Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Vaccine Construction
2.2. Cell Culture
2.3. Expression of Viral Proteins and Cytokines IL-7 and IL-15
2.3.1. Cell Transfection
2.3.2. E Enzyme-Linked Immunosorbent Assay: ELISA
2.3.3. Functional Analysis of Cytokines
2.4. Evaluation of Immunogenicity in Animal Models
2.4.1. Ethical Statements
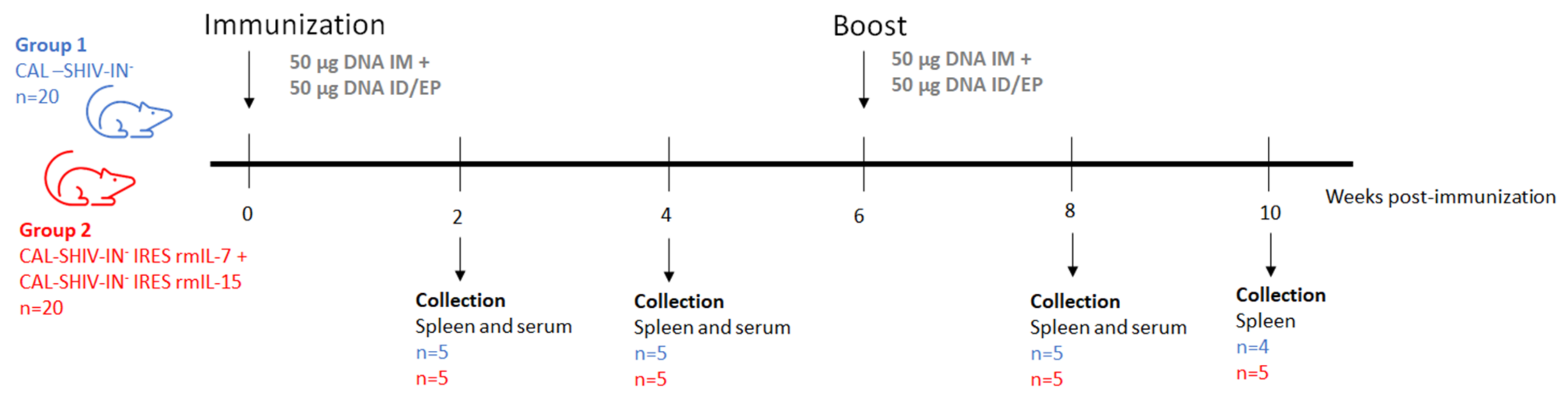
2.4.2. Mice
2.4.3. Macaques
2.4.4. Detection of IFN-γ Producing Cells by ELISpot Assay
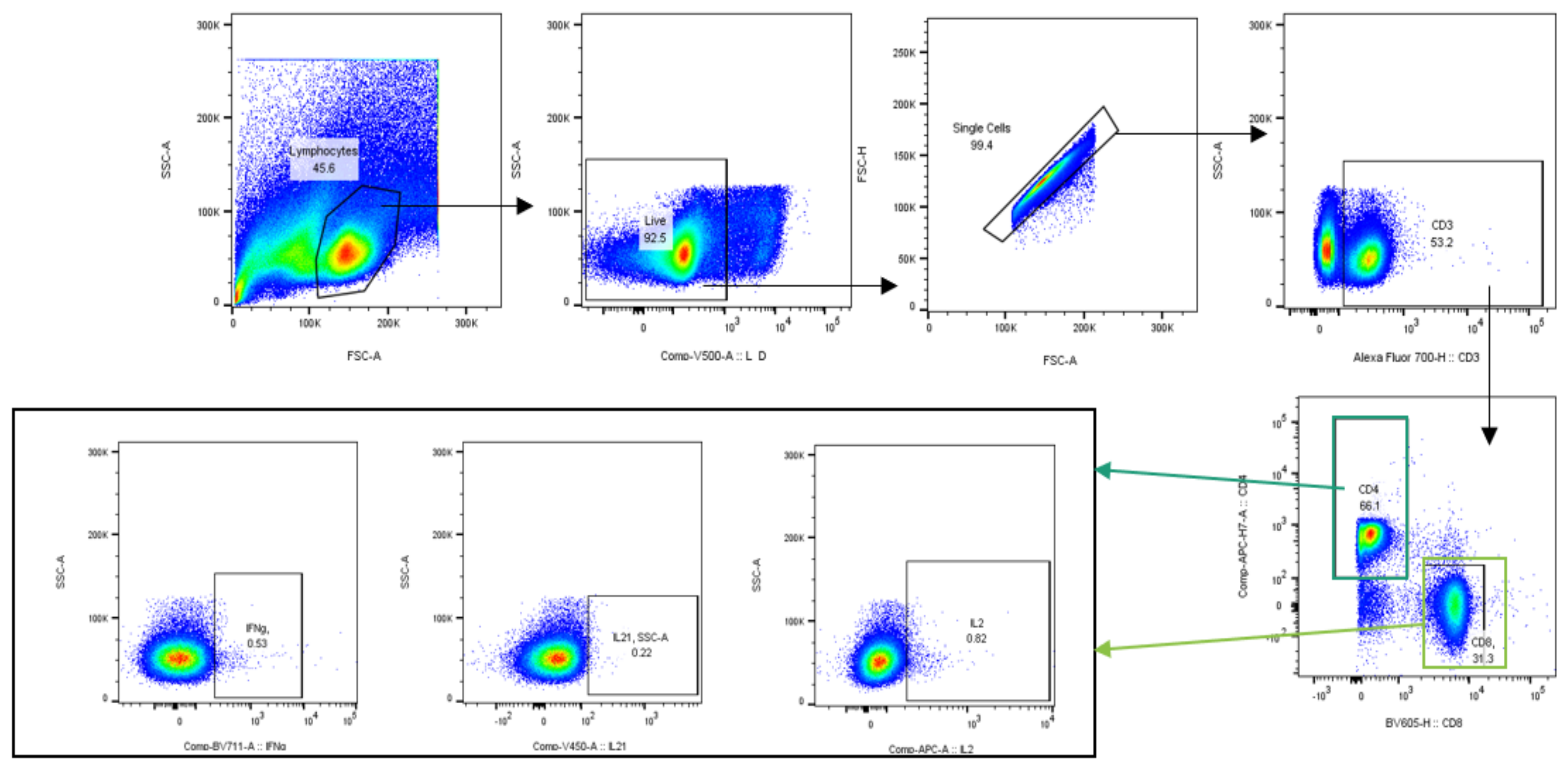
2.4.5. Multiparametric Flow Cytometry Assay
2.4.6. Detection of Total and HIV-Specific Antibodies
2.4.7. Neutralization Assay
2.4.8. Antibody-Dependent Cellular Cytotoxicity (ADCC) Assay
2.4.9. Statistical Analysis
3. Results
3.1. CAL-SHIV-IN− IRES-Cytokine Prototypes Produce Functional Viral Proteins
3.2. IL-7 and IL-15 Cytokines Inserted into CAL-SHIV-IN− Genome Are Expressed, Secreted and Functional
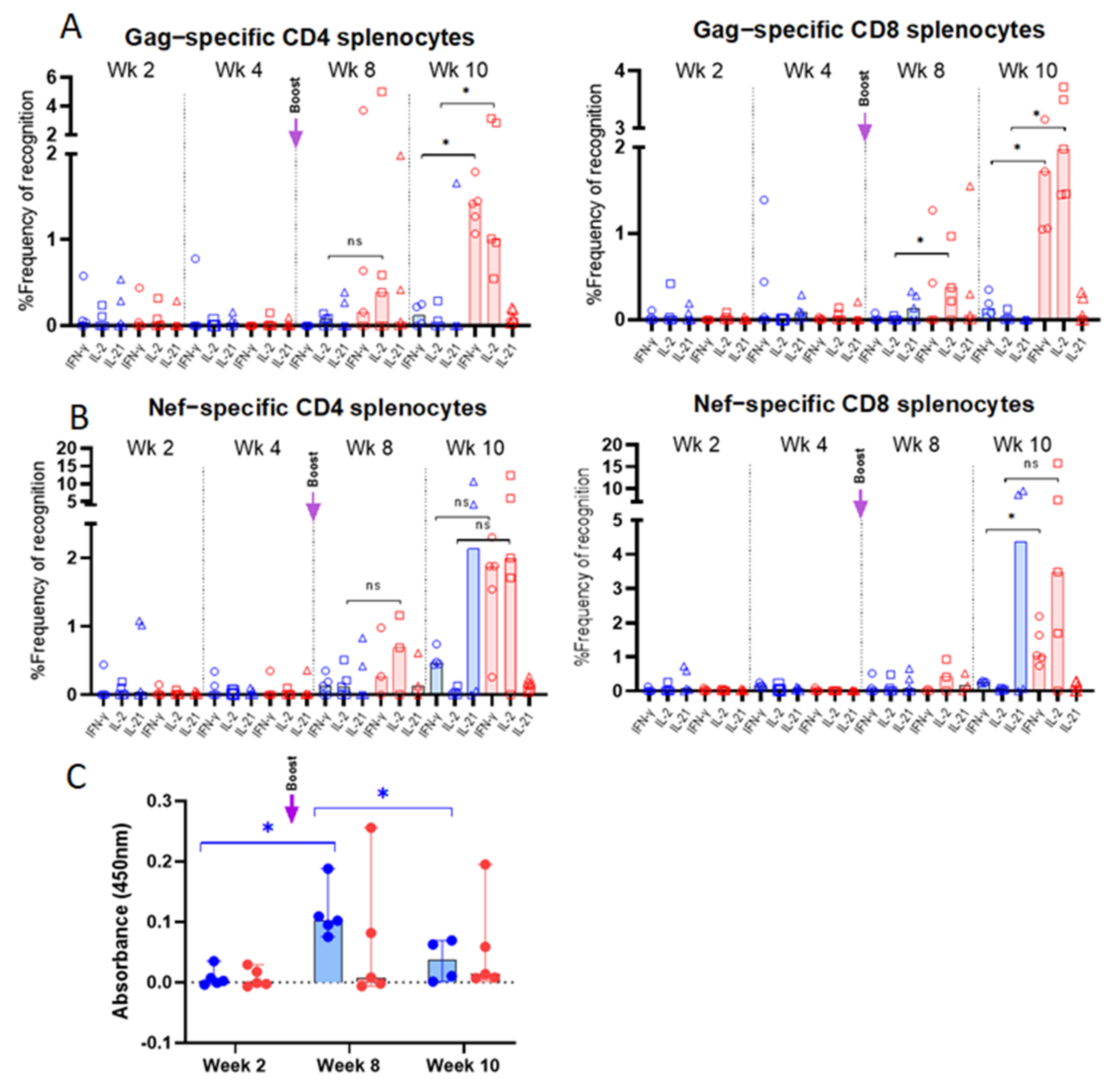
3.3. Vaccine-Specific T Cell Responses in Vaccinated Mice
3.4. Env-Specific Antibody Responses in Vaccinated Mice
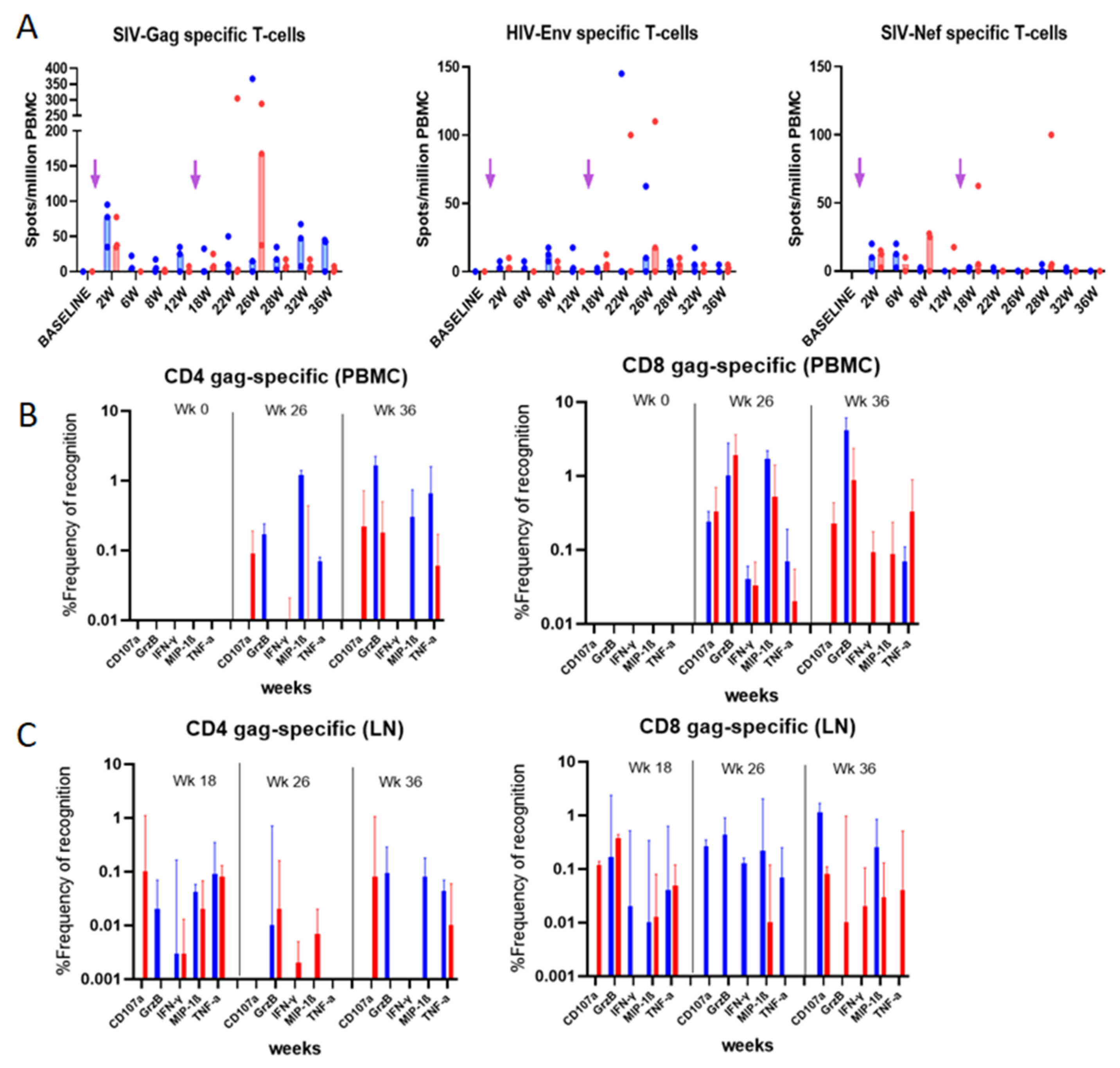
3.5. Evaluation of Vaccine-Specific T Cell Responses in Immunized Rhesus macaques
3.5.1. IFN-γ ELISpot Responses
3.5.2. Intracellular Cytokine Analysis
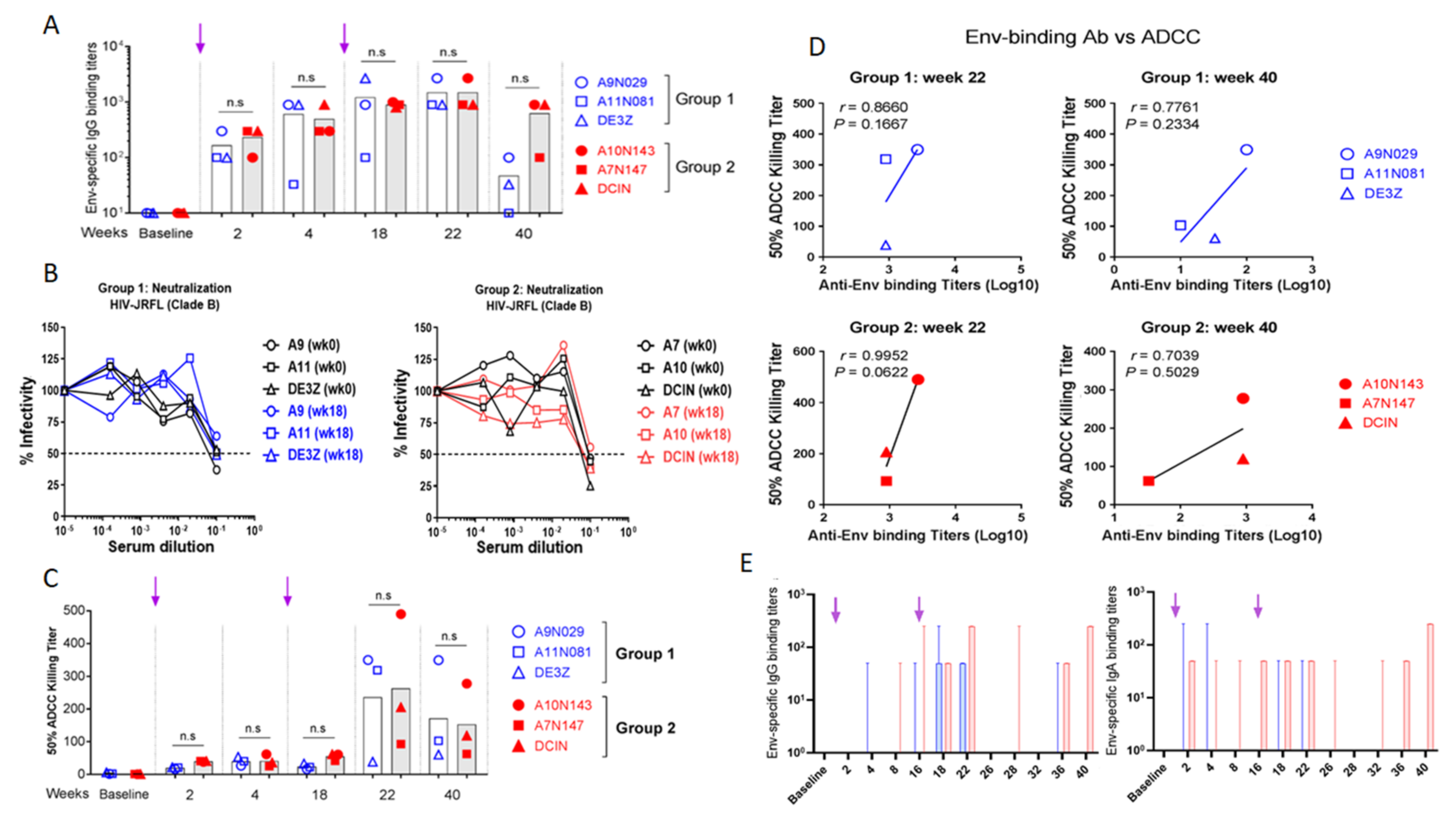
3.6. Evaluation of Vaccine-Specific Antibody Responses in Rhesus Macaques
3.6.1. Evaluation of Env-Specific Abs in Macaque Serum Samples
3.6.2. Evaluation of Env-Specific IgG and IgA in Rectal Secretions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Animal ID | Mamu-A*01 Allele | Mamu-B*08 Allele | Mamu-B*17 Allele | Body Weight (kg) | Age (yr) at Time of Study | Date of Birth | Gender | Group Assigned |
|---|---|---|---|---|---|---|---|---|
| A9N029 | + | - | - | 11.2 | 9.66 | 25 May 2009 | Male | 1 |
| A11N081 | - | - | - | 17.7 | 7.67 | 24 May 2011 | Male | 1 |
| DE3Z | - | - | - | 11.6 | 10.06 | 1 January 2009 | Male | 1 |
| A7N147 | - | - | - | 12.1 | 11.23 | 2 November 2007 | Male | 2 |
| A10N143 | + | - | - | 10.4 | 8.11 | 15 December 2010 | Male | 2 |
| DCIN | - | - | - | 15.2 | 11.06 | 1 January 2008 | Male | 2 |


References
