Efficacy and Immunogenicity of Recombinant Pichinde Virus-Vectored Turkey Arthritis Reovirus Subunit Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Preparation
2.2. Virus
2.3. Experimental Design
2.4. ELISA and Serum Neutralization Test (SNT)
2.5. Processing of Tissue Samples
2.6. Viral Nucleic Acid Extraction
2.7. Virus Gene Copy Number
2.8. Histopathology
2.9. Statistical Analysis
3. Results
3.1. Clinical Disease and Gross Lesions
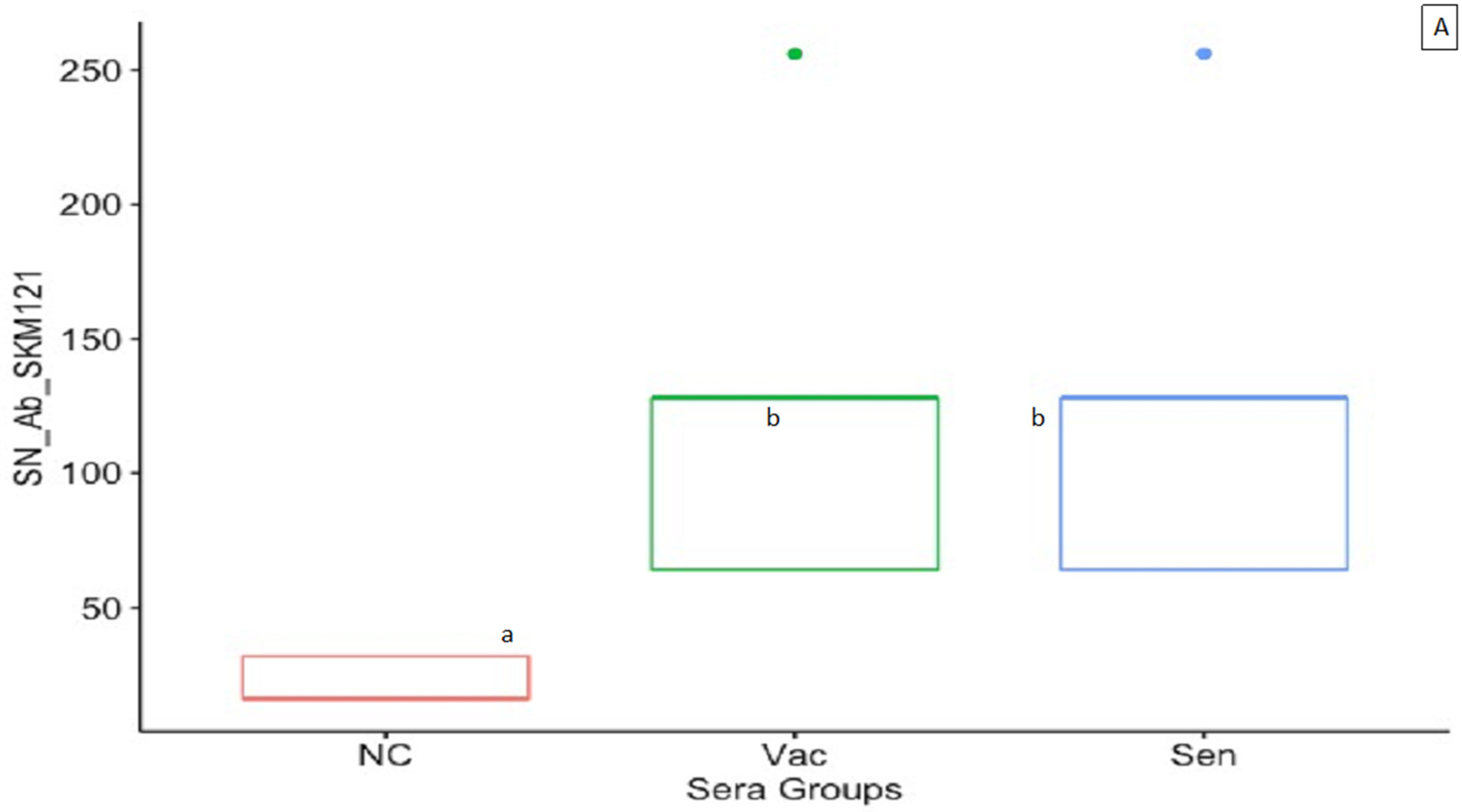
3.2. ELISA and Serum Neutralization Antibody Titers
3.3. Body Weight
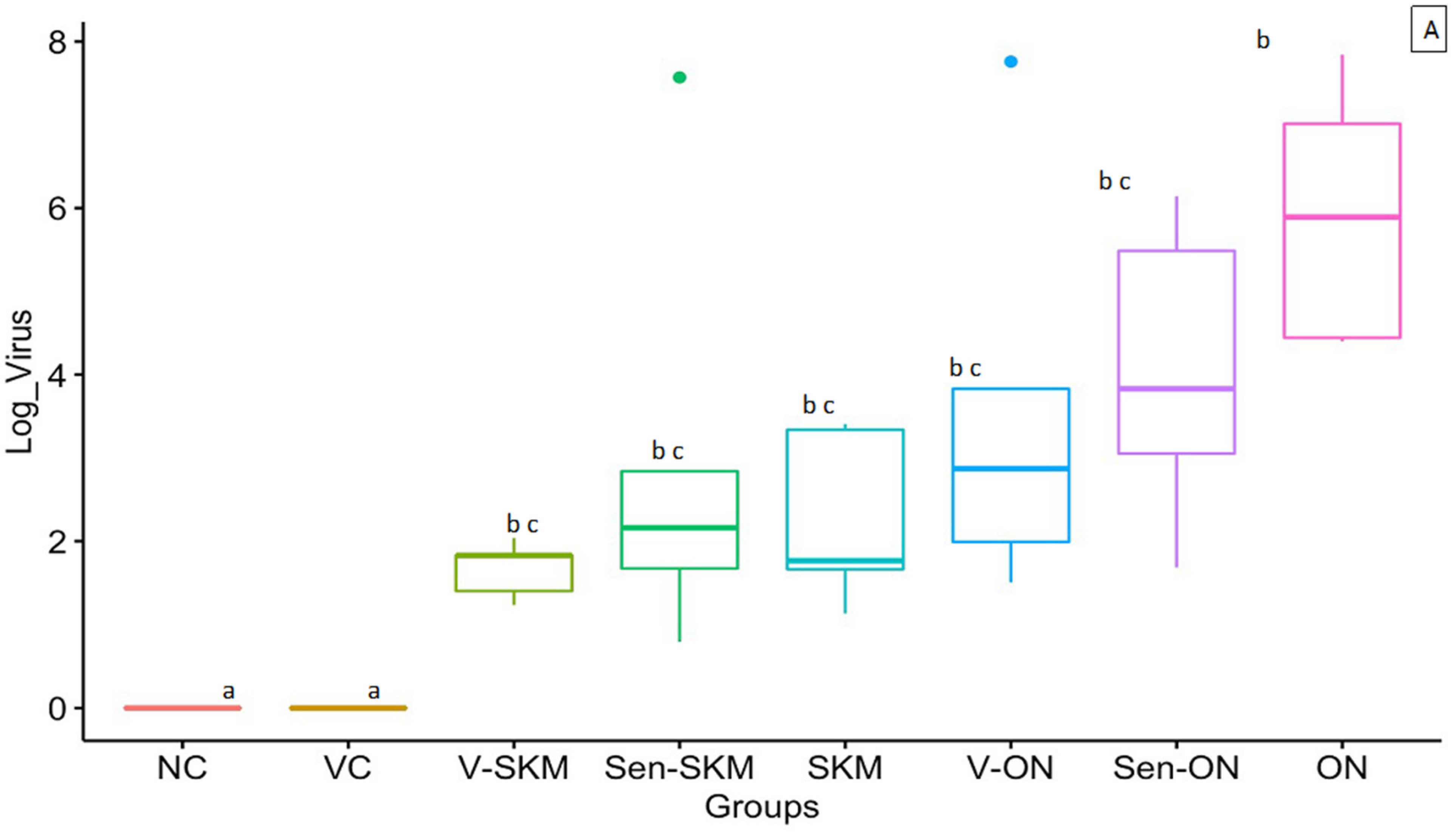
3.4. Virus Gene Copy Number in Intestine
3.5. Virus Gene Copy Number in Tendons
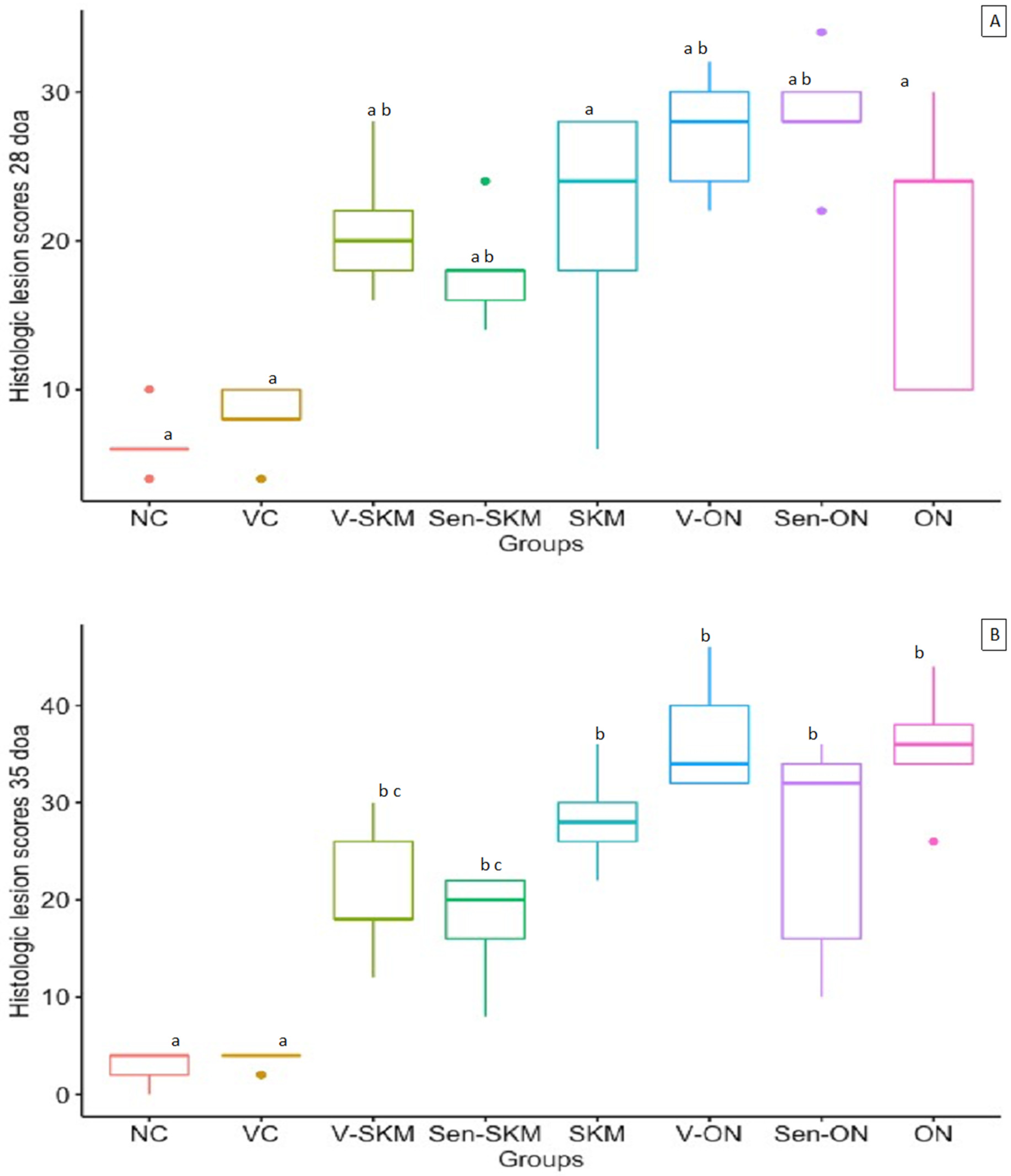
3.6. Histopathologic Lesion Scores in Gastrocnemius Tendons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levisohn, S.; Gur-Lavie, A.; Weisman, J. Infectious synovitis in Turkeys: Isolation of tenosynovitis virus-like agent. Avian Pathol. 1980, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Page, R.K.; Fletcher, O.J., Jr.; Villegas, P. Infectious tenosynovitis in young turkeys. Avian Dis. 1982, 26, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.K.; Sharafeldin, T.A.; Porter, R.E.; Ziegler, A.; Patnayak, D.P.; Goyal, S.M. Isolation and Characterization of a Turkey Arthritis Reovirus. Avian Dis. 2013, 57, 97–103. [Google Scholar] [CrossRef]
- Lu, H.; Tang, Y.; Dunn, P.A.; Wallner-Pendleton, E.A.; Lin, L.; Knoll, E.A. Isolation and molecular characterization of newly emerging avian reovirus variants and novel strains in Pennsylvania, USA, 2011–2014. Sci. Rep. 2015, 5, 14727. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, H.; Sebastian, A.; Yeh, Y.-T.; Praul, C.A.; Albert, I.U.; Zheng, S.-Y. Genomic characterization of a turkey reovirus field strain by Next-Generation Sequencing. Infect. Genet. Evol. 2015, 32, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, R. Turkey reoviral arthritis update. In Proceedings of the MPF Convention Turkey Health Workshop, Midwest Poultry Federation of the Conference, Minneapolis, MN, USA, 13–15 March 2018. [Google Scholar]
