A COVID-19 Vaccine for Dogs Prevents Reverse Zoonosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antigen
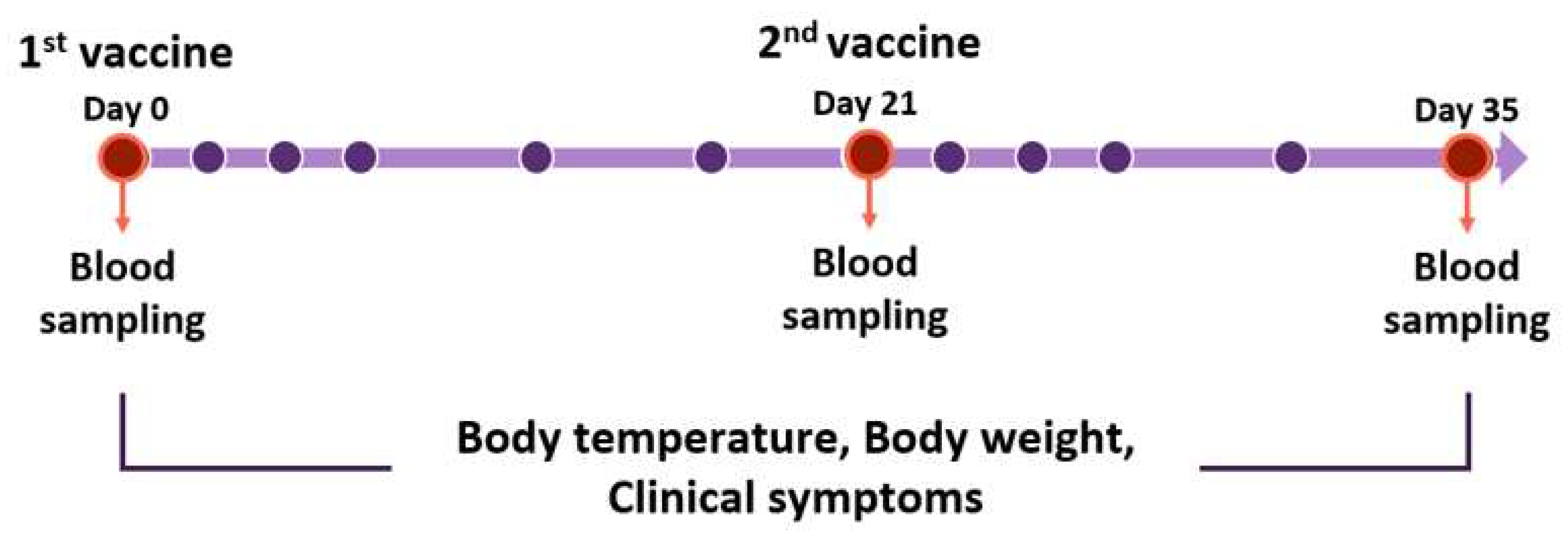
2.3. Vaccination
2.4. Cells and Viruses
2.5. Groups
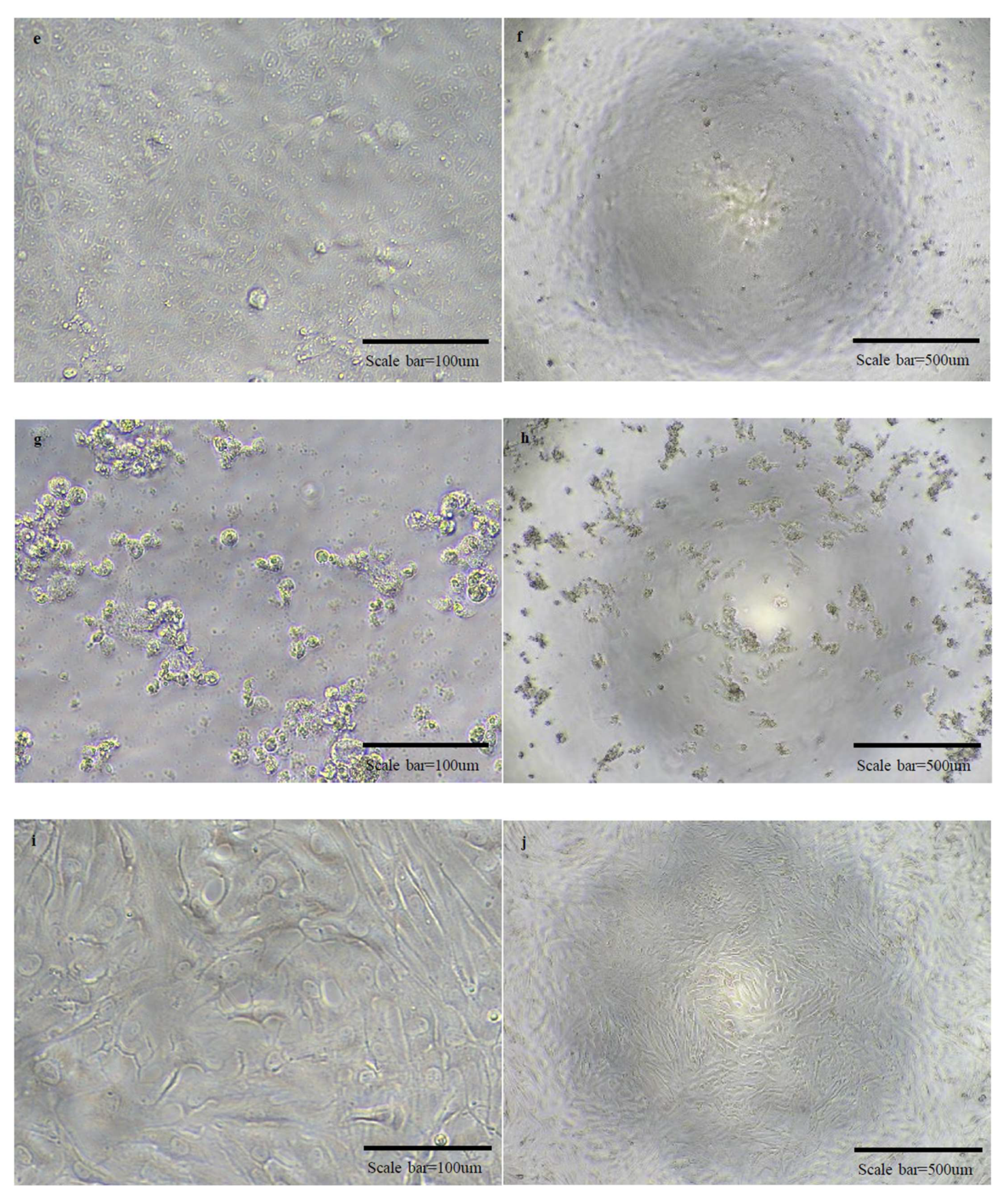
2.6. SN Test
3. Results
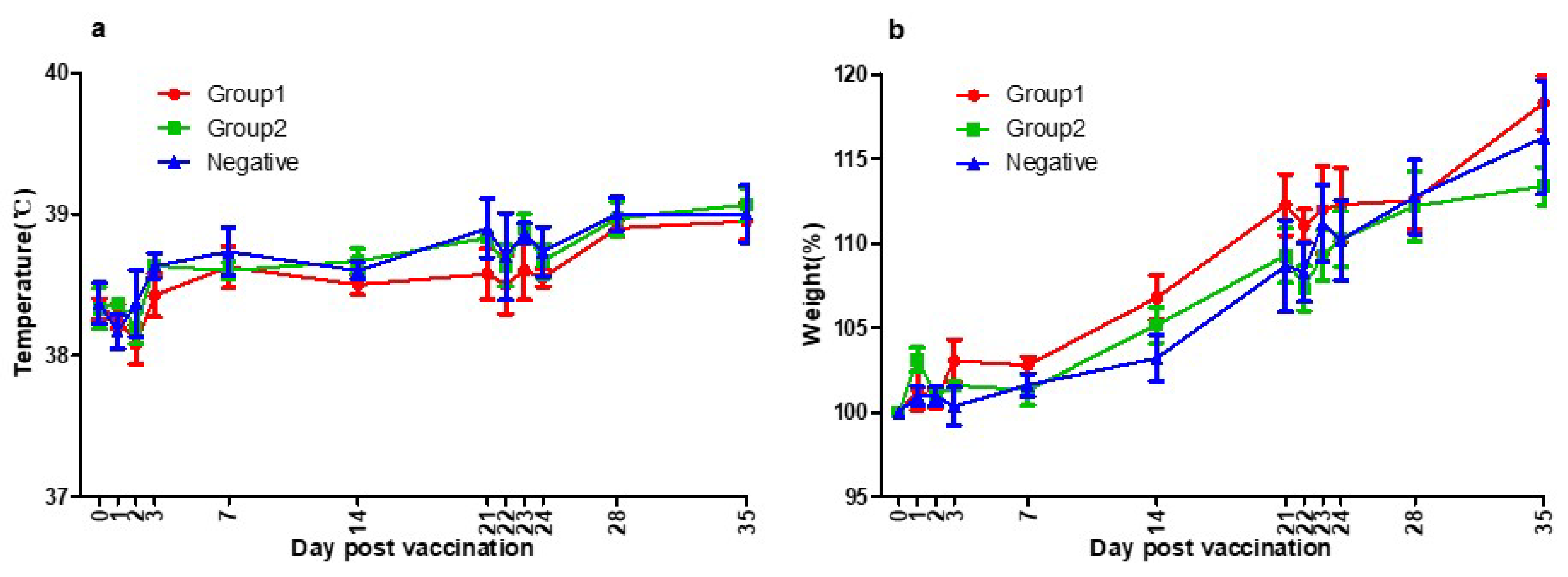
3.1. Side Effects of Vaccination
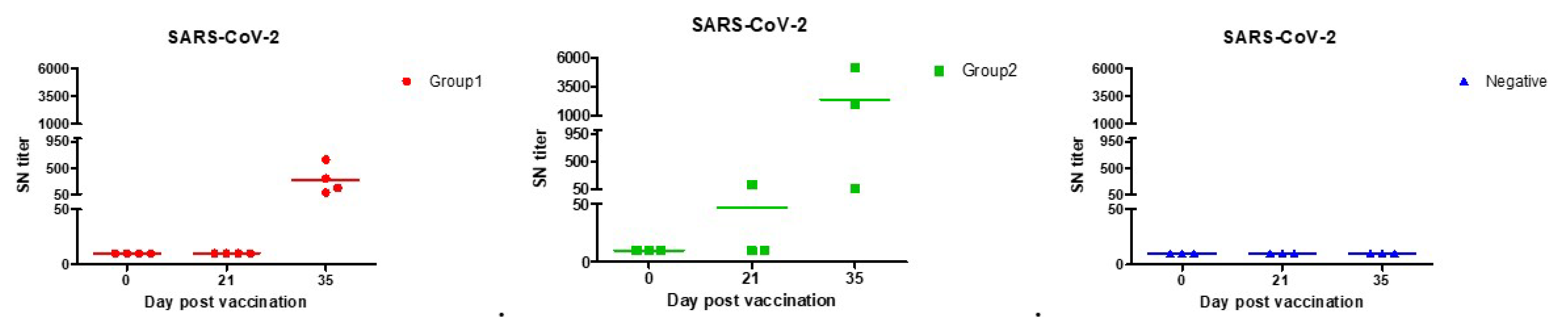
3.2. SN Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pratelli, A.; Tinelli, A.; Decaro, N.; Martella, V.; Camero, M.; Tempesta, M.; Martini, M.; Carmichael, L.; Buonavoglia, C. Safety and efficacy of a modified-live canine coronavirus vaccine in dogs. Vet. Microbiol. 2004, 99, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Priestnall, S.L.; Brownlie, J.; Dubovi, E.J.; Erles, K. Serological prevalence of canine respiratory coronavirus. Vet. Microbiol. 2006, 115, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009, 11, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Clark, M. Bovine coronavirus. Br. Vet. J. 1993, 149, 51–70. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J. Emergence of porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Song, D.S.; Park, B.K. Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J. Vet. Diagn. Investig. 2001, 13, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackwood, M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Li, B.; Yoo, D.; Qin, T.; Zhang, X.; Jia, Y.; Cui, S. Animal coronaviruses and SARS-CoV-2. Transbound. Emerg. Dis. 2021, 68, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Burki, T. The origin of SARS-CoV-2. Lancet Infect. Dis. 2020, 20, 1018–1019. [Google Scholar] [CrossRef]
- Domingo, E.; Holland, J. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 1997, 51, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Sit, T.H.; Brackman, C.J.; Ip, S.M.; Tam, K.W.; Law, P.Y.; To, E.M.; Veronica, Y.; Sims, L.D.; Tsang, D.N.; Chu, D.K. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Hatta, M.; Chiba, S.; Maemura, T.; Fan, S.; Takeda, M.; Kinoshita, N.; Hattori, S.-i.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K. Transmission of SARS-CoV-2 in domestic cats. N. Engl. J. Med. 2020, 383, 592–594. [Google Scholar] [CrossRef]