- Whyte-Allman, S.-K.; Bendayan, R. HIV-1 Sanctuary Sites—The Role of Membrane-Associated Drug Transporters and Drug Metabolic Enzymes. AAPS J. 2020, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.; Colomer-Lluch, M.; Serra-Moreno, R. Barriers for HIV Cure: The Latent Reservoir. AIDS Res. Hum. Retrovir. 2018, 34, 739–759. [Google Scholar] [CrossRef] [PubMed]
- Excler, J.-L.; Kim, J.H. Novel prime-boost vaccine strategies against HIV-1. Expert Rev. Vaccines 2019, 18, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Sekaly, R.-P. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J. Exp. Med. 2008, 205, 7–12. [Google Scholar] [CrossRef]
- McCarthy, M. AIDS vaccine fails in Thai trial. Lancet 2003, 362, 1728. [Google Scholar] [CrossRef]
- Almond, N.; Kent, K.; Stott, E.; Cranage, M.; Rud, E.; Clarke, B. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet 1995, 345, 1342–1344. [Google Scholar] [CrossRef]
- Baba, T.W.; Jeong, Y.S.; Pennick, D.; Bronson, R.; Greene, M.F.; Ruprecht, R.M. Pathogenicity of Live, Attenuated SIV after Mucosal Infection of Neonatal Macaques. Science 1995, 267, 1820–1825. [Google Scholar] [CrossRef]
- Learmont, J.C.; Geczy, A.F.; Mills, J.; Ashton, L.J.; Raynes-Greenow, C.H.; Garsia, R.J.; Dyer, W.B.; McIntyre, L.; Oelrichs, R.B.; Rhodes, D.I.; et al. Immunologic and Virologic Status after 14 to 18 Years of Infection with an Attenuated Strain of HIV-1—A Report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 1999, 340, 1715–1722. [Google Scholar] [CrossRef]
- Zaunders, J.; Dyer, W.B.; Churchill, M. The Sydney Blood Bank Cohort: Implications for viral fitness as a cause of elite control. Curr. Opin. HIV AIDS 2011, 6, 151–156. [Google Scholar] [CrossRef]
- Adnan, S.; Colantonio, A.D.; Yu, Y.; Gillis, J.; Wong, F.E.; Becker, E.A.; Piatak, M., Jr.; Reeves, R.K.; Lifson, J.D.; O’Connor, S.L.; et al. CD8 T cell response maturation defined by anentropic specificity and repertoire depth correlates with SIVΔnef-induced protection. PLoS Pathog. 2015, 11, e1004633. [Google Scholar] [CrossRef]
