Enhanced Potency and Persistence of Immunity to Varicella-Zoster Virus Glycoprotein E in Mice by Addition of a Novel BC02 Compound Adjuvant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Materials and Reagents
2.3. Preparation of Recombinant VZV gE Glycoprotein
2.4. Experimental Vaccine Formulations
2.5. Immunization and Anatomy of Mice
2.6. IFN-γ and IL-2 ELISpot Assays
2.7. Memory B Cells ELISpot Assays
2.8. VZV Fluorescent Antibody to Membrane Antigen (FAMA) Assays
2.9. Determination of VZV Neutralizing Antibody Titers by Plaque Reduction Test
2.10. Statistical Analysis
3. Results
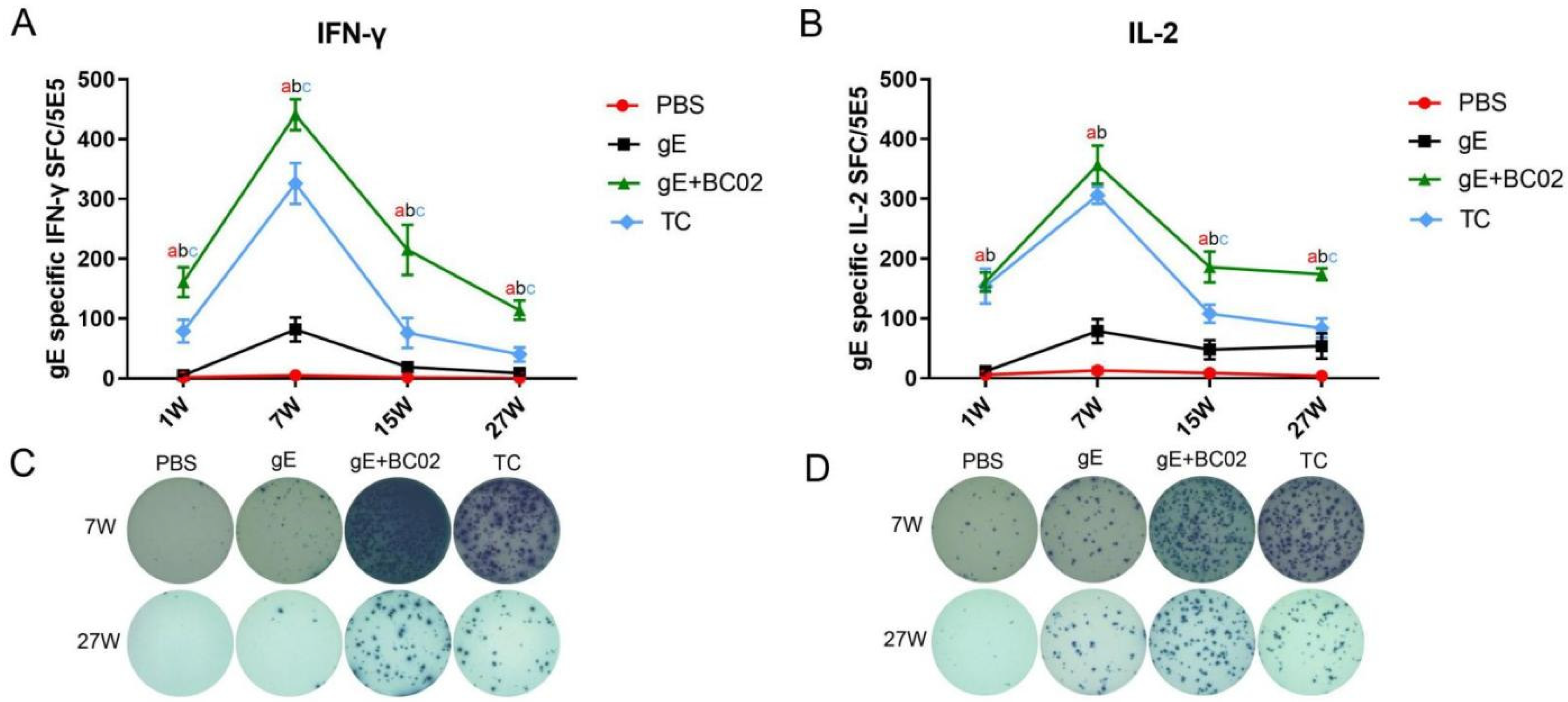
3.1. Higher Induction of gE-Specific IFN-γ and IL-2 by the Experimental Vaccine with BC02
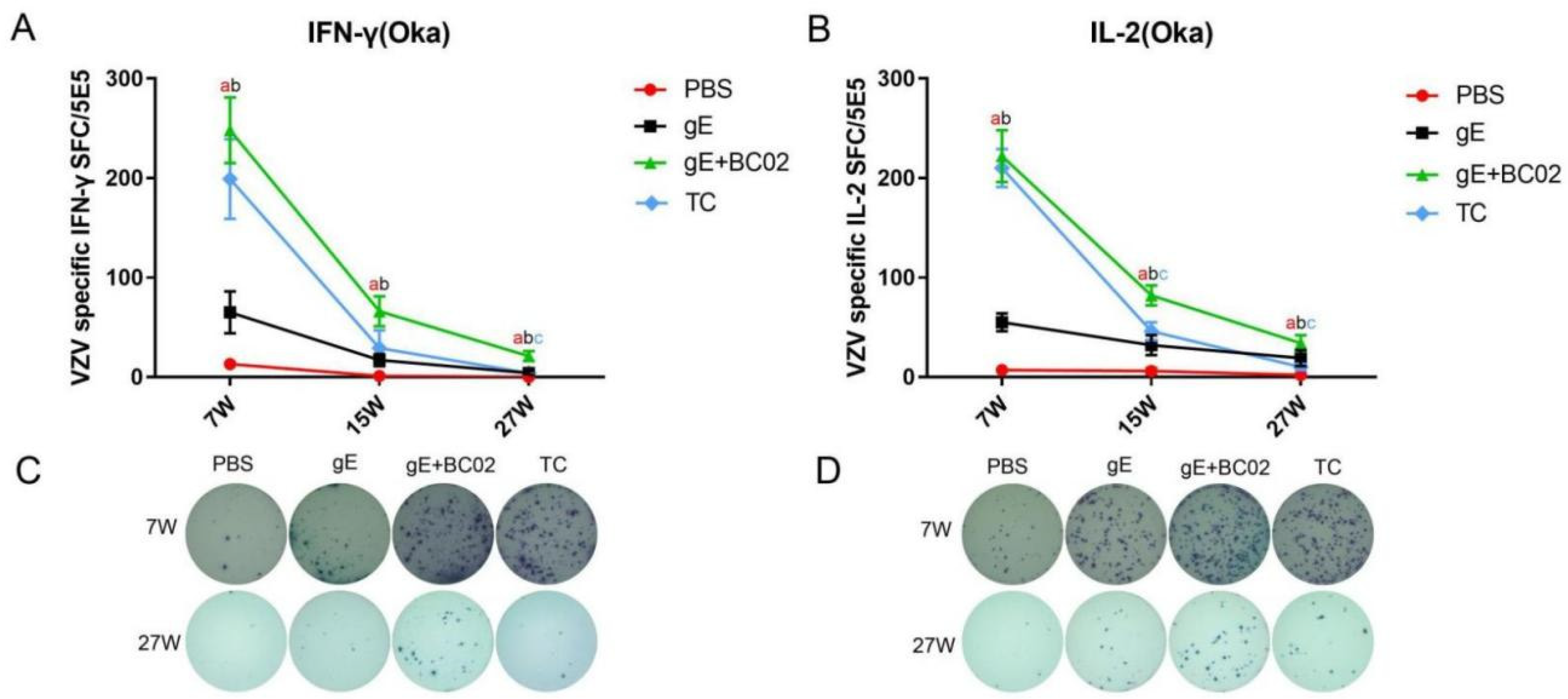
3.2. Higher Induction of Oka-Specific IFN-γ and IL-2 by the Experimental Vaccine with BC02
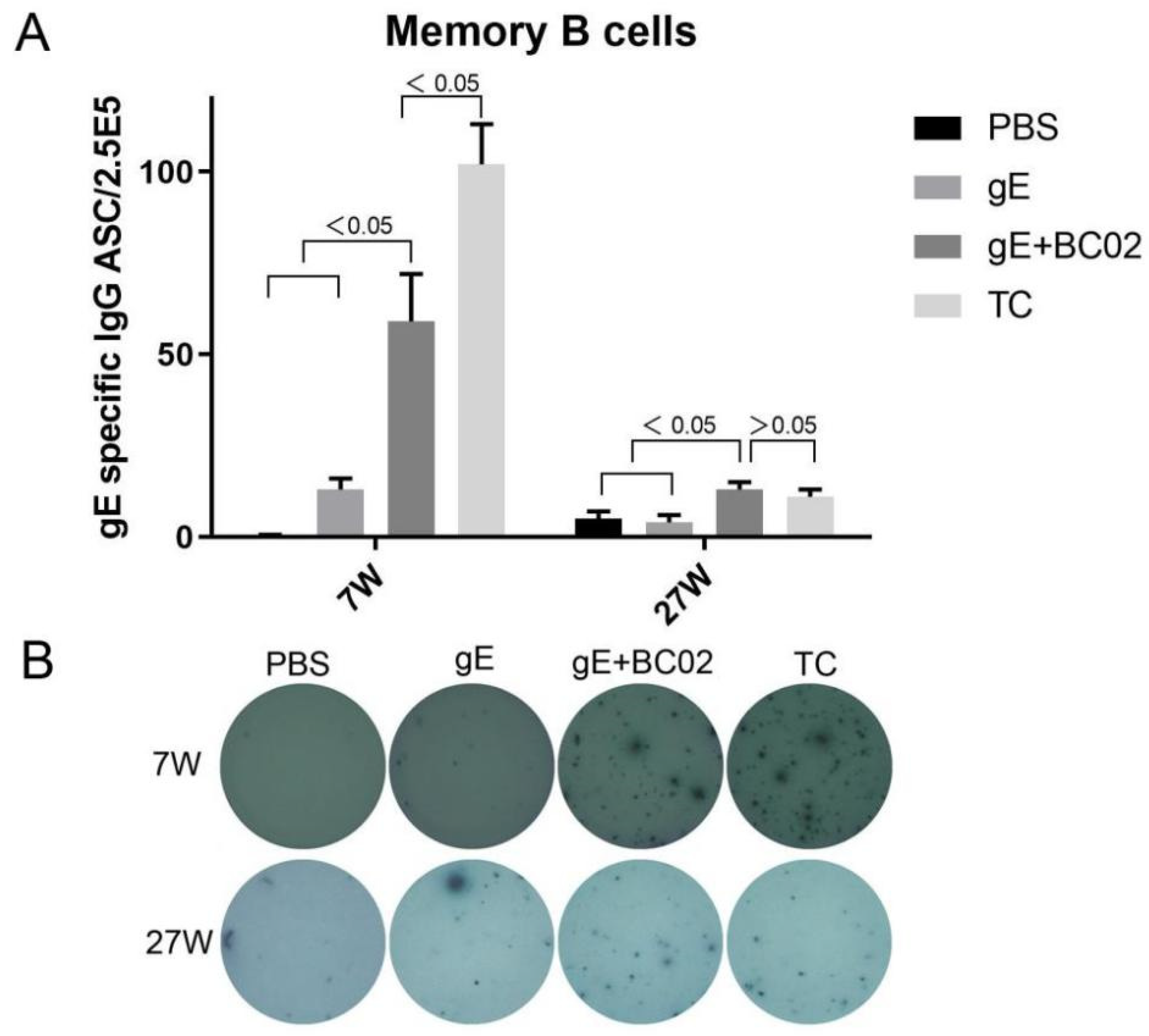
3.3. Higher Induction of Memory B Cells by the Experimental Vaccine with BC02
3.4. Efficient Induction of Anti-VZV Antibody by the Experimental Vaccine with BC02
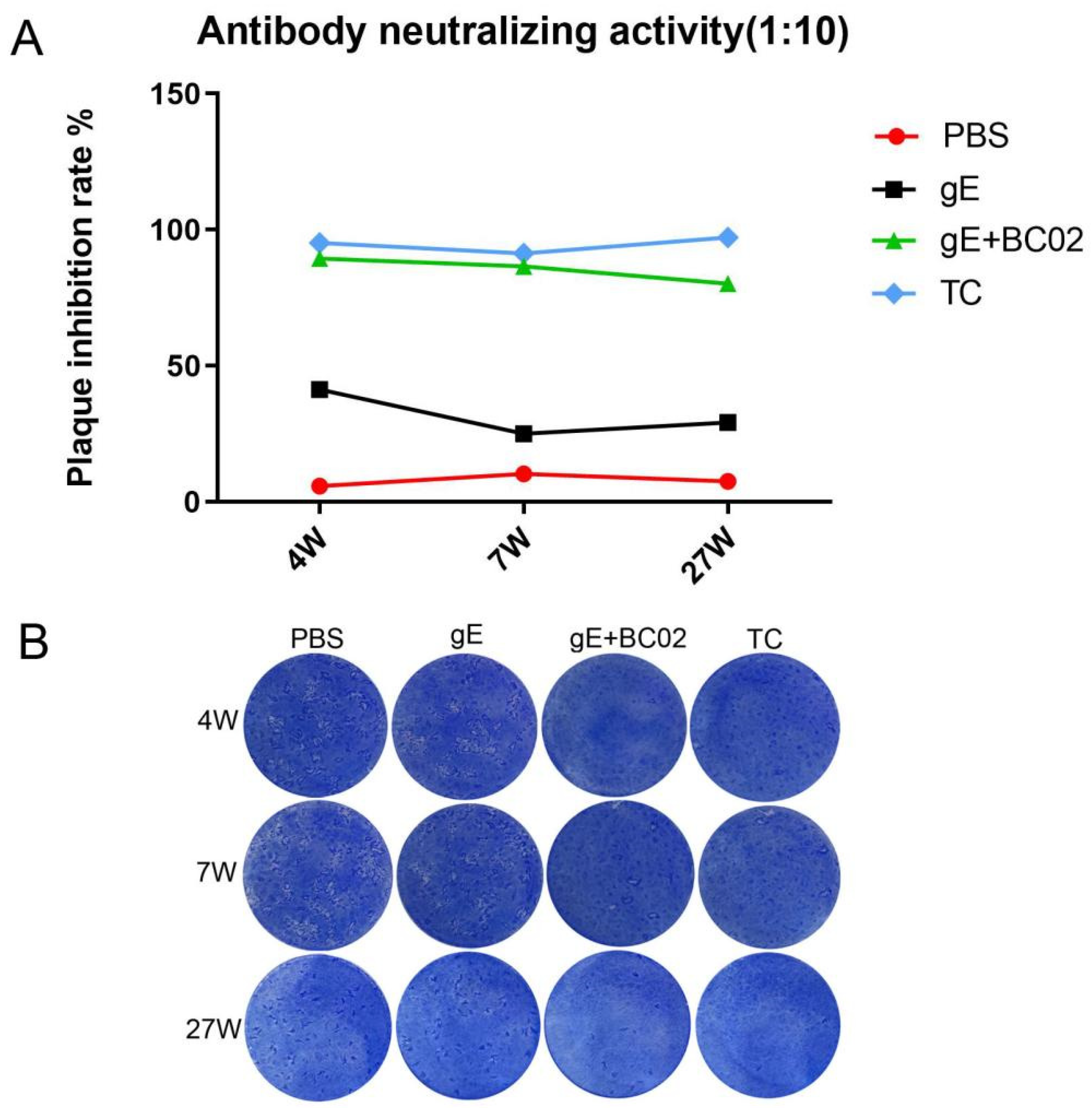
3.5. Efficient Induction of VZV Neutralizing Antibody by the Experimental Vaccine with BC02
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Genet. 2014, 12, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Gnann, J.W., Jr.; Whitley, R.J. Clinical practice. Herpes zoster. N. Engl. J. Med. 2002, 347, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Clinical practice: Herpes zoster. N. Engl. J. Med. 2013, 369, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gater, A.; Uhart, M.; McCool, R.; Préaud, E. The humanistic, economic and societal burden of herpes zoster in Europe: A critical review. BMC Public Health 2015, 15, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElhaney, J.; Gavazzi, G.; Flamaing, J.; Petermans, J. The role of vaccination in successful independent ageing. Eur. Geriatr. Med. 2016, 7, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Oxman, M.N.; Levin, M.J.; Shingles Prevention Study Group. