Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedures
2.2.1. Demographics and Clinical Data
2.2.2. Sample Collection and Study Protocol
2.2.3. Assessment of Antibody Responses against SARS-CoV-2
- Anti-SARS-CoV-2 spike total antibodies
- 2.
- SARS-CoV-2 neutralizing antibodies to ancestral type
- 3.
- Multiplex sVNT to ancestral type SARS-CoV-2 and VOCs
2.2.4. Assessment of T-Cell Response against SARS-CoV-2
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Immune Response
3.2.1. Anti-SARS-CoV-2 Spike Total Antibodies
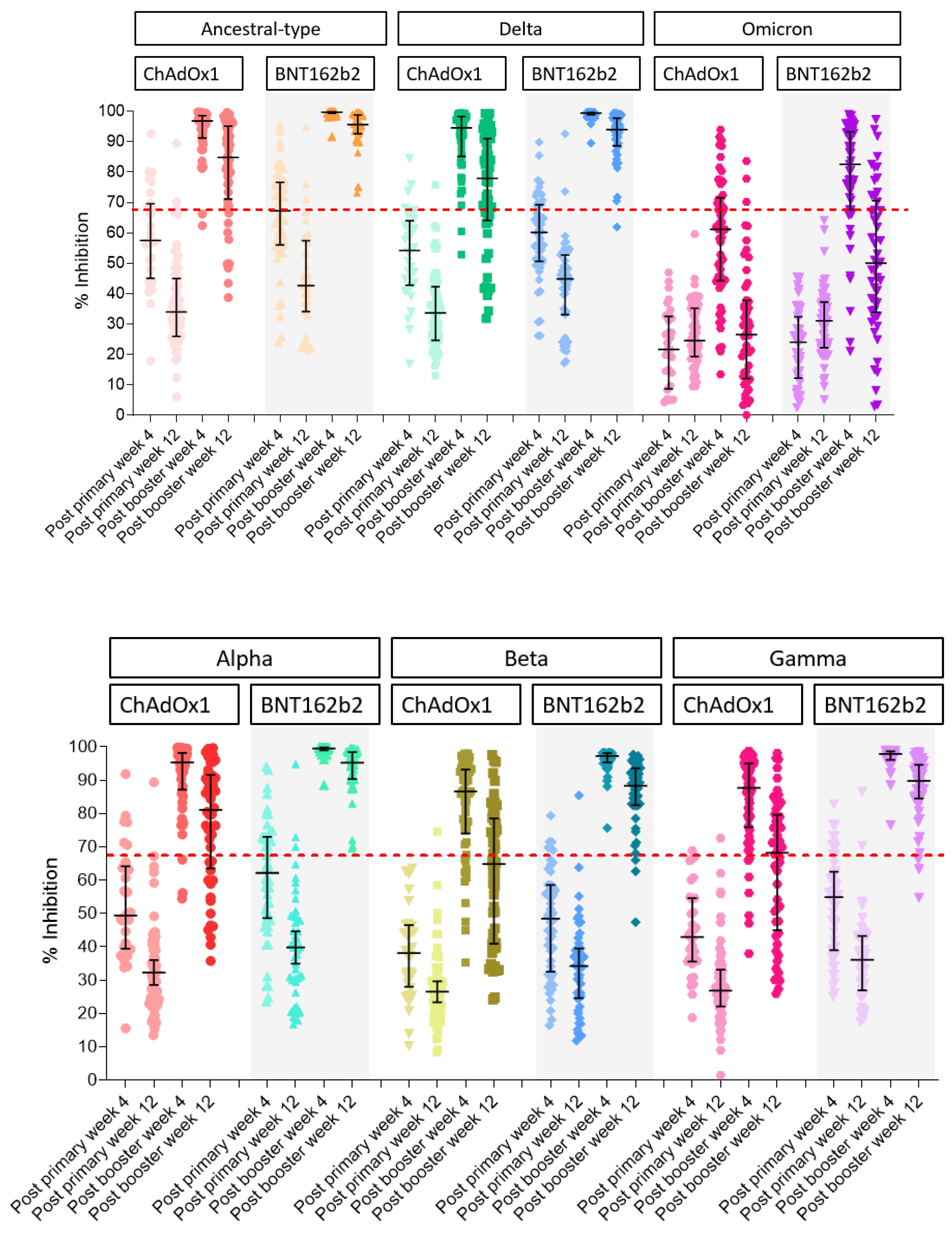
3.2.2. Surrogate Viral Neutralizing Antibody to Ancestral Type by GenScript and VOCs by Luminex
3.2.3. Assessment of T-Cell Response by ELISpot
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jantarabenjakul, W.; Chantasrisawad, N.; Puthanakit, T.; Wacharapluesadee, S.; Hirankarn, N.; Ruenjaiman, V.; Paitoonpong, L.; Suwanpimolkul, G.; Torvorapanit, P.; Pradit, R.; et al. Short-term immune response after inactivated SARS-CoV-2 (CoronaVac(R), Sinovac) and ChAdOx1 nCoV-19 (Vaxzevria(R), Oxford-AstraZeneca) vaccinations in health care workers. Asian Pac. J. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 5 March 2022).