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Munnink, B.B.O.; Hakze-van Der Honing, R.W.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S. SARS-CoV-2 infection in farmed minks, the Netherlands, April and 20. Eurosurveillance 2020, 25, 2001005. [Google Scholar] [CrossRef] [PubMed]
- McAloose, D.; Laverack, M.; Wang, L.; Killian, M.L.; Caserta, L.C.; Yuan, F.; Mitchell, P.K.; Queen, K.; Mauldin, M.R.; Cronk, B.D. From people to Panthera: Natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. MBio 2020, 11, e02220-20. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.M. Influenza as a model system for studying the cross–species transfer and evolution of the SARS coronavirus. Philos. Trans. Royal Soc. London Series B Biol. Sci. 2004, 359, 1067–1073. [Google Scholar] [CrossRef]
- Ma, W.; Kahn, R.E.; Richt, J.A. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J. Mol. Genet. Med. Int. J. Biomed. Res. 2009, 3, 158. [Google Scholar] [CrossRef]
- McLean, A.R.; May, R.M.; Pattison, J.; Weiss, R.A. SARS: A Case Study in Emerging Infections; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Wong, G.; Liu, W.; Liu, Y.; Zhou, B.; Bi, Y.; Gao, G.F. MERS, SARS, and Ebola: The role of super-spreaders in infectious disease. Cell Host Microbe 2015, 18, 398–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, R.L.; Baric, R.S. Recombination, reservoirs, and the modular spike: Mechanisms of coronavirus cross-species transmission. J. Virol. 2010, 84, 3134–3146. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Zhang, Z.; Gao, X.; Du, P.; Lu, J.; Yan, B.; Wang, C.; Zheng, Y.; Huang, H.; Sun, Q. N501Y mutation imparts cross-species transmission of SARS-CoV-2 to mice by enhancing receptor binding. Signal Trans. Targ. Ther. 2021, 6, 1–3. [Google Scholar] [CrossRef]