- Sharafeldin, T.A.; Mor, S.K.; Bekele, A.Z.; Verma, H.; Goyal, S.M.; Porter, R.E. The role of avian reoviruses in turkey tenosynovitis/arthritis. Avian Pathol. 2014, 43, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Sharafeldin, T.A.; Mor, S.K.; Bekele, A.Z.; Verma, H.; Noll, S.L.; Goyal, S.M.; Porter, R.E. Experimentally induced lameness in turkeys inoculated with a newly emergent turkey reovirus. Vet. Res. 2015, 46, 11. [Google Scholar] [CrossRef] [Green Version]
- Spandidos, D.A.; Graham, A.F. Physical and chemical characterization of an avian reovirus. J. Virol. 1976, 19, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Benavente, J.; Martinez-Costas, J. Avian reovirus: Structure and biology. Virus Res. 2007, 123, 105–119. [Google Scholar] [CrossRef]
- Schnitzer, T.J.; Ramos, T.; Gouvea, V. Avian Reovirus Polypeptides: Analysis of Intracellular Virus-Specified Products, Virions, Top Component, and Cores. J. Virol. 1982, 43, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Costas, J.; Grande, A.; Varela, R.; García-Martínez, C.; Benavente, J. Protein architecture of avian reovirus S1133 and identification of the cell attachment protein. J. Virol. 1997, 71, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande, A.; Costas, C.; Benavente, J. Subunit composition and conformational stability of the oligomeric form of the avian reovirus cell-attachment protein Sigma C. J. Gen. Virol. 2002, 83, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, R.; Meanger, J.; Enriquez, C.E.; Wilcox, G.E. Avian Reovirus Proteins Associated with Neutralization of Virus Infectivity. Virology 1993, 194, 688–696. [Google Scholar] [CrossRef]