- Billingsley, J.M.; Rajakumar, P.A.; Connole, M.A.; Salisch, N.C.; Adnan, S.; Kuzmichev, Y.V.; Hong, H.S.; Reeves, R.K.; Kang, H.J.; Li, W.; et al. Characterization of CD8+ T cell differentiation following SIVΔnef vaccination by transcription factor expression profiling. PLoS Pathog. 2015, 11, e1004740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barouch, D.H. Novel adenovirus vector-based vaccines for HIV-1. Curr. Opin. HIV AIDS 2010, 5, 386–390. [Google Scholar] [CrossRef]
- Gómez, C.E.; Nájera, J.L.; Perdiguero, B.; García-Arriaza, J.; Sorzano, C.O.S.; Jiménez, V.; González-Sanz, R.; Jiménez, J.L.; Munoz-Fernández, M.A.; López Bernaldo de Quirós, J.C.; et al. The HIV/AIDS vaccine candidate MVA-B administered as a single immunogen in humans triggers robust, polyfunctional, and selective effector memory T cell responses to HIV-1 antigens. J. Virol. 2011, 85, 11468–11478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad-Fernandez, M.; Goonetilleke, N. Human cytomegalovirus-vectored vaccines against HIV. Curr. Opin. HIV AIDS 2019, 14, 137–142. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.; Ewing, D.; Blevins, M.; Sun, P.; Sundaram, A.K.; Raviprakash, K.S.; Porter, K.R.; Sanders, J.W. Enhanced immunogenicity and protective efficacy of a tetravalent dengue DNA vaccine using electroporation and intradermal delivery. Vaccine 2019, 37, 4444–4453. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [Green Version]
- Collins, D.R.; Gaiha, G.D.; Walker, B.D. CD8+ T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 2020, 20, 471–482. [Google Scholar] [CrossRef]
- McBrien, J.; Kumar, N.A.; Silvestri, G. Mechanisms of CD8+T cell-mediated suppression of HIV/SIV replication. Eur. J. Immunol. 2018, 48, 898–914. [Google Scholar] [CrossRef] [Green Version]
- Diehl, M.C.; Lee, J.C.; Daniels, S.E.; Tebas, P.; Khan, A.S.; Giffear, M.; Sardesai, N.Y.; Bagarazzi, M.L. Tolerability of intramuscular and intradermal delivery by CELLECTRA((R)) adaptive constant current electroporation device in healthy volunteers. Hum. Vaccines Immunother 2013, 9, 2246–2252. [Google Scholar] [CrossRef] [Green Version]
- Adam, L.; Tchitchek, N.; Todorova, B.; Rosenbaum, P.; Joly, C.; Poux, C.; Chapon, C.; Spetz, A.L.; Ustav, M.; Le Grand, R.; et al. Innate Molecular and Cellular Signature in the Skin Preceding Long-Lasting T Cell Responses after Electroporated DNA Vaccination. J. Immunol. 2020, 204, 3375–3388. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Dean, D.A. Electroporation-Mediated Gene Delivery. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar] [PubMed]
