Cost per Responder Analysis of Secukinumab versus Adalimumab in the Treatment of Psoriatic Disease
Abstract
:1. Introduction
2. Materials and Methods
Cost per Responder Model
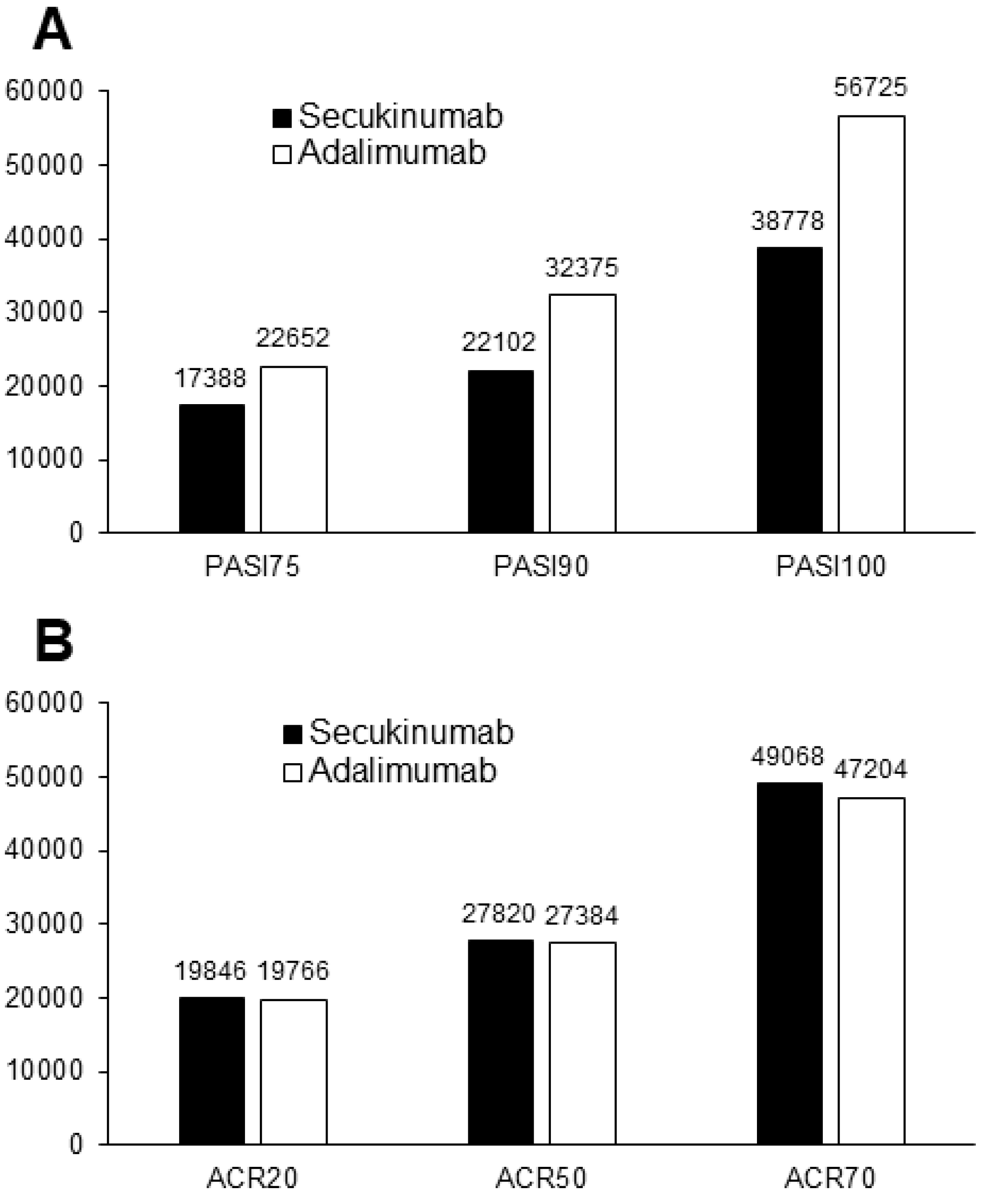
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The global, regional, and national burden of psoriasis: Results and insights from the global burden of disease 2019 study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Altomare, G.; Peris, K.; Martini, P.; Quarta, G.; Congedo, M.; Costanzo, A.; Di Cesare, A.; Lapucci, E.; Chimenti, S. Moderate and severe plaque psoriasis: Cost-of-illness study in Italy. Ther. Clin. Risk Manag. 2008, 4, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nast, A.; Gisondi, P.; Ormerod, A.D.; Saiag, P.; Smith, C.; Spuls, P.I.; Arenberger, P.; Bachelez, H.; Barker, J.; Dauden, E.; et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris--Update 2015--Short version--EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2277–2294. [Google Scholar] [CrossRef] [Green Version]
- Esposti, L.D.; Perrone, V.; Sangiorgi, D.; Buda, S.; Andretta, M.; Rossini, M.; Girolomoni, G. Analysis of drug utilization and health care resource consumption in patients with psoriasis and psoriatic arthritis before and after treatment with biological therapies. Biologics 2018, 12, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, A.; Merola, J.F. Psoriatic arthritis for dermatologists. J. Dermatolog. Treat. 2020, 31, 662–679. [Google Scholar] [CrossRef]
- Amin, M.; Lee, E.B.; Tsai, T.F.; Wu, J.J. Psoriasis and co-morbidity. Acta Derm Venereol. 2020, 100, adv00033. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.S.; Balkrishnan, R.; Camacho, F.T.; Anderson, R.T.; Feldman, S.R. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J. Drugs Dermatol. 2004, 3, 661–666. [Google Scholar] [CrossRef]