- Wu, H.; Williams, Y.; Gunn, K.S.; Singh, N.K.; Locy, R.D.; Giambrone, J.J. Yeast-Derived Sigma C Protein-Induced Immunity Against Avian Reovirus. Avian Dis. 2005, 49, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, L.H.; Shih, W.L.; Hu, Y.C.; Liu, H.J. Baculovirus surface display of σC and σB proteins of avian reovirus and immunogenicity of the displayed proteins in a mouse model. Vaccine 2008, 26, 6361–6367. [Google Scholar] [CrossRef]
- Bi, Z.; Zhu, Y.; Chen, Z.; Li, C.; Wang, Y.; Wang, G.; Liu, G. Induction of a robust immunity response against novel duck reovirus in ducklings using a subunit vaccine of sigma C protein. Sci. Rep. 2016, 6, 39092. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, D.; Lublin, A.; Rosenbluth, E.; Heller, E.D.; Pitcovski, J. Optimized polypeptide for a subunit vaccine against avian reovirus. Vaccine 2016, 34, 3178–3183. [Google Scholar] [CrossRef]
- Mor, S.K.; Verma, H.; Sharafeldin, T.A.; Porter, R.E.; Jindal, N.; Ziegler, A.; Goyal, S.M. Characterization of S class gene segments of a newly isolated turkey arthritis reovirus. Virology 2014, 464–465, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Mor, S.K.; Sharafeldin, T.A.; Porter, R.E.; Goyal, S.M. Molecular characterization of L class genome segments of a newly isolated turkey arthritis reovirus. Infect. Genet. Evol. 2014, 27, 193–201. [Google Scholar] [CrossRef]
- Mor, S.K.; Verma, H.; Sharafeldin, T.A.; Porter, R.E.; Ziegler, A.F.; Noll, S.L.; Goyal, S.M. Survival of turkey arthritis reovirus in poultry litter and drinking water. Poult. Sci. 2015, 94, 639–642. [Google Scholar] [CrossRef]
- Sellers, H.S. Current limitations in control of viral arthritis and tenosynovitis caused by avian reoviruses in commercial poultry. Vet. Microbiol. 