- Yuan, S.; Nelsen, C.J.; Murtaugh, M.P.; Schmitt, B.J.; Faaberg, K.S. Recombination between North American strains of porcine reproductive and respiratory syndrome virus. Virus Res. 1999, 61, 87–98. [Google Scholar] [CrossRef]
- Tolou, H.; Couissinier-Paris, P.; Durand, J.-P.; Mercier, V.; de Pina, J.-J.; De Micco, P.; Billoir, F.; Charrel, R.; De Lamballerie, X. Evidence for recombination in natural populations of dengue virus type 1 based on the analysis of complete genome sequences. J. Gen. Virol. 2001, 82, 1283–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, V.; Descamps, V.; François, C.; Fournier, C.; Brochot, E.; Capron, D.; Duverlie, G.; Castelain, S. Emergence of a genomic variant of the recombinant 2k/1b strain during a mixed hepatitis C infection: A case report. J. Clin. Virol. 2010, 47, 382–386. [Google Scholar] [CrossRef]
- Lai, M.; Baric, R.; Makino, S.; Keck, J.; Egbert, J.; Leibowitz, J.; Stohlman, S. Recombination between nonsegmented RNA genomes of murine coronaviruses. J. Virol. 1985, 56, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keck, J.; Matsushima, G.; Makino, S.; Fleming, J.; Vannier, D.; Stohlman, S.; Lai, M. In vivo RNA-RNA recombination of coronavirus in mouse brain. J. Virol. 1988, 62, 1810–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masters, P.S.; Koetzner, C.A.; Kerr, C.A.; Heo, Y. Optimization of targeted RNA recombination and mapping of a novel nucleocapsid gene mutation in the coronavirus mouse hepatitis virus. J. Virol. 1994, 68, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Karaca, K.; Parrish, C.; Naqi, S. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch. Virol. 1995, 140, 259–271. [Google Scholar] [CrossRef]
- Lai, M.M. Recombination in large RNA viruses: Coronaviruses. In Seminars in Virology; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Wu, Y. Strong evolutionary convergence of receptor-binding protein spike between COVID-19 and SARS-related coronaviruses. bioRxiv 2020. preprint. [Google Scholar] [CrossRef] [Green Version]