- Arrode-Bruses, G.; Moussa, M.; Baccard-Longere, M.; Villinger, F.; Chebloune, Y. Long-term central and effector SHIV-specific memory T cell responses elicited after a single immunization with a novel lentivector DNA vaccine. PLoS ONE 2014, 9, e110883. [Google Scholar] [CrossRef] [PubMed]
- Chebloune, Y.; Moussa, M.; Arrode-Brusés, G.; Ronfort, C.; Bose, D.; Gagnon, J.; Gumber, S.; Villinger, T.; Byrareddy, S.N.; Kozlowski, P.A.; et al. A single lentivector DNA based immunization contains a late heterologous SIVmac251 mucosal challenge infection. Vaccine 2020, 38, 3729–3739. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; Lin, J.X.; O’Shea, J.J. The gammac Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugli, E.; Brummelman, J.; Pilipow, K.; Roychoudhuri, R. Paths to expansion: Differential requirements of IRF4 in CD8(+) T-cell expansion driven by antigen and homeostatic cytokines. Eur. J. Immunol. 2018, 48, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Cieri, N.; Camisa, B.; Cocchiarella, F.; Forcato, M.; Oliveira, G.; Provasi, E.; Bondanza, A.; Bordignon, C.; Peccatori, J.; Ciceri, F.; et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013, 121, 573–584. [Google Scholar] [CrossRef]
- Logerot, S.; Figueiredo-Morgado, S.; Charmeteau-De-Muylder, B.; Sandouk, A.; Drillet-Dangeard, A.-S.; Bomsel, M.; Bourgault-Villada, I.; Couëdel-Courteille, A.; Cheynier, R.; Rancez, M. IL-7-Adjuvanted Vaginal Vaccine Elicits Strong Mucosal Immune Responses in Non-Human Primates. Front. Immunol. 2021, 12, 614115. [Google Scholar] [CrossRef]
- Calarota, S.A.; Dai, A.; Trocio, J.N.; Weiner, D.B.; Lori, F.; Lisziewicz, J. IL-15 as memory T-cell adjuvant for topical HIV-1 DermaVir vaccine. Vaccine 2008, 26, 5188–5195. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Gowthaman, U.; Jain, S.; Parihar, P.; Banskar, S.; Gupta, P.; Gupta, U.D.; Agrewala, J.N. Coadministration of Interleukins 7 and 15 with Bacille Calmette-Guérin Mounts Enduring T Cell Memory Response against Mycobacterium tuberculosis. J. Infect. Dis. 2010, 202, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Z.Y.; Petersen, E.; Liu, W.G.; Zhu, X.Q. Co-administration of interleukins 7 and 15 with DNA vaccine improves protective immunity against Toxoplasma gondii. Exp. Parasitol. 