2017, 206, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharafeldin, T.A.; Kumar, R.; Huang, Q.; Liang, Y.; Goyal, S.M.; Porter, R.E.; Ly, H.; Mor, S.K. Development of a Recombinant Pichinde Virus-Vectored Vaccine against Turkey Arthritis Reovirus and Its Immunological Response Characterization in Vaccinated Animals. Pathogens 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Ly, H.; Liang, Y. Recombinant tri-segmented pichinde virus as a novel live viral vaccine platform. In Recombinant Virus Vaccines; Humana Press: New York, NY, USA, 2017; pp. 169–179. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Giambrone, J.J.; Hathcock, T.L. Efficacy of Coarse-Spray Administration of a Reovirus Vaccine in Young Chickens. Avian Dis. 1991, 35, 204. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.; Muskett, J.; Thornton, D. Observations on the ability of avian reovirus vaccination of hens to protect their progeny against the effects of challenge with homologous and heterologous strains. J. Comp. Pathol. 1986, 96, 125–129. [Google Scholar] [CrossRef]
- Pitcovski, J.; Goyal, S.M. Avian Reovirus Infections. Dis. Poult. 2019, 382–400. [Google Scholar] [CrossRef]
- Dhanwani, R.; Zhou, Y.; Huang, Q.; Verma, V.; Dileepan, M.; Ly, H.; Liang, Y. A Novel Live Pichinde Virus-Based Vaccine Vector Induces Enhanced Humoral and Cellular Immunity after a Booster Dose. J. Virol. 2016, 90, 2551–2560. [Google Scholar] [CrossRef] [Green Version]
- Pitcovski, J.; Gutter, B.; Gallili, G.; Goldway, M.; Perelman, B.; Gross, G.; Krispel, S.; Barbakov, M.; Michael, A. Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine 2003, 21, 4736–4743. [Google Scholar] [CrossRef]
- Fingerut, E.; Gutter, B.; Gallili, G.; Michael, A.; Pitcovski, J. A subunit vaccine against the adenovirus egg-drop syndrome using part of its fiber protein. Vaccine 2003, 21, 2761–2766. [Google Scholar] [CrossRef]
- Pitcovski, J.; Fingerut, E.; Gallili, G.; Eliahu, D.; Finger, A.; Gutter, B. A subunit vaccine against hemorrhagic enteritis adenovirus. Vaccine 2005, 23, 4697–4702. [Google Scholar] [CrossRef]
