Local Response and Barrier Recovery in Elderly Skin Following the Application of High-Density Microarray Patches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. Procedure
2.3. Equipment
2.3.1. High-Density Microarray Patches (HD-MAP)
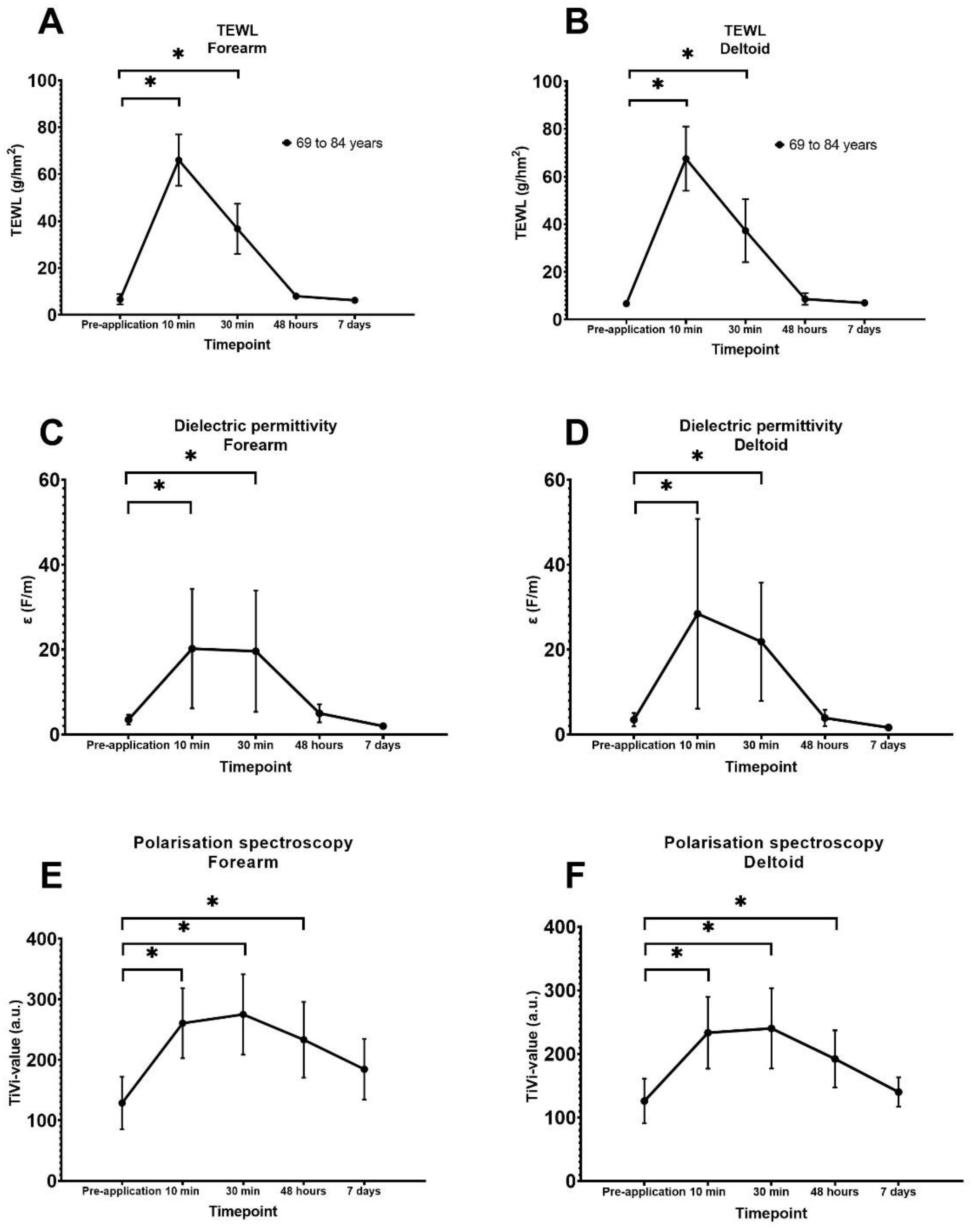
2.3.2. Evaporimetry
2.3.3. Dielectric Permittivity
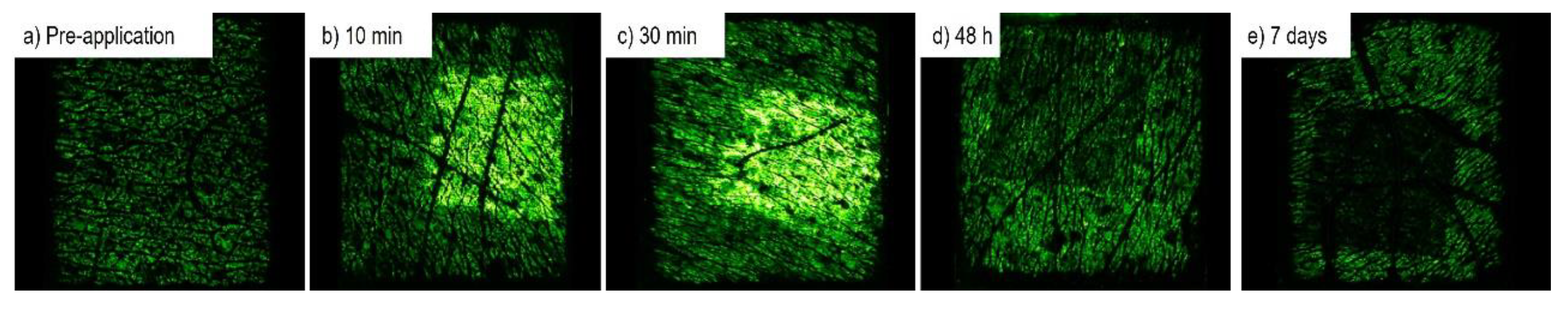
2.3.4. Polarisation Spectroscopy
2.3.5. Dermoscopy
2.3.6. Statistical Analysis
3. Results
3.1. Quantification of Transepidermal Waterloss
3.2. Quantification of Superficial Skin Hydration
3.3. Quantification of Red Blood Cell Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; García-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F. Vaccine delivery systems. Hum. Vaccines Immunother. 2017, 13, 17–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesse, E.M.; Atanasoff, S.; Hibbs, B.F.; Adegoke, O.J.; Ng, C.; Marquez, P.; Osborn, M.; Su, J.R.; Moro, P.L.; Shimabukuro, T.; et al. Shoulder Injury Related to Vaccine Administration (SIRVA): Petitioner claims to the National Vaccine Injury Compensation Program, 2010–2016. Vaccine 2020, 38, 1076–1083. [Google Scholar] [CrossRef]
- Arya, J.; Prausnitz, M.R. Microneedle patches for vaccination in developing countries. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Fernando, G.J.; Crichton, M.L.; Flaim, C.; Yukiko, S.R.; Fairmaid, E.J.; Corbett, H.J.; Primiero, C.A.; Ansaldo, A.B.; Frazer, I.H.; et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J. Control. Release Off. J. Control. Release Soc. 2011, 152, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.; Taba, M.; Deng, L.; Karatas, C.; Bag, S.; Ross, C.; Forster, A.; Booy, R.; Skinner, S.R. Usability, acceptability, and feasibility of a High-Density Microarray Patch (HD-MAP) applicator as a delivery method for vaccination in clinical settings. Hum. Vaccines Immunother. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.I.; Fernando, G.J.; Depelsenaire, A.C.; Kendall, M.A. Potent response of QS-21 as a vaccine adjuvant in the skin when delivered with the Nanopatch, resulted in adjuvant dose sparing. Sci. Rep. 2016, 6, 29368. [Google Scholar] [CrossRef]
- Ng, H.I.; Tuong, Z.K.; Fernando, G.J.P.; Depelsenaire, A.C.I.; Meliga, S.C.; Frazer, I.H.; Kendall, M.A.F. Microprojection arrays applied to skin generate mechanical stress, induce an inflammatory transcriptome and cell death, and improve vaccine-induced immune responses. NPJ Vaccines 2019, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Choo, J.J.Y.; Vet, L.J.; McMillan, C.L.D.; Harrison, J.J.; Scott, C.A.P.; Depelsenaire, A.C.I.; Fernando, G.J.P.; Watterson, D.; Hall, R.A.; Young, P.R.; et al. A chimeric dengue virus vaccine candidate delivered by high density microarray patches protects against infection in mice. NPJ Vaccines 2021, 6, 66. [Google Scholar] [CrossRef]