- Ortega, J.T.; Serrano, M.L.; Pujol, F.H.; Rangel, H.R. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. EXCLI J. 2020, 19, 410. [Google Scholar]
- Liu, Z.; Xiao, X.; Wei, X.; Li, J.; Yang, J.; Tan, H.; Zhu, J.; Zhang, Q.; Wu, J.; Liu, L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020, 92, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, M.; Song, W.; Zhou, H.; Xu, J.; Chen, S.; Xiang, Y.; Wang, X. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017, 27, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Gui, M.; Wang, X.; Xiang, Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018, 14, e1007236. [Google Scholar] [CrossRef] [PubMed]
- Kirchdoerfer, R.N.; Wang, N.; Pallesen, J.; Wrapp, D.; Turner, H.L.; Cottrell, C.A.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Cao, D.; Zhang, Y.; Ma, J.; Qi, J.; Wang, Q.; Lu, G.; Wu, Y.; Yan, J.; Shi, Y. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xiang, R.; Deng, X.; Wang, L.; Yu, Z.; Tian, S.; Liang, R.; Li, Y.; Ying, T.; Jiang, S. Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2. Signal Transd. Targ. Ther. 2020, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Noor, R. Developmental Status of the Potential Vaccines for the Mitigation of the COVID-19 Pandemic and a Focus on the Effectiveness of the Pfizer-BioNTech and Moderna mRNA Vaccines. Curr. Clin. Microbiol. Rep. 2021, 8, 178–185. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human coronavirus-229E,-OC43,-NL63, and-HKU1. Encyclopedia Virol. 2020, 428–440. [Google Scholar] [CrossRef]
- Zeng, Z.-Q.; Chen, D.-H.; Tan, W.-P.; Qiu, S.-Y.; Xu, D.; Liang, H.-X.; Chen, M.-X.; Li, X.; Lin, Z.-S.; Liu, W.-K. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: A study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erles, K.; Toomey, C.; Brooks, H.W.; Brownlie, J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 2003, 310, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Xu, Y.; Shi, F.; Hu, S. Vaccination at different anatomic sites induces different levels of the immune responses. Res. Vet. Sci. 2019, 122, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Minke, J.; Bouvet, J.; Cliquet, F.; Wasniewski, M.; Guiot, A.; Lemaitre, L.; Cariou, C.; Cozette, V.; Vergne, L.; Guigal, P. Comparison of antibody responses after vaccination with two inactivated rabies vaccines. Vet. Microbiol. 2009, 133, 283–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, H.S.; Calisher, C.H.; Mathews, J.H. Serum dilution neutralization test for California group virus identification and serology. J. Clin. Microbiol. 1976, 4, 503–510. [Google Scholar] [CrossRef]
- Yoon, J.; Joo, H.S.; Goyal, S.M.; Molitor, T.W. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J. Vet. Diagn. Investig. 1994, 6, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Prot. 2020, 15, 3699–3715. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Colavita, F.; Lapa, D.; Meschi, S.; Bordi, L.; Piselli, P.; Gagliardini, R.; Corpolongo, A.; Nicastri, E.; Antinori, A. SARS-CoV-2 Serum neutralization assay: A traditional tool for a brand-new virus. Viruses 2021, 13, 655. [Google Scholar] [CrossRef]
- Nelson, R.W.; Couto, C.G. Small Animal Internal Medicine, E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Cristiano, A.; Nuccetelli, M.; Pieri, M.; Sarubbi, S.; Pelagalli, M.; Calugi, G.; Tomassetti, F.; Bernardini, S. Serological anti-SARS-CoV-2 neutralizing antibodies association to live virus neutralizing test titers in COVID-19 paucisymptomatic/symptomatic patients and vaccinated subjects. Int. Immunopharmacol. 2021, 101, 108215. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Michalek, S.M.; Katz, J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun. 2003, 71, 2498–2507. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Hotez, P.J.; Bottazzi, M.E. Potential for developing a SARS-CoV receptor-binding domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19. Human Vacc. Immunother. 