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Denis, K.J.S.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Quach, T.H.T.; Tran, T.M.; Phuoc, H.N.; Nguyen, H.T.; Vo, T.K.; Van Vo, G. Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine. Biomed. Pharmacother. 2022, 147, 112650. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2021, 22, 483–495. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Q.; Deng, C.; Li, M.; Li, L.; Liu, D.; Liu, M.; Ruan, X.; Mei, J.; Mo, R.; et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov. 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Keskin, A.U.; Bolukcu, S.; Ciragil, P.; Topkaya, A.E. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J. Med. Virol. 2022, 94, 39–41. [Google Scholar] [CrossRef]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Çağlayan, D.; Süner, A.F.; Şiyve, N.; Güzel, I.; Irmak, Ç.; Işik, E.; Appak, Ö.; Çelik, M.; Öztürk, G.; Çavuş, S.A.; et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J. Med. Virol. 2022, 94, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Yorsaeng, R.; Suntronwong, N.; Phowatthanasathian, H.; Assawakosri, S.; Kanokudom, S.; Thongmee, T.; Vichaiwattana, P.; Auphimai, C.; Wongsrisang, L.; Srimuan, D.; et al. Immunogenicity of a third dose viral-vectored COVID-19 vaccine after receiving two-dose inactivated vaccines in healthy adults. Vaccine 2021, 40, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Elecsys Anti-SARS-CoV-2. Available online: https://www.fda.gov/media/137605/download (accessed on 14 March 2021).
- Jochum, S.; Kirste, I.; Hortsch, S.; Grunert, V.P.; Legault, H.; Eichenlaub, U.; Kashlan, B.; Pajon, R. Clinical Utility of Elecsys Anti-SARS-CoV-2 S Assay in COVID-19 Vaccination: An Exploratory Analysis of the mRNA-1273 Phase 1 Trial. Front. Immunol. 2021, 12, 798117. [Google Scholar] [CrossRef] [PubMed]
- De Santis, G.C.; Mendrone, A.; Langhi, D., Jr.; Covas, D.T.; Fabron, A., Jr.; Cortez, A.J.P.; Dinardo, C.L.; Ubiali, E.M.A.; Marques, J.F.C., Jr.; Bordin, J.O.; et al. Suggested guidelines for convalescent plasma therapy for the treatment of COVID-19. Hematol. Transfus. Cell Ther. 2021, 43, 212–213. [Google Scholar] [CrossRef]
- Bossart, K.N.; McEachern, J.A.; Hickey, A.C.; Choudhry, V.; Dimitrov, D.S.; Eaton, B.T.; Wang, L.-F. Neutralization assays for differential henipavirus serology using Bio-Plex Protein Array Systems. J. Virol. Methods 2007, 142, 29–40. [Google Scholar] [CrossRef]
- Lehmann, A.A.; Kirchenbaum, G.A.; Zhang, T.; Reche, P.A.; Lehmann, P.V. Deconvoluting the T Cell Response to SARS-CoV-2: Specificity Versus Chance and Cognate Cross-Reactivity. Front. Immunol. 2021, 12, 635942. [Google Scholar] [CrossRef]
- Kudlay, D.; Kofiadi, I.; Khaitov, M. Peculiarities of the T Cell Immune Response in COVID-19. Vaccines 2022, 10, 242. [Google Scholar] [CrossRef]