- Muller, D.A.; Depelsenaire, A.C.I.; Shannon, A.E.; Watterson, D.; Corrie, S.R.; Owens, N.S.; Agyei-Yeboah, C.; Cheung, S.T.M.; Zhang, J.; Fernando, G.J.P.; et al. Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays. Vaccines 2019, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Choo, J.J.Y.; McMillan, C.L.D.; Fernando, G.J.P.; Hall, R.A.; Young, P.R.; Hobson-Peters, J.; Muller, D.A. Developing a Stabilizing Formulation of a Live Chimeric Dengue Virus Vaccine Dry Coated on a High-Density Microarray Patch. Vaccines 2021, 9, 1301. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.P.; Hickling, J.; Flores, C.M.J.; Griffin, P.; Anderson, C.D.; Skinner, S.R.; Davies, C.; Witham, K.; Pryor, M.; Bodle, J.; et al. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). Vaccines 2018, 36, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.H.; Witham, K.; Depelsenaire, A.C.I.; Veitch, M.; Wells, J.W.; Wheatley, A.; Pryor, M.; Lickliter, J.D.; Francis, B.; Rockman, S.; et al. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: Results from a randomized, controlled phase I clinical trial. PLoS Med. 2020, 17, e1003024. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Chen, X.; Prow, N.A.; Fernando, G.J.; Tan, C.S.; Raphael, A.P.; Chang, D.; Ruutu, M.P.; Jenkins, D.W.; Pyke, A.; et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small 2010, 6, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Kakita, K.; Imafuku, K.; Takashima, S.; Haga, N.; Yamaguchi, Y.; Taguchi, K.; Oyamada, T. Safety and dose-sparing effect of Japanese encephalitis vaccine administered by microneedle patch in uninfected, healthy adults (MNA-J): A randomised, partly blinded, active-controlled, phase 1 trial. Lancet Microbe 2022, 3, e96–e104. [Google Scholar] [CrossRef]
- Muller, D.A.; Pearson, F.E.; Fernando, G.J.; Agyei-Yeboah, C.; Owens, N.S.; Corrie, S.R.; Crichton, M.L.; Wei, J.C.; Weldon, W.C.; Oberste, M.S.; et al. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci. Rep. 2016, 6, 22094. [Google Scholar] [CrossRef]
- Muller, D.A.; Fernando, G.J.P.; Owens, N.S.; Agyei-Yeboah, C.; Wei, J.C.J.; Depelsenaire, A.C.I.; Forster, A.; Fahey, P.; Weldon, W.C.; Oberste, M.S.; et al. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Sci. Rep. 2017, 7, 12644. [Google Scholar] [CrossRef]