2016, 162, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Im, S.J.; Araki, K.; Ahmed, R. Cytokine-Mediated Regulation of CD8 T-Cell Responses During Acute and Chronic Viral Infection. Cold Spring Harb. Perspect. Biol. 2017, 11, a028464. [Google Scholar] [CrossRef] [PubMed]
- Arrode, G.; Hegde, R.; Mani, A.; Jin, Y.; Chebloune, Y.; Narayan, O. Phenotypic and Functional Analysis of Immune CD8+ T Cell Responses Induced by a Single Injection of a HIV DNA Vaccine in Mice. J. Immunol. 2007, 178, 2318–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzotti-Kelsoe, M.; Bailer, R.T.; Turk, E.; Lin, C.-L.; Bilska, M.; Greene, K.M.; Gao, H.; Todd, C.A.; Ozaki, D.A.; Seaman, M.S.; et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Alpert, M.D.; Heyer, L.N.; Williams, D.E.J.; Harvey, J.D.; Greenough, T.; Allhorn, M.; Evans, D.T. A Novel Assay for Antibody-Dependent Cell-Mediated Cytotoxicity against HIV-1- or SIV-Infected Cells Reveals Incomplete Overlap with Antibodies Measured by Neutralization and Binding Assays. J. Virol. 2012, 86, 12039–12052. [Google Scholar] [CrossRef] [Green Version]
- Forthal, D.N.; Finzi, A. Antibody-dependent cellular cytotoxicity in HIV infection. AIDS 2018, 32, 2439–2451. [Google Scholar] [CrossRef]
- Sereti, I.; Dunham, R.M.; Spritzler, J.; Aga, E.; Proschan, M.A.; Medvik, K.; Battaglia, C.A.; Landay, A.L.; Pahwa, S.; Fischl, M.A.; et al. IL-7 administration drives T cell–cycle entry and expansion in HIV-1 infection. Blood 2009, 113, 6304–6314. [Google Scholar] [CrossRef] [Green Version]
- Younes, S.-A.; Freeman, M.L.; Mudd, J.C.; Shive, C.L.; Reynaldi, A.; Panigrahi, S.; Estes, J.D.; Deleage, C.; Lucero, C.; Anderson, J.; et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J. Clin. Investig. 2016, 126, 2745–2756. [Google Scholar] [CrossRef]
- Cockerham, L.R.; Siliciano, J.D.; Sinclair, E.; O’Doherty, U.; Palmer, S.; Yukl, S.A.; Strain, M.C.; Chomont, N.; Hecht, F.; Siliciano, R.F.; et al. CD4+ and CD8+ T Cell Activation Are Associated with HIV DNA in Resting CD4+ T Cells. PLoS ONE 2014, 9, e110731. [Google Scholar] [CrossRef] [Green Version]