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J. Infect. Dis. 2008, 197 (Suppl. 2), S228–S236. [Google Scholar] [CrossRef] [Green Version]
- Lang, P.O.; Govind, S.; Bokum, A.T.; Kenny, N.; Matas, E.; Pitts, D.; Aspinall, R. Immune Senescence and Vaccination in the Elderly. Curr. Top. Med. Chem. 2013, 13, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Alvarez-Pasquin, M.J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: A multi-disciplinary perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; Gebremeskel, B.G.G.; Acosta, C.J. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yawn, B.P.; Wollan, P.C.; Kurland, M.J.; Sauver, J.L.S.; Saddier, P. Herpes Zoster Recurrences More Frequent Than Previously Reported. Mayo Clin. Proc. 2011, 86, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.F.; Chi, M.; Smith, N.; Marcy, S.M.; Sy, L.S.; Jacobsen, S.J. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J. Infect. Dis. 2012, 15, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.W.; Rice, A.S. Clinical practice. Postherpetic neuralgia. N. Engl. J. Med. 2014, 371, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu (accessed on 14 March 2016).
- Schmader, K.E.; Levin, M.J.; Gnann, J.W., Jr.; McNeil, S.A.; Vesikari, T.; Betts, R.F.; Keay, S.; Stek, J.E.; Bundick, N.D.; Su, S.