- Jones, R.C. Avian reovirus infections. Rev. Sci. Tech. 2000, 19, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Roessler, D.E.; Rosenberger, J.K. In vitro and in vivo Characterization of Avian Reoviruses. III. Host Factors Affecting Virulence and Persistence. Avian Dis. 1989, 33, 555. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharafeldin, T.A.; Goyal, S.M.; Mor, S.K.; Porter, R.E. Infection and Transmission dynamics of turkey arthritis reovirus in different age turkeys. SSRN Electron. J. 2021; preprint. [Google Scholar] [CrossRef]
- Liu, H.J.; Kuo, L.C.; Hu, Y.C.; Liao, M.H.; Lien, Y.Y. Development of an ELISA for detection of antibodies to avian reovirus in chickens. J. Virol. Methods 2002, 102, 129–138. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Wang, C.-Y.; Lee, L.-H.; Chuang, K.-P.; Lien, Y.-Y.; Yin, H.-S.; Tong, D.-W.; Xu, X.-G.; Liu, H.-J. Development of ELISA kits for antibodies against avian reovirus using the σC and σB proteins expressed in the methyltropic yeast Pichia pastoris. J. Virol. Methods 2010, 163, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Lublin, A.; Goldenberg, D.; Rosenbluth, E.; Heller, E.D.; Pitcovski, J. Wide-range protection against avian reovirus conferred by vaccination with representatives of four defined genotypes. Vaccine 2011, 29, 8683–8688. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Georgiou, K. Reovirus-induced tenosynovitis in chickens the influence of age at infection. Avian Pathol. 1984, 13, 441–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.F.; Kulkarni, A.; Fletcher, O. Reovirus Infections in Young Broiler Chickens. Avian Dis. 2013, 57, 321–325. [Google Scholar] [CrossRef]
- Troxler, S.; Rigomier, P.; Bilic, I.; Liebhart, D.; Prokofieva, I.; Robineau, B.; Hess, M. Identification of a new reovirus causing substantial losses in broiler production in France, despite routine vaccination of breeders. Vet. Rec. 2013, 172, 556. [Google Scholar] [CrossRef]