- Coppola, C.; Hopkins, B.; Huhn, S.; Du, Z.; Huang, Z.; Kelly, W.J. Investigation of the Impact from IL-2, IL-7, and IL-15 on the Growth and Signaling of Activated CD4+ T Cells. Int. J. Mol. Sci. 2020, 21, 7814. [Google Scholar] [CrossRef]
- Leone, A.; Picker, L.J.; Sodora, D.L. IL-2, IL-7 and IL-15 as immuno-modulators during SIV/HIV vaccination and treatment. Curr. HIV Res. 2009, 7, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Picker, L.J.; Reed-Inderbitzin, E.F.; Hagen, S.I.; Edgar, J.B.; Hansen, S.G.; Legasse, A.; Planer, S.; Piatak, M.; Lifson, J.D.; Maino, V.C.; et al. IL-15 induces CD4+ effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Investig. 2006, 116, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Valentin, A.; von Gegerfelt, A.; Rosati, M.; Miteloudis, G.; Alicea, C.; Bergamaschi, C.; Jalah, R.; Patel, V.; Khan, A.S.; Draghia-Akli, R.; et al. Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit. Vaccine 2010, 28, 1962–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristillo, A.D.; Lisziewicz, J.; He, L.; Lori, F.; Galmin, L.; Trocio, J.N.; Unangst, T.; Whitman, L.; Hudacik, L.; Bakare, N.; et al. HIV-1 prophylactic vaccine comprised of topical DermaVir prime and protein boost elicits cellular immune responses and controls pathogenic R5 SHIV162P3. Virology 2007, 366, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Valentin, A.; Ng, S.; Beach, R.K.; Alicea, C.; Bergamaschi, C.; Felber, B.K.; Pavlakis, G.N. Differential effects of IL-15 on the generation, maintenance and cytotoxic potential of adaptive cellular responses induced by DNA vaccination. Vaccine 2015, 33, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Fujino, M.; Saito, Y.; Ahmed, N.; Sato, H.; Sugimoto, C.; Okamura, T.; Hanaki, K.; Nakayama, E.E.; Shioda, T.; et al. Protective Immune Responses Elicited by Deglycosylated Live-Attenuated Simian Immunodeficiency Virus Vaccine Are Associated with IL-15 Effector Functions. J. Immunol. 2020, 205, 1331–1344. [Google Scholar] [CrossRef]
- McGettigan, J.P.; Sarma, M.; Orenstein, J.M.; Porerantz, R.J.; Schnell, M.J. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J. Virol. 2001, 75, 8724–8732. [Google Scholar] [CrossRef] [Green Version]