-C.; et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin. Infect. Dis. 2012, 54, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, M.J.; Oxman, M.N.; Zhang, J.H.; Johnson, G.R.; Stanley, H.; Hayward, A.R.; Caulfield, M.J.; Irwin, M.; Smith, J.G.; Clair, J.; et al. Varicella-Zoster Virus–Specific Immune Responses in Elderly Recipients of a Herpes Zoster Vaccine. J. Infect. Dis. 2008, 197, 825–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adju-vanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- James, S.F.; Chahine, E.B.; Sucher, A.J.; Hanna, C. Shingrix: The New Adjuvanted Recombinant Herpes Zoster Vaccine. Ann. Pharmacother. 2018, 52, 673–680. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.-J.; Diez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, L.; Wang, G.; Subbian, S.; Qin, C.; Zhao, A. Unmethylated CpG motif-containing genomic DNA fragment of Bacillus calmette-guerin promotes macrophage functions through TLR9-mediated activation of NF-κB and MAPKs signaling pathways. Innate Immun. 2020, 26, 183–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Zhang, X.; Li, Q.; Fu, L.; Wang, M.; Liu, S.; Wu, J.; Nie, J.; Zhang, L.; Zhao, C.; et al. Unmethylated CpG motif-containing genomic DNA fragments of bacillus calmette-guerin improves immune response towards a DNA vaccine for COVID-19. Vaccine 2021, 39, 6050–6056. [Google Scholar] [CrossRef]
- Chen, L.; Xu, M.; Wang, Z.Y.; Chen, B.W.; Du, W.X.; Su, C.; Shen, X.B.; Zhao, A.H.; Dong, N.; Wang, Y.J.; et al. The development and preliminary evaluation of a new Mycobacterium tuberculosis vaccine comprising Ag85b, HspX and CFP-10:ESAT-6 fusion protein with CpG DNA and aluminum hydroxide adjuvants. FEMS Immunol Med. Microbiol. 2010, 59, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, Y.C.; Zhang, J.; Bo, S.Y.; Xin, X.F.; Wang, G.Z. Immune effect of STAg combined with BCG-DNA and aluminum hydroxide adjuvant in mice. Chin. J. Biol. 2011, 24, 1177–1179. [Google Scholar]