- McMillan, C.L.D.; Choo, J.J.Y.; Idris, A.; Supramaniam, A.; Modhiran, N.; Amarilla, A.A.; Isaacs, A.; Cheung, S.T.M.; Liang, B.; Bielefeldt-Ohmann, H.; et al. Complete protection by a single-dose skin patch-delivered SARS-CoV-2 spike vaccine. Sci. Adv. 2021, 7, eabj8065. [Google Scholar] [CrossRef]
- Muller, D.A.; Henricson, J.; Baker, S.B.; Togö, T.; Jayashi Flores, C.M.; Lemaire, P.A.; Forster, A.; Anderson, C.D. Innate local response and tissue recovery following application of high density microarray patches to human skin. Sci. Rep. 2020, 10, 18468. [Google Scholar] [CrossRef]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 2015, 57, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Gravenstein, S.; Davidson, H.E.; Taljaard, M.; Ogarek, J.; Gozalo, P.; Han, L.; Mor, V. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: A cluster-randomised trial. Lancet Respir. Med. 2017, 5, 738–746. [Google Scholar] [CrossRef]
- Henricson, J.; Muller, D.A.; Baker, S.B.; Iredahl, F.; Togö, T.; Anderson, C.D. Micropuncture closure following high density microarray patch application in healthy subjects. Ski. Res. Technol. 2022, 28, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.; Elliott, S.; Krauer, K.; Davies, C.; Rachel Skinner, S.; Anderson, C.D.; Forster, A. Safety, acceptability and tolerability of uncoated and excipient-coated high density silicon micro-projection array patches in human subjects. Vaccine 2017, 35, 6676–6684. [Google Scholar] [CrossRef] [PubMed]
- Logger, J.G.M.; Münchhoff, C.U.; Olydam, J.I.; Peppelman, M.; Van Erp, P.E.J. Anatomical site variation of water content in human skin measured by the Epsilon: A pilot study. Ski. Res. Technol. 2019, 25, 333–338. [Google Scholar] [CrossRef] [Green Version]
- O’Doherty, J.; Henricson, J.; Anderson, C.; Leahy, M.J.; Nilsson, G.E.; Sjöberg, F. Sub-epidermal imaging using polarized light spectroscopy for assessment of skin microcirculation. Ski. Res. Technol. 2007, 13, 472–484. [Google Scholar] [CrossRef]
- Depelsenaire, A.C.I.; Witham, K.; Veitch, M.; Wells, J.W.; Anderson, C.D.; Lickliter, J.D.; Rockman, S.; Bodle, J.; Treasure, P.; Hickling, J.; et al. Cellular responses at the application site of a high-density microarray patch delivering an influenza vaccine in a randomized, controlled phase I clinical trial. PLoS ONE 2021, 16, e0255282. [Google Scholar] [CrossRef]