- Oliveira Mde, F.; Rocha Bde, O.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. An. Bras. Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.J.; Feldman, S.R.; Ko, J.; Marangell, L.B. Epidemiology of mental health comorbidity in psoriasis. J. Dermatolog. Treat. 2018, 29, 487–495. [Google Scholar] [CrossRef]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and suicidality: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Schupp, C.; Wu, J.; Bebo, B. Quality of life and work productivity impairment among psoriasis patients: Findings from the National Psoriasis Foundation survey data 2003–2011. PLoS ONE 2012, 7, e52935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisondi, P.; Geat, D.; Pizzolato, M.; Girolomoni, G. State of the art and pharmacological pipeline of biologics for chronic plaque psoriasis. Curr. Opin. Pharmacol. 2019, 46, 90–99. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Behrens, F.; Mease, P.J.; Kavanaugh, A.; Ritchlin, C.; Nash, P.; Masmitja, J.G.; Goupille, P.; Korotaeva, T.; Gottlieb, A.B.; et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): A double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020, 395, 1496–1505. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Merola, J.F.; Reich, K.; Behrens, F.; Nash, P.; Griffiths, C.E.M.; Bao, W.; Pellet, P.; Pricop, L.; McInnes, I.B.; et al. Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: Results from EXCEED, a randomized, double-blind head-to-head monotherapy study. Br. J. Dermatol. 2021, 185, 1124–1134. [Google Scholar] [CrossRef]
- Gossec, G.; McGonagle, D.; Korotaeva, T.; Lubrano, E.; de Miguel, E.; Østergaard, M.; Behrens, F. Minimal Disease Activity as a Treatment Target in Psoriatic Arthritis: A Review of the Literature. J. Rheum. 2018, 45, 6–13. [Google Scholar] [CrossRef]
- Available online: https://www.gazzettaufficiale.it/eli/id/2016/11/11/16A07913/sg. (accessed on 3 February 2022).
- Available online: https://www.gazzettaufficiale.it/eli/id/2021/07/28/21A04520/SG#:~:text=Prezzo%20al%20pubblico%20(iva%20inclusa,E%20(in%20base%2010) (accessed on 3 February 2022).