- Li, J.L.; Fu, L.L.; Wang, G.Z.; Yang, X.M.; Zhao, A.H. Synergistic enhancement of macrophage innate immune response with BC02 complex adjuvant. Chin. J. Biol. 2018, 31, 941–948. [Google Scholar]
- Li, J.L.; Fu, L.L.; Yang, Y.; Wang, G.Z.; Zhao, A.H. Analysis of synergistic enhancement of innate immune response by BC02 compound adjuvant components. Chin. J. Biol. 2022, 35, 11–18. [Google Scholar]
- Kutinová, L.; Hainz, P.; Ludvíková, V.; Maresová, L.; Nĕmecková, S. Immune response to vaccinia virus recombinants expressing glycoproteins gE, gB, gH, and gL of Varicella-zoster virus. Virology 2001, 280, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Forghani, B.; Dupuis, K.W.; Schmidt, N.J. Epitopes functional in neutralization of varicella-zoster virus. J. Clin. Microbiol. 1990, 28, 2500–2506. [Google Scholar] [CrossRef] [Green Version]
- Giller, R.H.; Winistorfer, S.; Grose, C. Cellular and Humoral Immunity to Varicella Zoster Virus Glycoproteins in Immune and Susceptible Human Subjects. J. Infect. Dis. 1989, 160, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.R.; Burger, R.; Scheper, R.; Arvin, A.M. Major histocompatibility complex restriction of T-cell responses to var-icella-zoster virus in guinea pigs. J. Virol. 1991, 65, 1491–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forghani, B.; Ni, L.; Grose, C. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology 1994, 199, 458–462. [Google Scholar] [CrossRef]
- Grose, C. Glycoproteins encoded by varicella-zoster virus: Biosynthesis, phosphorylation, and intracellular trafficking. Annu. Rev. Microbiol. 1990, 44, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I.; Ali, M.A.; Bayat, A.; Steinberg, S.P.; Park, H.; Gershon, A.A.; Burbelo, P.D. Detection of antibodies to varicellazoster virus in recipients of the varicella vaccine by using a luciferase immunoprecipitation system assay. Clin. Vaccine Immunol. 2014, 21, 1288–1291. [Google Scholar] [CrossRef] [Green Version]