- Salsberg, J.; Andriessen, A.; Abdulla, S.; Ahluwalia, R.; Beecker, J.; Sander, M.; Schachter, J. A review of protection against exposome factors impacting facial skin barrier function with 89% mineralizing thermal water. J. Cosmet. Dermatol. 2019, 18, 815–820. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevenini, E.; Monti, D.; Franceschi, C. Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Garagnani, P.; Santoro, F.; Rappuoli, R.; Franceschi, C.; Medaglini, D. Shelter from the cytokine storm: Pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Semin. Immunopathol. 2020, 42, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Garland, M.J.; Morrow, D.I.; Migalska, K.; Singh, T.R.; Majithiya, R.; Woolfson, A.D. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release Off. J. Control. Release Soc. 2010, 147, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Enfield, J.; O’Connell, M.L.; Lawlor, K.; Jonathan, E.; O’Mahony, C.; Leahy, M. In-vivo dynamic characterization of microneedle skin penetration using optical coherence tomography. J. Biomed. Opt. 2010, 15, 046001. [Google Scholar] [CrossRef]

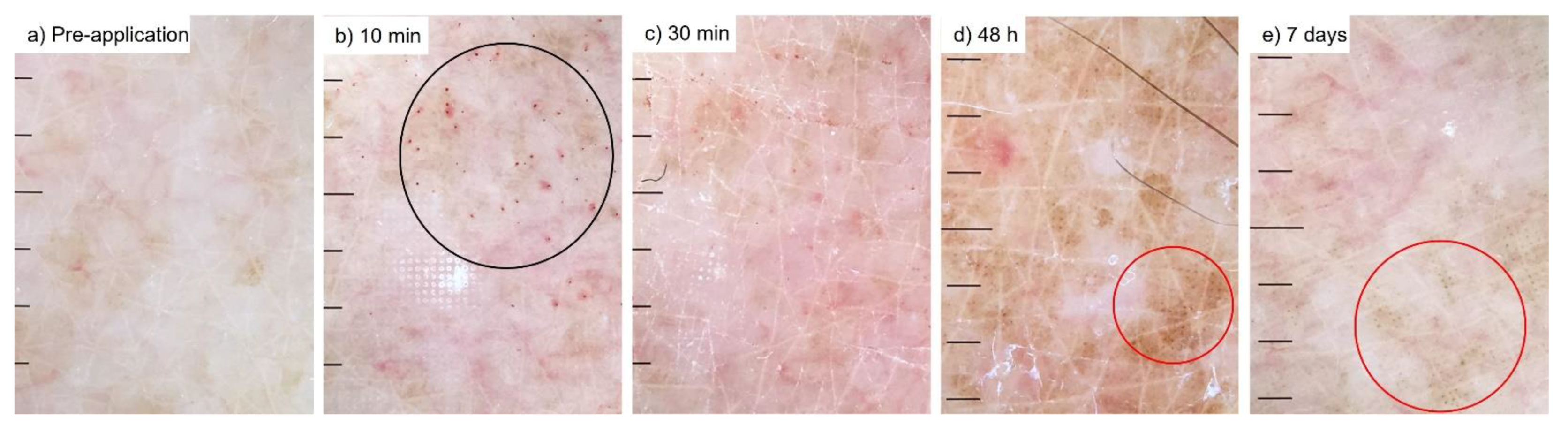
| Petechiae | Black Dots | Flaking | Wet Bleeding | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 0 |
| Pre-treatment | - | - | - | 24 | - | - | - | 24 | 7 | 2 | - | 15 | - | 24 |
| 10 min | 13 | 9 | - | 2 | - | - | - | 24 | 13 | 1 | - | 10 | 12 | 12 |
| 30 min | 12 | 8 | - | 4 | 1 | - | - | 23 | 13 | 2 | - | 9 | - | 24 |
| 48 h | 1 | 1 | - | 22 | 10 | 3 | - | 11 | 14 | 2 | - | 8 | - | 24 |
| 7 days | - | - | - | 8 | 7 | - | - | 1 | 7 | - | - | 1 | - | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iredahl, F.; Muller, D.A.; Togö, T.; Jonasson, H.; Baker, B.; Anderson, C.D.; Henricson, J. Local Response and Barrier Recovery in Elderly Skin Following the Application of High-Density Microarray Patches. Vaccines 2022, 10, 583. https://doi.org/10.3390/vaccines10040583
Iredahl F, Muller DA, Togö T, Jonasson H, Baker B, Anderson CD, Henricson J. Local Response and Barrier Recovery in Elderly Skin Following the Application of High-Density Microarray Patches. Vaccines. 2022; 10(4):583. https://doi.org/10.3390/vaccines10040583
Chicago/Turabian StyleIredahl, Fredrik, David A. Muller, Totte Togö, Hanna Jonasson, Ben Baker, Chris D. Anderson, and Joakim Henricson. 2022. "Local Response and Barrier Recovery in Elderly Skin Following the Application of High-Density Microarray Patches" Vaccines 10, no. 4: 583. https://doi.org/10.3390/vaccines10040583