- Prasithsirikul, W.; Pongpirul, K.; Nopsopon, T.; Phutrakool, P.; Pongpirul, W.; Samuthpongtorn, C.; Suwanwattana, P.; Jongkaewwattana, A. Immunogenicity of ChAdOx1 nCoV-19 Booster Vaccination Following Two CoronaVac Shots in Healthcare Workers. Vaccines 2022, 10, 217. [Google Scholar] [CrossRef]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.; Malik, A.A.; De la Cruz, E.; Jorge, A.; et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022, 28, 481–485. [Google Scholar] [CrossRef]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.; De la Cruz, E.; Jorge, A.; De los Santos, M.; et al. Immunogenicity of heterologous BNT162b2 booster in fully vaccinated individuals with CoronaVac against SARS-CoV-2 variants Delta and Omicron: The Dominican Republic Experience. medRxiv 2021. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Althaus, T.; Tan, C.W.; Costantini, A.; Chia, W.N.; Chau, N.V.V.; Mattiuzzo, G.; Rose, N.J.; Voiglio, E.; Wang, L.F. WHO international standard for SARS-CoV-2 antibodies to determine markers of protection. Lancet Microbe 2022, 3, e81–e82. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature 2022, 603, 493–496. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Kannan, S.R.; Spratt, A.N.; Sharma, K.; Chand, H.S.; Byrareddy, S.N.; Singh, K. Omicron SARS-CoV-2 variant: Unique features and their impact on pre-existing antibodies. J. Autoimmun. 2022, 126, 102779. [Google Scholar] [CrossRef]
- He, Q.; Mao, Q.; An, C.; Zhang, J.; Gao, F.; Bian, L.; Li, C.; Liang, Z.; Xu, M.; Wang, J. Heterologous prime-boost: Breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021, 10, 629–637. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Leick, M.B.; Larson, R.C.; Berger, T.R.; Katsis, K.; Yam, J.Y.; Brini, G.; Grauwet, K.; MGH COVID-19 Collection & Processing Team; Maus, M.V. SARS-CoV-2 T-cell immunity to variants of concern following vaccination. bioRxiv 2021. [Google Scholar] [CrossRef]


| Characteristics | ChAdOx1 Booster | BNT 162b2 Booster | p-Value |
|---|---|---|---|
| N = 56 | N = 42 | ||
| Age, years, median (IQR) | 47 (34–53) | 32 (30–45) | 0.001 |
| Age group, n(%) | 0.01 | ||
| 20–30 years | 7 (12.5) | 15 (35.7) | |
| 31–40 years | 12 (21.4) | 14 (33.3) | |
| 41–50 years | 13 (23.2) | 5 (11.9) | |
| 51–60 years | 24 (42.9) | 8 (19.1) | |
| Female, n(%) | 44 (78.6) | 34 (81) | 0.005 |
| BMI (kg/m2), median (IQR) | 22.6 (20.3–25.9) | 21.5 (20–25.8) | 0.45 |
| Median (IQR) | Anti-SARS-CoV-2 Spike Total Antibodies (U/mL) | Surrogate Viral Neutralizing Antibody to Ancestral Type (%Inhibition) by GenScript | ||||
|---|---|---|---|---|---|---|
| Primary immunization | (N = 56) | (N = 42) | p-value | (N = 56) | (N = 42) | p-value |
| Week 4 | 179 (92–301) | 242 (163–462) | 0.04 | 66.8 (47.4–86.1) | 83.4 (66.8–91.0) | 0.04 |
| Week 12 | 56 (41–126) | 117 (61–198) | 0.001 | 39.4 (18.6–57.8) | 41.3 (31.7–59.2) | 0.26 |
| Booster | ChAdOx1 | BNT162b2 | p-value | ChAdOx1 | BNT162b2 | p-value |
| Week 4 | 7768 (5349–11,142) | 25,129 (17,531–39,434) | <0.001 | 98.1 (97.9–98.2) | 98.5 (98.5–98.6) | <0.001 |
| Week 12 | 3139 (2185–4660) | 6558 (3836–8885) | <0.001 | 97.9 (95.8–98.1) | 97.9 (97.8–98.2) | 0.03 |