- Thomsson, E.; Persson, L.; Grahn, A.; Snäll, J.; Ekblad, M.; Brunhage, E.; Svensson, F.; Jern, C.; Hansson, G.C.; Bäckström, M.; et al. Recombinant glycoprotein E produced in mammalian cells in large-scale as an antigen for varicel-la-zoster-virus serology. J. Virol. Methods 2011, 175, 53–59. [Google Scholar] [CrossRef]
- Patterson-Bartlett, J.; Levin, M.J.; Lang, N.; Schödel, F.P.; Vessey, R.; Weinberg, A. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine 2007, 25, 7087–7093. [Google Scholar] [CrossRef]
- Vermeulen, J.N.; Lange, J.M.; Tyring, S.K.; Peters, P.H.; Nunez, M.; Poland, G.; Levin, M.J.; Freeman, C.; Chalikonda, I.; Li, J.; et al. Safety, tolerability, and immunogenicity after 1 and 2 doses of zoster vaccine in healthy adults ≥60 years of age. Vaccine 2012, 30, 904–910. [Google Scholar] [CrossRef]
- Sei, J.J.; Cox, K.S.; Dubey, S.A.; Antonello, J.M.; Krah, D.L.; Casimiro, D.R.; Vora, K.A. Effector and Central Memory Poly-Functional CD4+ and CD8+ T Cells are Boosted upon ZOSTAVAX® Vaccination. Front. Immunol. 2015, 6, 553. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Heineman, T.C.; Lal, H.; Godeaux, O.; Chlibek, R.; Hwang, S.-J.; McElhaney, J.E.; Vesikari, T.; Andrews, C.; Choi, W.S.; et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J. Infect. Dis. 2018, 217, 1750–1760. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of Adaptive Immunity by the Human Vaccine Adjuvant AS01 Depends on Activated Dendritic Cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Detienne, S.; Welsby, I.; Collignon, C.; Wouters, S.; Coccia, M.; Delhaye, S.; Van Maele, L.; Thomas, S.; Swertvaegher, M.; Detavernier, A.; et al. Central Role of CD169+ Lymph Node Resident Macrophages in the Adjuvanticity of the QS-21 Component of AS01. Sci. Rep. 2016, 6, 39475. [Google Scholar] [CrossRef]