- Freel, S.A.; Lamoreaux, L.; Chattopadhyay, P.K.; Saunders, K.; Zarkowsky, D.; Overman, R.G.; Ochsenbauer, C.; Edmonds, T.G.; Kappes, J.C.; Cunningham, C.K.; et al. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J. Virol. 2010, 84, 4998–5006. [Google Scholar] [CrossRef] [Green Version]
- Hidajat, R.; Xiao, P.; Zhou, Q.; Venzon, D.; Summers, L.E.; Kalyanaraman, V.S.; Montefiori, D.C.; Robert-Guroff, M. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J. Virol. 2009, 83, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Zhao, J.; Patterson, L.J.; Brocca-Cofano, E.; Venzon, D.; Kozlowski, P.A.; Hidajat, R.; Demberg, T.; Robert-Guroff, M. Multiple Vaccine-Elicited Nonneutralizing Antienvelope Antibody Activities Contribute to Protective Efficacy by Reducing both Acute and Chronic Viremia following Simian/Human Immunodeficiency Virus SHIV 89.6P Challenge in Rhesus Macaques. J. Virol. 2010, 84, 7161–7173. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Excler, J.L.; Michael, N.L. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu. Rev. Med. 2015, 66, 423–437. [Google Scholar] [CrossRef] [Green Version]
- Mabuka, J.; Nduati, R.; Odem-Davis, K.; Peterson, D.; Overbaugh, J. HIV-Specific Antibodies Capable of ADCC Are Common in Breastmilk and Are Associated with Reduced Risk of Transmission in Women with High Viral Loads. PLOS Pathog. 2012, 8, e1002739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruprecht, R.M.; Marasini, B.; Thippeshappa, R. Mucosal Antibodies: Defending Epithelial Barriers against HIV-1 Invasion. Vaccines 2019, 7, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliyari, Z.; Alami, F.; Mostafavi, T.; Nasrabadi, H.T.; Soleimanirad, J.; Charoudeh, H.N. The Roles of IL-2, IL-7, and IL15 Ligands in B Cells Development from Cord Blood Mononuclear Cells. Iran. J. Pediatr. Hematol. Oncol. 2015, 5, 155–160. [Google Scholar]
- Hegde, R.; Liu, Z.; Mackay, G.; Smith, M.; Chebloune, Y.; Narayan, O.; Singh, D.K. Antigen Expression Kinetics and Immune Responses of Mice Immunized with Noninfectious Simian-Human Immunodeficiency Virus DNA. J. Virol. 2005, 79, 14688–14697. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Extracellular Markers | Fluorochrome | Clone | Company |