| Post Booster | Primary Immunization with CoronaVac and Booster with ChAdOx1 | Primary Immunization with CoronaVac and Booster with BNT162b2 | ||||
|---|---|---|---|---|---|---|
| GM | GMR | % Decrease | GM | GMR | % Decrease | |
| Anti-SARS-CoV-2 spike total antibodies | ||||||
| week 4 | 7292.9 (6049.7–8791.6) | ref | 29,268.9 (23,921.4–35,812.1) | ref | ||
| week 12 | 2960.5 (2421.0–3620.2) | 0.41 (0.37–0.45) | 59% | 6200.0 (5043.5–7621.8) | 0.21 (0.18–0.25) | 79% |
| Surrogate viral neutralizing antibody(sVNT) | ||||||
| Ancestral type | ||||||
| week 4 | 92.8 (90.3–95.3) | Ref | 99.5 (99.4–99.7) | ref | ||
| week 12 | 79.2 (74.1–84.6) | 0.85 (0.81–0.89) | 15% | 93.8 (91.7–96.0) | 0.94 (0.92–0.96) | 6% |
| Alpha | ||||||
| week 4 | 90.3 (87.0–93.8) | ref | 90.3 (87.0–93.8) | ref | ||
| week 12 | 74.8 (69.4–80.6) | 0.83 (0.79–0.87) | 17% | 74.8 (69.4–80.6) | 0.83 (0.79–0.87) | 17% |
| Beta | ||||||
| week 4 | 79.7 (75.0–84.8) | ref | 96.4 (95.7–97.2) | ref | ||
| week 12 | 58.1 (52.3–64.4) | 0.73 (0.68–0.78) | 27% | 85.2 (81.5–89.0) | 0.88 (0.85–0.92) | 12% |
| Gamma | ||||||
| week 4 | 81.9 (77.4–86.7) | ref | 97.1 (96.4–97.8) | ref | ||
| week 12 | 59.8 (53.8–66.4) | 0.73 (0.68–0.78) | 27% | 86.7 (83.3–90.3) | 0.89 (0.86–0.93) | 11% |
| Delta | ||||||
| week 4 | 89.6 (86.3–93.1) | ref | 99.1 (98.9–99.3) | ref | ||
| week 12 | 71.4 (65.6–77.7) | 0.8 (0.75–0.84) | 20% | 91.1 (88.3–94) | 0.92 (0.89–0.95) | 8% |
| Omicron | ||||||
| week 4 | 54.5 (48.6–61.3) | ref | 80.9 (75.1–87.1) | ref | ||
| week 12 | 20.8 (15.3–28.2) | 0.38 (0.29–0.49) | 62% | 44.4 (35.3–55.7) | 0.55 (0.43–0.69) | 45% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantarabenjakul, W.; Sodsai, P.; Chantasrisawad, N.; Jitsatja, A.; Ninwattana, S.; Thippamom, N.; Ruenjaiman, V.; Tan, C.W.; Pradit, R.; Sophonphan, J.; et al. Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers. Vaccines 2022, 10, 639. https://doi.org/10.3390/vaccines10050639
Jantarabenjakul W, Sodsai P, Chantasrisawad N, Jitsatja A, Ninwattana S, Thippamom N, Ruenjaiman V, Tan CW, Pradit R, Sophonphan J, et al. Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers. Vaccines. 2022; 10(5):639. https://doi.org/10.3390/vaccines10050639
Chicago/Turabian StyleJantarabenjakul, Watsamon, Pimpayao Sodsai, Napaporn Chantasrisawad, Anusara Jitsatja, Sasiprapa Ninwattana, Nattakarn Thippamom, Vichaya Ruenjaiman, Chee Wah Tan, Rakchanok Pradit, Jiratchaya Sophonphan, and et al. 2022. "Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers" Vaccines 10, no. 5: 639. https://doi.org/10.3390/vaccines10050639
APA StyleJantarabenjakul, W., Sodsai, P., Chantasrisawad, N., Jitsatja, A., Ninwattana, S., Thippamom, N., Ruenjaiman, V., Tan, C. W., Pradit, R., Sophonphan, J., Wacharapluesadee, S., Wang, L.-F., Puthanakit, T., Hirankarn, N., & Putcharoen, O. (2022). Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers. Vaccines, 10(5), 639. https://doi.org/10.3390/vaccines10050639