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; Van Helden, M.J.; Dutta, S.; Genito, C.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines 2017, 2, 25. [Google Scholar] [CrossRef]
- Zaia, J.A.; Oxman, M.N. Antibody to Varicella-Zoster Virus-Induced Membrane Antigen: Immunofluorescence Assay Using Monodisperse Glutaraldehyde-Fixed Target Cells. J. Infect. Dis. 1977, 136, 519–530. [Google Scholar] [CrossRef]
- Iltis, J.P.; Castellano, G.A.; Gerber, P.; Le, C.; Vujcic, L.K.; Quinnan, G.V., Jr. Comparison of the Raji cell line fluorescent an-tibody to membrane antigen test and the enzyme-linked immunosorbent assay for determination of immunity to varicella-zoster virus. J. Clin. Microbiol. 1982, 16, 878–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, V.; Gershon, A.; Brunell, P.A. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J. Infect. Dis. 1974, 130, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Schmid, D.S.; Gershon, A.A. Use and Limitations of Varicella-Zoster Virus–Specific Serological Testing to Evaluate Breakthrough Disease in Vaccinees and to Screen for Susceptibility to Varicella. J. Infect. Dis. 2008, 197, S147–S151. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hwang, J.Y.; Shim, H.M.; Lee, E.; Park, S.; Park, H. Evaluation of a commercial glycoprotein enzymelinked im-munosorbent assay for measuring vaccine immunity to varicella. Yonsei Med. J. 2014, 55, 459–466. [Google Scholar] [CrossRef]
- Gershon, A.A.; LaRussa, P.; Steinberg, S. Detection of antibodies to varicella-zoster virus using a latex agglutination assay. Clin. Diagn. Virol. 1994, 2, 271–277. [Google Scholar] [CrossRef]