2020, 16, 1239–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, W.; Zhang, X.; Drelich, A.; Shi, J.; Hsu, J.C.; Luchsinger, L.; Hillyer, C.D.; Tseng, C.-T.K.; Jiang, S.; Du, L. A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 2020, 30, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.M.; Goodwin, E.C.; Verma, A.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E.; Gouma, S.; McAllister, C.M.; Christensen, S.R.; Weaver, J. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 2021, 184, 1858–1864.e10. [Google Scholar] [CrossRef] [PubMed]


| Per Dose | ||
|---|---|---|
| Antigen | S1 (Spike Protein 1) | 60 μg |
| RBD (Receptor binding domain) | 60 μg | |
| Adjuvant | Montanide gel (PR02) | 10%(vol) |
| Stimulant | MPL (Sigma) | 50 μg |
| Group | Immunization Route | Vaccine | Number of Dogs |
|---|---|---|---|
| Group 1 | S.C | FCoV-19 | 4 |
| Group 2 | S.C | FCoV-19+stl 1 | 3 |
| Unvaccinated | S.C | PBS | 3 |
| Paucisymptomatic Patients | Symptomatic Patients | Vaccinated People | Group 1 | Group 2 | Negative | ||
|---|---|---|---|---|---|---|---|
| SN titer | 1:80 | 1:160 | SN titer (1st) | 40 | 10 | 47 | 10 |
| SN titer (2nd) | 160 | 226 | 833 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ga, E.; Won, Y.; Hwang, J.; Moon, S.; Yeom, M.; Lyoo, K.; Song, D.; Han, J.; Na, W. A COVID-19 Vaccine for Dogs Prevents Reverse Zoonosis. Vaccines 2022, 10, 676. https://doi.org/10.3390/vaccines10050676
Ga E, Won Y, Hwang J, Moon S, Yeom M, Lyoo K, Song D, Han J, Na W. A COVID-19 Vaccine for Dogs Prevents Reverse Zoonosis. Vaccines. 2022; 10(5):676. https://doi.org/10.3390/vaccines10050676
Chicago/Turabian StyleGa, Eulhae, Yongkwan Won, Jaehyun Hwang, Suyun Moon, Minju Yeom, Kwangsoo Lyoo, Daesub Song, Jeonghee Han, and Woonsung Na. 2022. "A COVID-19 Vaccine for Dogs Prevents Reverse Zoonosis" Vaccines 10, no. 5: 676. https://doi.org/10.3390/vaccines10050676