|---|---|---|---|
| CD3 | Alexa Fluor 700 | 17a2 | BD Pharmingen |
| CD4 | APC-H7 | GK1.5 | BD Pharmingen |
| CD8a | Brilliant Violet 605 | 53–6.7 | BD Horizon |
| CD127 | PE-Cy7 | Sb/199 | BD Pharmingen |
| CD62L | PE-CF594 | Mel-14 | BD Horizon |
| Intracellular Markers | Fluorochrome | Clone | Company |
| IFN-γ | Brilliant Violet 711 | Xmg1.2 | BD Horizon |
| IL-21 | eFluor450 | Ffa21 | eBioscience |
| IL-2 | APC | Jes6-5H4 | eBioscience |
| Extracellular Markers | Fluorochrome | Clone | Company |
|---|---|---|---|
| CD3 | Alexa Fluor 700 | SP34-2 | BD Pharmingen |
| CD4 | FITC | L200 | BD Pharmingen |
| CD8 | APC-Cy7 | SK1 | BD Pharmingen |
| CD28 | ECD | n/a | IO Test |
| CD95 | PerCp-Cy5.5 | DX2 | BD Pharmingen |
| CD45 | APC | D058-1283 | BD Horizon |
| CD107a | BV650 | H4A3 | Biolegend |
| Intracellular Markers | Fluorochrome | Clone | Company |
| IFN-γ | PE-Cy7 | B27 | BD Pharmingen |
| TNF-α | BV785 | Mab11 | Biolegend |
| MIP-1β | V450 | D21-1351 | BD Horizon |
| Granzyme B | PE | GB11 | BD Pharmingen |
| Brilliant buffer plus | BD horizon | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroy, L.-A.; Mac Donald, A.; Kandlur, A.; Bose, D.; Xiao, P.; Gagnon, J.; Villinger, F.; Chebloune, Y. Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models. Vaccines 2022, 10, 461. https://doi.org/10.3390/vaccines10030461
Leroy L-A, Mac Donald A, Kandlur A, Bose D, Xiao P, Gagnon J, Villinger F, Chebloune Y. Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models. Vaccines. 2022; 10(3):461. https://doi.org/10.3390/vaccines10030461
Chicago/Turabian StyleLeroy, Laury-Anne, Alice Mac Donald, Aditi Kandlur, Deepanwita Bose, Peng Xiao, Jean Gagnon, François Villinger, and Yahia Chebloune. 2022. "Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models" Vaccines 10, no. 3: 461. https://doi.org/10.3390/vaccines10030461
APA StyleLeroy, L.-A., Mac Donald, A., Kandlur, A., Bose, D., Xiao, P., Gagnon, J., Villinger, F., & Chebloune, Y. (2022). Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models. Vaccines, 10(3), 461. https://doi.org/10.3390/vaccines10030461