| Sample 1 | Time | FAMA Titration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 32 | 64 | 128 | 256 | 512 | 16 | 32 | 64 | 128 | 256 | 512 | |||
| PBS | 1W | − | 7W | − | ||||||||||
| gE | + | − | + | + | − | |||||||||
| gE + BC02 | + | − | + | + | + | + | + | − | ||||||
| TC 2 | + | − | + | + | + | + | + | + | ||||||
| PBS | 2W | − | 15W | − | ||||||||||
| gE | + | − | + | − | ||||||||||
| gE + BC02 | + | − | + | + | + | + | + | − | ||||||
| TC | + | + | − | + | + | + | + | + | − | |||||
| PBS | 4W | − | 27W | − | ||||||||||
| gE | + | + | − | + | − | |||||||||
| gE + BC02 | + | + | + | − | + | + | + | + | + | − | ||||
| TC | + | + | + | + | + | − | + | + | + | + | + | − | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Fu, L.; Yang, Y.; Wang, G.; Zhao, A. Enhanced Potency and Persistence of Immunity to Varicella-Zoster Virus Glycoprotein E in Mice by Addition of a Novel BC02 Compound Adjuvant. Vaccines 2022, 10, 529. https://doi.org/10.3390/vaccines10040529
Li J, Fu L, Yang Y, Wang G, Zhao A. Enhanced Potency and Persistence of Immunity to Varicella-Zoster Virus Glycoprotein E in Mice by Addition of a Novel BC02 Compound Adjuvant. Vaccines. 2022; 10(4):529. https://doi.org/10.3390/vaccines10040529
Chicago/Turabian StyleLi, Junli, Lili Fu, Yang Yang, Guozhi Wang, and Aihua Zhao. 2022. "Enhanced Potency and Persistence of Immunity to Varicella-Zoster Virus Glycoprotein E in Mice by Addition of a Novel BC02 Compound Adjuvant" Vaccines 10, no. 4: 529. https://doi.org/10.3390/vaccines10040529
APA StyleLi, J., Fu, L., Yang, Y., Wang, G., & Zhao, A. (2022). Enhanced Potency and Persistence of Immunity to Varicella-Zoster Virus Glycoprotein E in Mice by Addition of a Novel BC02 Compound Adjuvant. Vaccines, 10(4), 529. https://doi.org/10.3390/vaccines10040529