- Perelman, B.; Krispin, H.; Solomon, A.; Elrom, K.; Farnoushi, Y. Use of Controlled Exposure as a Novel Method for Reovirus Arthritis/Tenosynovitis Prevention. A Preliminary Report. Isr. J. Vet. Med. 2019, 74, 163–172. [Google Scholar]
- Kerr, K.M.; Olson, N.O. Control of Infectious Synovitis. 14. The Effect of Age of Chickens on the Susceptibility to Three Agents. Avian Dis. 1964, 8, 256. [Google Scholar] [CrossRef]
- Kibenge, F.S.B.; Jones, R.C.; Savage, C.E. Effects of experimental immunosuppression on reovirus-induced tenosynovitis in light-hybrid chickens. Avian Pathol. 1987, 16, 73–92. [Google Scholar] [CrossRef]
- van Loon, A.A.; Kosman, W.; van Zuilekom, H.I.; van Riet, S.; Frenken, M.; Schijns, E.J. The contribution of humoral im- munity to the control of avian reoviral infection in chickens after vaccination with live reovirus vaccine (strain 2177) at an early age. Avian Pathol. 2003, 32, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shapouri, M.R.S.; Frenette, D.; LaRochelle, R.; Arella, M.; Silim, A. Characterization of monoclonal antibodies against avian reovirus strain S1133. Avian Pathol. 1996, 25, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.; Lublin, A.; Rosenbluth, E.; Heller, E.D.; Pitcovski, J. Differentiating infected from vaccinated animals, and among virulent prototypes of reovirus. J. Virol. Methods 2011, 177, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Hsu, A.-P.; Shien, J.-H.; Chang, T.-J.; Liao, J.-W.; Chen, J.-R.; Lin, C.-F.; Hsu, W.-L. Avian reovirus sigma C enhances the mucosal and systemic immune responses elicited by antigen-conjugated lactic acid bacteria. Vaccine 2012, 30, 5019–5029. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, T.A.; Mor, S.K.; Sobhy, N.M.; Xing, Z.; Reed, K.M.; Goyal, S.M.; Porter, R.E. A Newly Emergent Turkey Arthritis Reovirus Shows Dominant Enteric Tropism and Induces Significantly Elevated Innate Antiviral and T Helper-1 Cytokine Responses. PLoS ONE 2015, 10, e0144085. [Google Scholar] [CrossRef]