- D’Angiolella, L.S.; Cortesi, P.A.; Lafranconi, A.; Micale, M.; Mangano, S.; Cesana, G.; Mantovani, L.G. Cost and cost effectiveness of treatments for psoriatic arthritis: A systematic literature review. Pharmacoeconomics 2018, 36, 567–589. [Google Scholar] [CrossRef]
- Ogdie, A.; Coates, L.C.; Gladman, D.D. Treatment guidelines in psoriatic arthritis. Rheumatology 2020, 59, i37–i46. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, V.; Sullivan, W.; Graham, C.; Miles, L.; Jugl, S.M.; Gunda, P.; Halliday, A.; Kirkham, B. Cost effectiveness of secukinumab for the treatment of active psoriatic arthritis in the UK. Pharmacoeconomics 2018, 36, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Morales, A.; Cáliz, R.; Aceituno, S.; Prades, M.; Blanch, C. A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis. Reumatol. Clin. 2021, 17, 536–542. [Google Scholar] [CrossRef]
- Gandjour, A.; Ostwald, D.A. Cost effectiveness of secukinumab versus other biologics and apremilast in the treatment of active psoriatic arthritis in Germany. Appl. Health Econ. Health Policy 2020, 18, 109–125. [Google Scholar] [CrossRef]
- Aiello, E.; Bianculli, P.M.; Bhattacharyya, D.; Gunda, P.; Citera, G. Cost-effectiveness of secukinumab versus other biologics in the treatment of psoriatic arthritis: An Argentinean perspective. Value Health Reg. Issues 2019, 20, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Purmonen, T.; Puolakka, K.; Bhattacharyya, D.; Jain, M.; Martikainen, J. Cost-effectiveness analysis of secukinumab versus other biologics and apremilast in the treatment of active psoriatic arthritis: A Finnish perspective. Cost Eff. Resour. Alloc. 2018, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Goeree, R.; Chiva-Razavi, S.; Graham, C.N.; Miles, L.; Nikoglou, E.; Jugl, S.M.; Gladman, D.D. Cost-effectiveness analysis of secukinumab for the treatment of active psoriatic arthritis: A Canadian perspective. J. Med. Econ. 2018, 21, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gunda, P.; Nikoglou, E.; Jugl, S.M.; Murphy, A. A Cost Per Responder Analysis of Secukinumab Vs. Adalimumab Based on a Matching-Adjusted Indirect Comparison of Efficacy Data for the Treatmetn of Psoriatic Arthritis at 48 Weeks from the Irish Payer Perspective. Value Health 2017, 20, A532–A533. [Google Scholar] [CrossRef]
- Puig, L.; Notario, J.; Jimenez-Morales, A.; Moreno-Ramírez, D.; López-Ferrer, A.; Gozalbo, I.; Prades, M.; Lizán, L.; Blanch, C. Secukinumab is the most efficient treatment for achieving clear skin in psoriatic patients: A cost-consequence study from the Spanish National Health Service. J. Dermatol. Treat. 2017, 28, 623–630. [Google Scholar] [CrossRef]
- Augustin, M.; McBride, D.; Gilloteau, I.; O’Neill, C.; Neidhardt, K.; Graham, C.N. Cost-effectiveness of secukinumab as first biologic treatment, compared with other biologics, for moderate to severe psoriasis in Germany. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2191–2199. [Google Scholar] [CrossRef]
- Augustin, M.; Krieger, T.; McBride, D.; Graham, C.N.; Melzer, N.; Kneidl, J.; Neidhardt, K. Cost-effectiveness of secukinumab as first biologic treatment for psoriasis compared with initiating other biologic therapy in Germany. Value Health 2016, 19, A568–A569. [Google Scholar] [CrossRef]
- Colombo, D.; Bianchi, L.; Fabbrocini, G.; Corrao, S.; Offidani, A.; Stingeni, L.; Costanzo, A.; Pellacani, G.; Peris, K.; Bardazzi, F.; et al. Real-world evidence of biologic treatments in moderate-severe psoriasis in Italy: Results of the CANOVA (EffeCtiveness of biologic treAtmeNts for plaque psOriasis in Italy: An obserVAtional longitudinal study of real-life clinical practice) study. Dermatol. Ther. 2022, 35, e15166. [Google Scholar] [CrossRef]
- Zagni, E.; Bianchi, L.; Fabbrocini, G.; Corrao, S.; Offidani, A.; Stingeni, L.; Costanzo, A.; Pellacani, G.; Peris, K.; Bardazzi, F.; et al. A real-world economic analysis of biologic therapies for moderate-to-severe plaque psoriasis in Italy: Results of the CANOVA observational longitudinal study. BMC Health Serv. Res. 2021, 21, 924. [Google Scholar] [CrossRef]
