B.; Mulard, L.A. Synthesis of the Zwitterionic Repeating Unit of the O-Antigen from Shigella sonnei and Chain Elongation at Both Ends. Org. Lett. 2014, 16, 4892–4895. [Google Scholar] [CrossRef] [PubMed]
- Dhara, D.; Mulard, L.A. Exploratory N-Protecting Group Manipulation for the Total Synthesis of Zwitterionic Shigella sonnei Oligosaccharides. Chem. Eur. J. 2021, 27, 5694–5711. [Google Scholar] [CrossRef] [PubMed]
- Mulard, L.A.; Dhara, D.; Paoletti, J.; Pfister, H.B.; Phalipon, A.; Guerreiro, C. Protected Disaccharides, Their Process of Preparation and Their Use in the Synthesis of Zwitterionic Oligosaccharides and Conjugates Thereof. Filed Patent PCT/EP2021/082559, 22 November 2021. [Google Scholar]
- Mulard, L.A.; Chassagne, P.; Sansonetti, P.J.; Phalipon, A.; Traincard, F.; Nato, F. Glycoconjugates and Their Use as Potential Vaccines against Infection by Shigella flexneri. U.S. Patent 10,376,593, 13 August 2019. [Google Scholar]
- Boutet, J.; Blasco, P.; Guerreiro, C.; Thouron, F.; Dartevelle, S.; Nato, F.; Canada, F.J.; Arda, A.; Phalipon, A.; Jimenez-Barbero, J.; et al. Detailed Investigation of the Immunodominant Role of O-Antigen Stoichiometric O-Acetylation as Revealed by Chemical Synthesis, Immunochemistry, Solution Conformation and STD-NMR Spectroscopy for Shigella flexneri 3a. Chem. Eur. J. 2016, 22, 10892–10911. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; White, A.F.B.; Mulard, L.A. Efficient Iterative Synthesis of O-Acetylated Tri- to Pentadecasaccharides Related to the Lipopolysaccharide of Shigella flexneri Type 3a through Di- and Trisaccharide Glycosyl Donors. Chem. Asian J. 2017, 12, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Cornil, J.; Hu, Z.Y.; Bouchet, M.; Mulard, L.A. Multigram synthesis of an orthogonally-protected pentasaccharide for use as a glycan precursor in a Shigella flexneri 3a conjugate vaccine: Application to a ready-for-conjugation decasaccharide. Org. Chem. Front. 2021, 8, 6279–6299. [Google Scholar] [CrossRef]
- Hu, Z.; Cornil, J.; Ligeour, C.; Thouron, F.; Hoos, S.; Guerreiro, C.; Phalipon, A.; Mulard, L.A. Toward a bivalent synthetic carbohydrate-based vaccine candidate against shigellosis. Abstr. Pap. Am. Chem. Soc. 2017, 254, 1. [Google Scholar]
- Hargreaves, J.M.; Le Guen, Y.; Guerreiro, C.; Descroix, K.; Mulard, L.A. Linear synthesis of the branched pentasaccharide repeats of O-antigens from Shigella flexneri 1a and 1b demonstrating the major steric hindrance associated with type-specific glucosylation. Org. Biomol. Chem. 2014, 12, 7728–7749. [Google Scholar] [CrossRef] [Green Version]
- Seeberger, P.H. Discovery of Semi- and Fully-Synthetic Carbohydrate Vaccines Against Bacterial Infections Using a Medicinal Chemistry Approach. Chem. Rev. 2021, 121, 3598–3626. [Google Scholar] [CrossRef]
- Verez-Bencomo, V.; Fernandez-Santana, V.; Hardy, E.; Toledo, M.E.; Rodriguez, M.C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 2004, 305, 522–525. [Google Scholar] [CrossRef]
- Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M.R.; Adamo, R. Glycoconjugate vaccines: Current approaches towards faster vaccine design. Expert Rev. Vaccines 2019, 18, 881–895. [Google Scholar] [CrossRef]
- Salamone, S.; Guerreiro, C.; Cambon, E.; Andre, I.; Remaud-Simeon, M.; Mulard, L.A. Programmed chemo-enzymatic synthesis of the oligosaccharide component of a carbohydrate-based antibacterial vaccine candidate. Chem. Commun. 2015, 51, 2581–2584. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phalipon, A.; Mulard, L.A. Toward a Multivalent Synthetic Oligosaccharide-Based Conjugate Vaccine against Shigella: State-of-the-Art for a Monovalent Prototype and Challenges. Vaccines 2022, 10, 403. https://doi.org/10.3390/vaccines10030403
Phalipon A, Mulard LA. Toward a Multivalent Synthetic Oligosaccharide-Based Conjugate Vaccine against Shigella: State-of-the-Art for a Monovalent Prototype and Challenges. Vaccines. 2022; 10(3):403. https://doi.org/10.3390/vaccines10030403
Chicago/Turabian StylePhalipon, Armelle, and Laurence A. Mulard. 2022. "Toward a Multivalent Synthetic Oligosaccharide-Based Conjugate Vaccine against Shigella: State-of-the-Art for a Monovalent Prototype and Challenges" Vaccines 10, no. 3: 403. https://doi.org/10.3390/vaccines10030403