- Dobson, K.N.; Glisson, J.R. Economic Impact of a Documented Case of Reovirus Infection in Broiler Breeders. Avian Dis. 1992, 36, 788. [Google Scholar] [CrossRef]




| Group Name | Group Description (n) | Oral Vac (Days) | I/N Vac (Days) | Ch-SKM121 (Days) | Ch-ON (Days) | Sampling (Age) |
|---|---|---|---|---|---|---|
| NC | Neg ctrl (12 + 5) | - | - | - | - | 21–28–35 |
| VC | V-ONLY (12 + 5) | 2 | 9 | - | - | 21–28–35 |
| V-SKM | V-Ch-SKM121 (12 + 5) | 2 | 9 | 15 | - | 21–28–35 |
| Sen-SKM | V + Sent-Ch-SKM121 (10 + 12) | 2 | 9 | 15 | 21–28–35 | |
| SKM | Ch- SKM121 (12 + 5) | - | - | 15 | - | 21–28–35 |
| V-ON | V-Ch-ON (12 + 5) | 2 | 9 | - | 15 | 21–28–35 |
| Sen-ON | Vac + Sent-Ch-ON (10 + 12) | 2 | 9 | - | 15 | 21–28–35 |
| ON | Ch-ON (12 + 5) | - | - | - | 15 | 21–28–35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Porter, R.E.; Mor, S.K.; Goyal, S.M. Efficacy and Immunogenicity of Recombinant Pichinde Virus-Vectored Turkey Arthritis Reovirus Subunit Vaccine. Vaccines 2022, 10, 486. https://doi.org/10.3390/vaccines10040486
Kumar R, Porter RE, Mor SK, Goyal SM. Efficacy and Immunogenicity of Recombinant Pichinde Virus-Vectored Turkey Arthritis Reovirus Subunit Vaccine. Vaccines. 2022; 10(4):486. https://doi.org/10.3390/vaccines10040486
Chicago/Turabian StyleKumar, Rahul, Robert E. Porter, Sunil K. Mor, and Sagar M. Goyal. 2022. "Efficacy and Immunogenicity of Recombinant Pichinde Virus-Vectored Turkey Arthritis Reovirus Subunit Vaccine" Vaccines 10, no. 4: 486. https://doi.org/10.3390/vaccines10040486
APA StyleKumar, R., Porter, R. E., Mor, S. K., & Goyal, S. M. (2022). Efficacy and Immunogenicity of Recombinant Pichinde Virus-Vectored Turkey Arthritis Reovirus Subunit Vaccine. Vaccines, 10(4), 486. https://doi.org/10.3390/vaccines10040486