- Sbidian, E.; Chaimani, A.; Garcia-Doval, I.; Doney, L.; Dressler, C.; Hua, C.; Hughes, C.; Naldi, L.; Afach, S.; Le Cleach, L. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 4, CD011535. [Google Scholar] [CrossRef]
- Reich, K.; Warren, R.B.; Coates, L.C.; Di Comite, G. Long-term efficacy and safety of secukinumab in the treatment of the multiple manifestations of psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Soliman, A.M.; Yang, H.; Wang, J.; Freimark, J.; Puig, L. Impact of PASI response on work productivity and the effect of risankizumab on indirect costs using machine learning in patients with moderate-to-severe psoriasis. J. Dermatolog. Treat. 2022, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.B.; Halliday, A.; Graham, C.N.; Gilloteau, I.; Miles, L.; McBride, D. Secukinumab significantly reduces psoriasis-related work impairment and indirect costs compared with ustekinumab and etanercept in the United Kingdom. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A. Safety of secukinumab in the treatment of psoriasis. Expert Opin. Drug Saf. 2016, 15, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Thatiparthi, A.; Martin, A.; Liu, J.; Egeberg, A.; Wu, J.J. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: A review. Am. J. Clin. Dermatol. 2021, 22, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; López-Ferrer, A. Biosimilars for the treatment of psoriasis. Expert Opin. Biol. Ther. 2019, 19, 993–1000. [Google Scholar] [CrossRef]
- Gisondi, P.; Geat, D.; Conti, A.; Dapavo, P.; Piaserico, S.; De Simone, C.; Bianchi, L.; Costanzo, A.; Malagoli, P.; Malara, G.; et al. TNF-α inhibitors biosimilars as first line systemic treatment for moderate-to-severe chronic plaque psoriasis. Expert Rev. Clin. Immunol. 2020, 16, 591–598. [Google Scholar] [CrossRef]
- Barker, J.; Girolomoni, G.; Egeberg, A.; Goncalves, J.; Pieper, B.; Kang, T. Anti-TNF biosimilars in psoriasis: From scientific evidence to real-world experience. J. Dermatolog. Treat. 2020, 31, 794–800. [Google Scholar] [CrossRef]

| Secukinumab | Adalimumab | P ^ | |
|---|---|---|---|
| ACR 20 | 76.4 | 68.3 | 0.07 |
| ACR 50 | 54.5 | 49.3 | 0.22 |
| ACR 70 | 30.9 | 28.6 | 0.29 |
| Minimal disease activity | 44.5 | 34.7 | 0.14 |
| PASI 75 | 87.2 | 59.6 | 0.01 |
| PASI 90 | 68.6 | 41.7 | 0.01 |
| PASI 100 | 39.1 | 23.8 | 0.01 |
| Drug | Dosage | Number of Administrations at Week 52 |
|---|---|---|
| Secukinumab | 300 mg by subcutaneous injection at weeks 0, 1, 2, 3, and 4 followed by 300 mg every 4 weeks | 16 |
| Adalimumab | 80 mg by subcutaneous injection at week 0, followed by 40 mg every other week beginning one week after initial dose | 27 |
| Drug (Trade Name) | Ex-Factory Price per Package ^ | Discount | Discounted Price per Package | Costs at 16 Weeks | Costs at 52 Weeks |
|---|---|---|---|---|---|
| Secukinumab 150 mg | 1050.0 | 5% and 5% | 947.6 | 6633.4 | 15162.1 |
| Adalimumab 40 mg | 1068.5 | 5% and 5% | 964.3 | 4821.6 | 13500.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisondi, P.; Geat, D.; Maurelli, M.; Degli Esposti, L.; Bellinato, F.; Girolomoni, G. Cost per Responder Analysis of Secukinumab versus Adalimumab in the Treatment of Psoriatic Disease. Vaccines 2022, 10, 646. https://doi.org/10.3390/vaccines10050646
Gisondi P, Geat D, Maurelli M, Degli Esposti L, Bellinato F, Girolomoni G. Cost per Responder Analysis of Secukinumab versus Adalimumab in the Treatment of Psoriatic Disease. Vaccines. 2022; 10(5):646. https://doi.org/10.3390/vaccines10050646
Chicago/Turabian StyleGisondi, Paolo, Davide Geat, Martina Maurelli, Luca Degli Esposti, Francesco Bellinato, and Giampiero Girolomoni. 2022. "Cost per Responder Analysis of Secukinumab versus Adalimumab in the Treatment of Psoriatic Disease" Vaccines 10, no. 5: 646. https://doi.org/10.3390/vaccines10050646
APA StyleGisondi, P., Geat, D., Maurelli, M., Degli Esposti, L., Bellinato, F., & Girolomoni, G. (2022). Cost per Responder Analysis of Secukinumab versus Adalimumab in the Treatment of Psoriatic Disease. Vaccines, 10(5), 646. https://doi.org/10.3390/vaccines10050646







